
Using preoperative four-dimensional automated left atrial quantification echocardiography to predict postoperative atrial fibrillation in patients undergoing cardiac surgery
Introduction
Postoperative atrial fibrillation (POAF) is a common complication in adults after cardiac surgery. POAF has an overall approximate incidence of 30%; however, its exact incidence depends on the type of surgery performed; for example, the incidence of coronary artery bypass grafting (CABG) is approximately 20%, while that of valve surgery is approximately 40–50% (1). POAF generally occurs in the first 5 days after surgery, and has the highest incidence between the second and third days after surgery (2). Patients who develop POAF have longer hospital stays, increased expenses, and higher rates of both short- and long-term complications, such as stroke, myocardial infarction, and mortality (3). Thus, identifying patients with a higher risk of POAF and implementing appropriate prophylactic strategies during the perioperative period is critical. The mechanism underlying AF development is not yet fully understood; however, left atrial (LA) remodeling has been shown to play a significant role in atrial fibrillation (AF) development (4). LA remodeling results in LA enlargement and dysfunction. Thus, the analysis of LA structural and functional abnormalities before surgery may be useful in predicting patients at higher risk of developing POAF.
Several previous studies have reported that LA enlargement as assessed by two-dimensional echocardiography (2DE) is an independent predictor of POAF after cardiac surgery (5-7). However, the application of a simple geometric model to a non-symmetrical chamber in 2DE technology to assess LA enlargement is indirect and limited. Atrial strain is defined as the fractional change of the length/width/thickness of one object in comparison to its original length/width/thickness. Atrial strain describes the deformation capacity of atrial myocardium, reflects the atrial mechanical function, has been recently used to assess LA function, and is linked to an increased risk of POAF (8-10). Previous studies measured atrial strain using two-dimensional speckle-tracking echocardiography (STE); however, it could only capture longitudinal strain and failed to capture deformation along the circumferential muscle fibers of the LA wall (11-13). Given the presence of both circumferential and longitudinal fibers in the LA myocardium, LA circumferential strain has the potential to provide complementary information (14). The four-dimensional automated LA quantification (4D auto LAQ) tool is a novel automated adaptive LA analysis technique that can be used to quantify and assess LA structure and function simply and rapidly. The biggest advantage of this tool over 2DE is that it can assess the real three-dimensional volume and circumferential strain of LA.
This study aimed to prospectively assess the value of 4D auto LAQ tool-derived risk factors, such as volume and strain parameters, as predictors of POAF following cardiac surgery, and to establish a POAF prediction model that combined clinical and 4D auto LAQ parameters. We present this article in accordance with the TRIPOD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1915/rc).
Methods
Study population
Patients undergoing cardiac surgery were consecutively enrolled in the study between January 2023 and December 2023. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Biomedical Research Ethics Committee of The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University (No. IIT-O-2024-230), and all participants provided written informed consent. Participants had to be in sinus rhythm as documented by a 12-lead electrocardiogram (ECG). Eligible patients included those undergoing CABG surgery, as well as surgery involving the valve, ascending aorta, pericardium, cardiac masses, and myomectomy. Patients were excluded from the study if they met any of the following exclusion criteria: were aged <18 years; had present or prior episodes of AF; had taken antiarrhythmic drugs (other than β-blockers) preoperatively; had congenital heart defects; had thyroid abnormalities; had images with a poor acoustic window, of insufficient quality, in which part of the LA was missing, or in which the endocardial boundaries were blurred, making 4D auto LAQ analysis unfeasible.
To prevent POAF, our study only examined preoperative and intraoperative risk factors. All the patients underwent conventional 2DE and 4D auto LAQ echocardiography evaluations before surgery. The patients were followed up for the development of POAF during their in-hospital stay until discharge. In detail, a comprehensive preoperative clinical examination of the patients was performed, and data on patients’ demographic characteristics, cardiovascular risk factors, and the preoperative use of medication were prospectively collected and recorded in a computerized database. In addition, blood sample tests were performed before surgery to measure patients’ erythrocyte sedimentation rate, white blood cell (WBC) count, neutrophil-lymphocyte ratio, creatinine, and pro-brain natriuretic peptide (pro-BNP). During the surgical procedure, data on the following parameters were collected: red blood cell (RBC) transfusion, surgical approach (minimally invasive or median sternotomy), and type of surgery (valve or non-valve). After surgery, data on in-hospital death, severe postoperative complications (e.g., pericardial tamponade, acute myocardial infarction, and acute renal failure), length of hospitalization stay, and costs were collected.
Echocardiographic analysis
The echocardiographic examinations were conducted at rest, using a high-quality ultrasound machine (Vivid E95, GE Medical Systems, Horten, Norway) with the participants in the left lateral decubitus position. A three-lead ECG was used to continually record heart rate (HR).
2DE and Doppler image acquisition and analysis
A M5S transducer was used for the conventional ultrasonic parameters. The measurements involved followed the current guidelines of the American Society of Echocardiography (15). The LA diameter (LAD), left ventricle end-diastolic diameter (LVEDD), and left ventricle end-systolic diameter (LVESD) were examined on the LV long-axis plane. The left ventricle ejection fraction (LVEF) was calculated using the modified Simpson’s method. The peak value of the early diastolic velocity of mitral inflow (E) was examined by pulse doppler on the apical four-chamber plane. The peak early diastolic velocity of the mitral annulus on the left ventricular septum and lateral wall (e’) was measured by tissue doppler on the apical four-chamber plane. The mean e’ and E/e’ were calculated.
4D auto LAQ image acquisition and analysis
A 4Vc-D transducer was employed for the 4D LA parameters. A 4D probe was used to collect full-volume LA dynamic images from the apical four-chamber view, where the frame rate was adjusted to be >40% of the participants’ HRs, and the participants were encouraged to hold their breath to obtain the dynamic images. Dynamic images were continuously collected for at least five consecutive cardiac cycles for each participant, and the three best-quality images were selected for the offline analysis.
The offline analysis was then performed using commercially available semiautomatic software packages (4D auto LAQ, Echo PAC, GE Healthcare, Horten, Norway). By entering the 4D auto LAQ analysis interface, and setting the target point at the intersection of the mitral valve center, the software automatically detected and tracked the LA endocardial boundaries, and was manually adjusted when the automatic identification was unsatisfactory. The results were used to obtain the 4D LA parameters. The volume parameters included the LA minimum volume (LAVmin), LA maximum volume (LAVmax), LA pre-systolic volume (LAVpreA), LAVmax indicator (LAVI), LA emptying volume (LAEV), LA ejection fraction (LAEF). The LAEV was calculated as follows: LAEV = LAVmax − LAVmin. The LAEF was calculated as follows: LAEF = (LAVmax − LAVmin)/LAVmax. The strain parameters included the LA reservoir longitudinal strain (LASr), LA conduit longitudinal strain (LAScd), and LA contraction longitudinal strain (LASct), and the LA reservoir circumferential strain (LASr-c), LA conduit circumferential strain (LAScd-c), and LA contraction circumferential strain (LASct-c). LASr and LASr-c represent the differences in the strain value at the mitral valve opening minus the ventricular end diastole; LAScd and LAScd-c represent the differences in the strain value at the onset of atrial contraction minus the mitral valve opening; LASct and LASct-c represent the differences in the strain value at the ventricular end diastole minus the onset of atrial contraction. Notably, LAScd/LAScd-c, and LASct/LASct-c have negative values, as the atrial muscle fibers shorten during ventricular diastole, and only the absolute value reflects the function condition. Figure 1 shows the 4D auto LAQ method used to analyze the 4D LA parameters in the research participants.
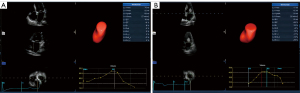
Endpoint
The endpoint of the study was the incidence of AF following the procedure up until discharge. Each participant received treatment in a critical care unit, and was continuously monitored, and their data were recorded. During the hospital stay in the ward, the cardiac rhythm was evaluated by daily ECG, and an additional ECG was performed as soon as there was any suspicion of arrhythmia. The diagnosis of AF was made using the ECG trace, which showed an erratic rhythm without any detectable P waves for at least 30 seconds.
Statistical analysis
The continuous variables are expressed as the median (interquartile range), or the mean ± standard deviation, and were compared using the Mann-Whitney U test or the t-test. The categorical variables are expressed as the number and percentage, and were compared using the χ2 or Fisher’s exact test as appropriate. The multiple imputation method was employed to address missing data. For easier clinical application, all the continuous variables were converted into binary variables in the logistic regression analysis. A receiver operating characteristic (ROC) curve analysis was performed on all the continuous variables. The best cut-off values were used as the boundaries for the statistically significant continuous variables, while the usual clinical or ultrasound values were used as the boundaries for the non-statistically significant continuous variables. A univariate analysis was performed, and variables with P values <0.05 were included in the least absolute shrinkage and selection operator (LASSO) regression analysis. To optimize the potential collinearity and avoid overfitting of variables, LASSO regression was performed to screen out risk factors, after which, a multivariate logistic analysis using a stepwise approach was performed to build a prediction model. The discrimination of the model was evaluated using ROC curves, and the calibration was assessed by the Hosmer-Lemeshow (H-L) test. Areas under the curve (AUCs) were compared using DeLong’s test. The model was internally validated using the five-fold cross-validation method. To assess the repeatability and reproducibility of the 4D auto LAQ parameters measurements, 15 patients were randomly selected. The analysis was repeated 1 week later by the same observer and by a second independent observer, and a Bland-Altman analysis was performed to evaluate the intra- and inter-observer agreement. The statistical analysis was performed using SPSS software (version 26.0) and R software (version 4.3.2).
Results
General data and occurrence of POAF
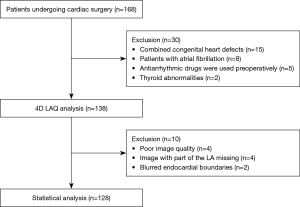
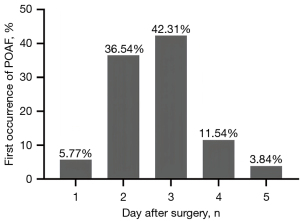
Initially, 168 cardiac surgery patients were identified for inclusion in the study, but 40 patients were subsequently excluded. Thus, ultimately, 128 patients were enrolled in the study (Figure 2). The following surgeries were performed: valve surgery (60.1%), CABG (11.7%), cardiac mass excision (10.2%), combined valve and ascending aorta surgery (10.2%), combined valve and CABG surgery (3.9%), ascending aorta surgery (2.3%), and combined valve, CABG, and ascending aorta surgery (1.6%). POAF occurred in 52 patients (40.6%), mostly within the first 48 or 72 hours of surgery. Histograms showing the distribution of the occurrence of the first episode of POAF are depicted in Figure 3. These patients formed the POAF group, and the remaining 76 formed the no-POAF group.
The baseline and pre-operative and operative characteristics of both groups are shown in Table 1. There were statistically significant differences between the two groups in terms of age, pro-BNP level, RBC transfusion during surgery, the surgical approach, and the type of surgery. However, there were no statistically significant differences between the two groups in terms of the other demographic, clinical, medication, and laboratory characteristics.
Table 1
| Variables | No POAF (n=76) | POAF (n=52) | P value |
|---|---|---|---|
| Demographic characteristics | |||
| Male | 49 (64.5) | 32 (61.5) | 0.735 |
| Age (years) | 52.5 [13.0] | 60.0 [14.0] | <0.001* |
| BMI (kg/m2) | 23.0 [4.8] | 23.0 [3.7] | 0.579 |
| Cardiovascular risk factors | |||
| Smoking | 25 (32.9) | 18 (34.6) | 0.840 |
| Hyperlipidemia | 29 (28.2) | 13 (25.0) | 0.119 |
| Hypertension | 24 (31.6) | 19 (36.5) | 0.560 |
| Diabetes | 10 (13.2) | 7 (13.5) | 0.960 |
| CHD | 17 (22.4) | 9 (17.3) | 0.485 |
| COPD | 11 (14.5) | 10 (19.2) | 0.475 |
| Stroke or TIA | 18 (23.7) | 12 (23.1) | 0.937 |
| Preoperative laboratory data | |||
| ESR (mm/h) | 21.5 [28] | 21.5 [28] | 0.648 |
| WBC (×109/L) | 6.8 [2.19] | 5.98 [3.14] | 0.178 |
| NLR | 3.36 [1.81] | 3.51 [2.32] | 0.341 |
| Creatinine (μmol/L) | 79.3 [34.5] | 81.0 [29.4] | 0.543 |
| Pro-BNP >300 pg/mL | 28 (36.8) | 30 (57.7) | 0.020* |
| Preoperative medication | |||
| β-blockers | 19 (25.0) | 19 (36.5) | 0.161 |
| ACEI/ARB | 13 (17.1) | 11 (21.2) | 0.564 |
| Calcium channel blockers | 10 (13.2) | 7 (13.5) | 0.960 |
| Statins | 17 (22.4) | 13 (25.0) | 0.730 |
| Operative data | |||
| Minimally invasive | 36 (47.4) | 15 (28.8) | 0.036* |
| Valve surgery | 50 (65.8) | 47 (90.4) | 0.001* |
| RBC transfusion | 12 (15.8) | 18 (34.6) | 0.014* |
| Hospitalization data | |||
| Hospitalization stays (days) | 15.92 [6] | 17.54 [9] | 0.039* |
| Hospitalization cost (CNY) | 99,100.20 [29,441.42] | 123,004.25 [67,639.17] | <0.001* |
| Preoperative echocardiographic data | |||
| LAD (mm) | 39.0 [10] | 44.0 [9.0] | <0.001* |
| LVEDD (mm) | 53 [13] | 56 [16] | 0.043* |
| LVESD (mm) | 34.5 [10] | 36.5 [16] | 0.06 |
| LVEF (%) | 63 [8] | 60.5 [13] | 0.103 |
| E/e' | 10.74 [6.21] | 14.47 [14.54] | <0.001* |
| LAVmin (mL) | 30 [25] | 57 [30] | <0.001* |
| LAVmax (mL) | 52.5 [34] | 81 [37] | <0.001* |
| LAVpreA (mL) | 44.5 [29] | 72.5 [36] | <0.001* |
| LAVI (mL/m2) | 32.5 [17] | 49 [27] | <0.001* |
| LAEV (mL) | 22 [11] | 23 [12] | 0.426 |
| LAEF (%) | 41.24±9.06 | 30.73±9.80 | <0.001* |
| LASr (%) | 16 [8] | 9 [8] | <0.001* |
| |LAScd| (%) | 6 [8] | 4 [6] | 0.045* |
| |LASct| (%) | 9 [6] | 5 [6] | <0.001* |
| LASr-c (%) | 18.5 [12] | 10.5 [11] | <0.001* |
| |LAScd-c| (%) | 6 [10] | 5 [6] | 0.077 |
| |LASct-c| (%) | 11 [8] | 5 [10] | <0.001* |
Data are presented as n (%), median [IQR], or mean ± SD. *, P<0.05. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; CHD, coronary heart disease; CNY, China Yuan; COPD, chronic obstructive pulmonary disease; ESR, erythrocyte sedimentation rate; IQR, interquartile range; LAD, left atrial diameter; LAEF, left atrial ejection fraction; LAEV, left atrial emptying volume; LAScd, left atrial conduit longitudinal strain; LAScd-c, left atrial conduit circumferential strain; LASct, left atrial contraction longitudinal strain; LASct-c, left atrial contraction circumferential strain; LASr, left atrial reservoir longitudinal strain; LASr-c, left atrial reservoir circumferential strain; LAVI, left atrial maximum volume indicator; LAVmax, left atrial maximum volume; LAVmin, left atrial minimum volume; LAVpreA, left atrial pre-systolic volume; LVEDD, left ventricle end-diastolic diameter; LVEF, left ventricle ejection fraction; LVESD, left ventricle end-systolic diameter; NLR, neutrophil-to-lymphocyte ratio; POAF, postoperative atrial fibrillation; RBC, red blood cell; SD, standard deviation; TIA, transient ischemic attack; WBC, white blood cell.
Compared to the no-POAF patients, the hospitalization stays of the POAF patients were longer, and their expenses were higher (Table 1). During hospitalization, the rate of acute renal injury or ventricular arrhythmia was 3.13% (four patients), the rate of acute myocardial infarction, re-exploration for bleeding or low cardiac output syndrome was 2.34% (three patients), the rate of stroke was 1.56% (two patients), and the rate of lung infection and deep venous thrombosis was 0.78% (one patient); there was no significant difference in the total complications between the two groups (P>0.05). The in-hospital mortality rate was 0.78% (one patient).
Echocardiographic data
Table 1 also sets out the all preoperative 2DE and 4D auto LAQ parameters. The POAF patients had a significantly higher LVEDD, E/e’ ratio, and LA size (including LAD, LAVmin, LAVmax, LAVpreA, and LAVI). They also had significantly lower LAEF and LA strains (including LASr, |LAScd|, |LASct|, LASr-c, and |LASct-c|).
Repeatability and reproducibility
The inter-observer correlation coefficients for the 4D auto LAQ parameters evaluated by the two separate investigators were all >0.9, and the intra-observer correlation coefficients measured by the same investigator on two different occasions were also all >0.9 (Table 2), indicating good intra- and inter-observer agreement in the LA volume and strain measurements.
Table 2
| Variables | Inter-observer | Intra-observer | |||||
|---|---|---|---|---|---|---|---|
| Correlation coefficient | 95% CI | P value | Correlation coefficient | 95% CI | P value | ||
| LAVmin | 0.998 | 0.988–0.999 | <0.001 | 0.999 | 0.996–1.000 | <0.001 | |
| LAVmax | 0.998 | 0.994–0.999 | <0.001 | 0.998 | 0.994–0.999 | <0.001 | |
| LAVpreA | 0.998 | 0.994–0.999 | <0.001 | 0.999 | 0.998–1.000 | <0.001 | |
| LAVI | 0.998 | 0.993–0.999 | <0.001 | 0.998 | 0.995–0.999 | <0.001 | |
| LAEV | 0.985 | 0.957–0.995 | <0.001 | 0.990 | 0.972–0.997 | <0.001 | |
| LAEF | 0.981 | 0.945–0.994 | <0.001 | 0.988 | 0.965–0.996 | <0.001 | |
| LASr | 0.945 | 0.836–0.982 | <0.001 | 0.964 | 0.892–0.988 | <0.001 | |
| LAScd | 0.952 | 0.859–0.984 | <0.001 | 0.986 | 0.959–0.995 | <0.001 | |
| LASct | 0.976 | 0.929–0.992 | <0.001 | 0.988 | 0.964–0.996 | <0.001 | |
| LASr-c | 0.990 | 0.969–0.997 | <0.001 | 0.994 | 0.983–0.998 | <0.001 | |
| LAScd-c | 0.974 | 0.924–0.991 | <0.001 | 0.981 | 0.944–0.994 | <0.001 | |
| LASct-c | 0.984 | 0.952–0.995 | <0.001 | 0.991 | 0.972–0.997 | <0.001 | |
CI, confidence interval; LAEF, left atrial ejection fraction; LAEV, left atrial emptying volume; LAScd, left atrial conduit longitudinal strain; LAScd-c, left atrial conduit circumferential strain; LASct, left atrial contraction longitudinal strain; LASct-c, left atrial contraction circumferential strain; LASr, left atrial reservoir longitudinal strain; LASr-c, left atrial reservoir circumferential strain; LAVI, left atrial maximum volume indicator; LAVmax, left atrial maximum volume; LAVmin, left atrial minimum volume; LAVpreA, left atrial pre-systolic volume.
Conversion of continuous variables to binary variables
The statistically significant continuous variables in the ROC analysis included age, LAD, LVEDD, LVESD, E/e’, LAVmin, LAVmax, LAVpreA, LAVI, LAEV, LAEF, LASr, |LAScd|, |LASct|, LASr-c, |LAScd-c|, and |LASct-c|, which had best cut-off values of 52 years, 43 mm, 56 mm, 36 mm, 14, 50 mL, 75 mL, 65 mL, 35 mL/m2, 30 mL, 37%, 13%, 6%, 10%, 16%, 8%, and 8%, respectively. The usual clinical or ultrasound parameters were used as the boundaries for the remaining non-statistically significant continuous variables; for example, the WBC count was bounded by 10×109/L, and LVEF was bounded by 50%.
Establishing the POAF model
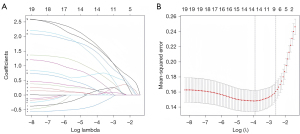
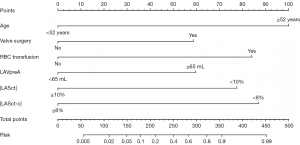
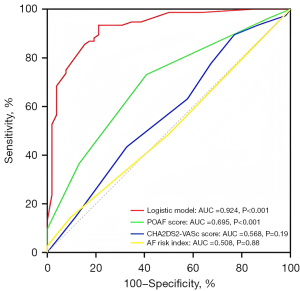
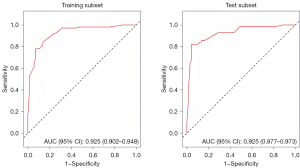
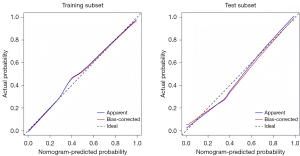
The univariate analysis results revealed statistically significant differences in 19 variables between the two groups (Table 3). A LASSO regression analysis of these 19 variables was conducted, and nine risk factors were screened out; that is, age, RBC transfusion, valve surgery, LAVpreA, LAVI, LAEF, LASr, LASct, and LASct-c (Figure 4). Ultimately, only an age ≥52 years [odds ratio (OR) =11.628; P<0.001], RBC transfusion during surgery (OR =8.084; P=0.005), valve surgery (OR =4.870; P=0.033), LAVpreA ≥65 mL (OR =3.779; P=0.034), |LASct| <10% (OR =6.290; P=0.017), and |LASct-c| <8% (OR =6.915; P=0.003) remained independently associated with POAF in the multivariate analysis (Table 3). The results of the multivariate analysis were presented by an intuitive predictive nomogram (Figure 5). The H-L test results showed that our model had good calibration (P=0.672>0.05). The model had an AUC value of 0.924, a sensitivity of 78.8%, and a specificity of 93.4%. Our model showed high discriminatory ability in comparison to three commonly used clinical models; that is, the POAF score (which includes seven factors of age, chronic obstructive pulmonary diseases, estimated glomerular filtration rate, emergency, preoperative intra-aortic balloon pump, LVEF, and valve surgery), the CHA2DS2-VASc score (which includes seven factors of congestive heart failure/left ventricular dysfunction, hypertension, age, diabetes mellitus, stroke/transient ischemic attack/thromboembolism, vascular disease, and sex), and the AF risk index (which includes four factors of age, weight, height, and peripheral and vascular disease) (16-18), and which had AUC values of 0.695, 0.568, and 0.508, respectively (Figure 6). Delong’s test indicated that the difference in the AUC values between our model and the three models was statistically significant (all P<0.001). According to the five-fold cross-validation results, the AUC values of the training and test subsets were equally 0.925, the model had strong discriminatory ability (Figure 7). The calibration curves of the training and test subsets showed that the prediction probability contrast lines were near the ideal complete agreement lines, indicating good model calibration (Figure 8).
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| β value | OR (95% CI) | P value | β value | OR (95% CI) | P value | ||
| Male | 0.126 | 1.134 (0.547–2.354) | 0.735 | ||||
| Age ≥52 years old | 1.652 | 5.218 (2.170–12.547) | <0.001* | 2.453 | 11.628 (3.161–42.766) | <0.001* | |
| BMI ≥24 kg/m2 | −0.234 | 0.792 (0.383–1.635) | 0.528 | ||||
| Smoking | 0.077 | 1.080 (0.513–2.275) | 0.840 | ||||
| Hyperlipidemia | 0.616 | 0.540 (0.248–1.178) | 0.122 | ||||
| Hypertension | 0.221 | 1.247 (0.593–2.623) | 0.560 | ||||
| Diabetes | 0.026 | 1.027 (0.364–2.898) | 0.960 | ||||
| CHD | −0.320 | 0.726 (0.296–1.784) | 0.486 | ||||
| COPD | 0.341 | 1.407 (0.549–3.602) | 0.477 | ||||
| Stroke or TIA | 0.034 | 0.967 (0.420–2.226) | 0.937 | ||||
| ESR ≥20 mm/h | 0.102 | 1.107 (0.546–2.244) | 0.778 | ||||
| WBC >10×109/L | 1.159 | 3.186 (0.646–15.722) | 0.155 | ||||
| NLR >4 | 0.396 | 1.486 (0.603–3.664) | 0.389 | ||||
| Creatinine (male ≥111 μmol/L, female ≥73 μmol/L) | 0.118 | 1.125 (0.481–2.629) | 0.786 | ||||
| Pro-BNP >300 pg/mL | 0.849 | 2.338 (1.137–4.808) | 0.021* | ||||
| Minimally invasive | −0.798 | 0.450 (0.213–0.954) | 0.037* | ||||
| RBC transfusion | 1.038 | 2.824 (1.218–6.544) | 0.016* | 2.090 | 8.084 (1.876–34.830) | 0.005* | |
| Valve surgery | 1.587 | 4.888 (1.734–13.783) | 0.003* | 1.583 | 4.870 (1.140–20.812) | 0.033* | |
| LAD ≥43 mm | 1.821 | 6.176 (2.837–13.447) | <0.001* | ||||
| LVEDD ≥56 mm | 0.828 | 2.288 (1.112–4.707) | 0.024* | ||||
| LVESD ≥36 mm | 0.260 | 1.296 (0.639–2.629) | 0.472 | ||||
| LVEF <50% | 0.724 | 2.063 (0.716–5.947) | 0.180 | ||||
| E/e' >14 | 1.117 | 3.056 (1.455–6.415) | 0.003* | ||||
| LAVmin ≥50 mL | 2.039 | 7.681 (3.440–17.154) | <0.001* | ||||
| LAVmax ≥75 mL | 1.792 | 6.003 (2.721–13.243) | <0.001* | ||||
| LAVpreA ≥65 mL | 1.955 | 7.063 (3.178–15.696) | <0.001* | 1.329 | 3.779 (1.108–12.855) | 0.034* | |
| LAVI ≥35 mL/m2 | 1.937 | 6.935 (2.959–16.255) | <0.001* | ||||
| LAEV ≥30 mL | 0.223 | 1.250 (0.542–2.883) | 0.601 | ||||
| LAEF <37% | 1.896 | 6.662 (3.029–14.653) | <0.001* | 0.836 | 2.307 (0.734–7.247) | 0.152 | |
| LASr <13% | 2.097 | 8.143 (3.647–18.180) | <0.001* | ||||
| |LAScd| <6% | 0.763 | 2.146 (1.041–4.422) | 0.039* | ||||
| |LASct| <10% | 2.741 | 15.496 (4.442–54.057) | <0.001* | 1.839 | 6.290 (1.389–28.493) | 0.017* | |
| LASr-c <16% | 1.812 | 6.120 (2.780–13.475) | <0.001* | ||||
| |LAScd-c| <8% | 0.939 | 2.558 (1.163–5.629) | 0.020* | ||||
| |LASct-c| <8% | 2.300 | 9.977 (4.343–22.927) | <0.001* | 1.934 | 6.915 (1.949–24.540) | 0.003* | |
*, P<0.05. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; CHD, coronary heart disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ESR, erythrocyte sedimentation rate; LAEF, left atrial ejection fraction; LAEV, left atrial emptying volume; LAScd, left atrial conduit longitudinal strain; LAScd-c, left atrial conduit circumferential strain; LASct, left atrial contraction longitudinal strain; LASct-c, left atrial contraction circumferential strain; LASr, left atrial reservoir longitudinal strain; LASr-c, left atrial reservoir circumferential strain; LAVI, left atrial maximum volume indicator; LAVmax, left atrial maximum volume; LAVmin, left atrial minimum volume; LAVpreA, left atrial pre-systolic volume; LAD, left atrial diameter; LVEDD, left ventricle end-diastolic diameter; LVEF, left ventricle ejection fraction; LVESD, left ventricle end-systolic diameter; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; POAF, postoperative atrial fibrillation; RBC, red blood cell; TIA, transient ischemic attack; WBC, white blood cell.
Discussion
The rate of POAF (40.6%) in our study was slightly higher than that reported in most previous studies (19-21). Most of the patients (75.8%) in the present study underwent valve or concomitant valvular surgery, which might explain this discrepancy. In most patients (42.31%), POAF developed on the third day after surgery. Consistent with the results of previous studies (22-24), the POAF patients had increased expenses and prolonged hospital stays.
The poor accuracy of the three established clinical prediction models (i.e., the POAF score, the CHA2DS2-VASc score, and the AF risk index) indicates that the use of clinical predictors alone may be insufficient. These three prediction models only include clinical factors, such as age, sex, and cardiovascular disease, and do not include LA echocardiographic parameters that intuitively reflect the presence or absence of LA remodeling. Thus, models that include comprehensive LA parameters need to be established to improve the predictive ability of clinical models of POAF.
Recent guidelines suggest that advanced echocardiography, such as three-dimensional echocardiography (3DE) and STE, should be used to assess the LA volume and function of AF patients (25). Thus, we developed a logistic model based on a 4D auto LAQ technique that quantifies the risk of POAF following cardiac surgery. Our model included six clinical combined echocardiographic predictors (i.e., age, valve surgery, RBC transfusion during surgery, LAVpreA, LASct, and LASct-c). The last three predictors represent the LA 3DE volume, longitudinal and circumferential strain, respectively, which reflect the LA structure and function. The model showed good performance in terms of discrimination and calibration, and had a high specificity.
LA enlargement is associated with higher levels of fibrosis, which impairs the myocardium electrical conduction, which in turn results in the creation of tiny re-entrant circuits and increased vulnerability to AF (26,27). Our study showed that the LA size parameters were higher in the POAF group than the no-POAF group, and a larger LAVpreA was an independent risk factor of POAF. Previous studies (28-30) have shown that LA dilation is asymmetrical, and the LA volume measured by 3DE is closer to the true LA volume. Our findings support the results of previous studies (28-30).
Recently, some studies have focused on the importance of impaired LA contraction function in POAF patients (31-33). AF causes a progressive decrease in myocardial contractility, which affects the LA constrain function, as a result of the atrial muscle fibrosis and the loss of electrical activity. The 4D auto LAQ tool uses LA contraction strain parameters to quickly and accurately depict the LA constrain function. In this study, we found that preoperative impaired LA contraction longitudinal and constrain circumferential strain (LASct and LASct-c) were independent risk factors for POAF. Some studies have reported that the LASct and LASct-c are the best predictors for evaluating LA function in cardio-cerebrovascular disease patients and healthy individuals (34,35). To the best of our knowledge, this is the first study to use constrained circumferential strain to evaluate LA functional abnormalities to predict POAF. Our findings may provide novel ideas for upcoming studies on the prediction of POAF.
In this study, the patients with POAF were significantly older than those without POAF (OR =11.628; P<0.001). The best cut-off value for age was 52 years, which was younger than that reported in previous studies (36,37). This might be because our study population was relatively younger than that of previous studies. Advanced age has been shown to be a reliable risk factor for AF in both general and cardiac surgery populations (38,39). Myocardial fibrosis and collagen deposition in the atrium accompany aging. Electrical conduction is impeded as a result of the cardiac fiber coupling structure being weakened by fibrosis and degeneration in the LA. Further, LA remodeling may also result from age-related atrial fibrosis. Thus, POAF may have an electrophysiologic and/or anatomic basis.
The incidence of POAF was influenced by the surgery type. In this study, the patients undergoing valve surgery had a higher incidence of POAF, and valve surgery was an independent risk factor of POAF. Valve surgery patients may have a higher incidence of POAF due to increased surgical trauma and the longer cardiopulmonary bypass time, which raises the sympathetic tone. The effective refractory period of atrial myocytes linked to POAF is shortened by activated sympathetic neurons (40).
We found that the patients who received RBC transfusions during surgery had an 8.084-fold increased risk of developing POAF in comparison to those who did not. The increased risk of POAF events in patients with receive RBC transfusion is not well understood. It may be that RBC transfusion elicits a proinflammatory state by the direct infusion of inflammatory mediators, and through the augmentation of the inflammatory process that further amplifies the intense inflammatory response, the inflammatory reaction leads to electrophysiological alterations in atrial myocytes (41). Notably, atrial fibrosis increases with age, leading to LA enlargement and dysfunction; circulatory and urological complications after RBC transfusion may also lead to LA dysfunction. Therefore, age and RBC transfusion may also interact with the LA volume and strain values.
Our study had a number of limitations. First, the absence of any external validation is a significant limitation of the study. Thus, more samples need to be collected and multi-center studies need to be conducted in the future for external validation to strengthen our results. Second, only patients who had echocardiographic images of an adequate quality were included in the study. Third, the patients did not undergo continuous ECG monitoring in the ward, and as a result, episodes of silent or asymptomatic POAF might have gone unnoticed, which might have led to an underestimation of the incidence of POAF. Fourth, this study included patients with mitral valve disease, and hemodynamic changes solely related to mitral valve pathology might have had an effect on the LA volume and strain measurements. Finally, due to the poor quality of the images postoperatively, the 4D auto LAQ parameters could not be continuously monitored postoperatively.
Conclusions
4D auto LAQ echocardiography is a novel, non-invasive tool for the analysis of LA structure and function in the preoperative setting of cardio surgery. The 4D auto LAQ parameters (including LAVpreA, LASct, and LASct-c), age, RBC transfusion, and valve surgery are independent predictors of the occurrence of POAF. The predictive model that includes the 4D auto LAQ parameters is more conducive to the risk stratification of POAF after cardiac surgery. However, our study had a small sample size, and lacked a validation group; thus, further studies need to be conducted to verify the efficacy and reliability of our predictive model in the future.
Acknowledgments
We would like to thank the staff at the Ultrasound Department of The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, for their support and cooperation in this study.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1915/rc
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1915/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Biomedical Research Ethics Committee of The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University (No. IIT-O-2024-230), and all participants provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gaudino M, Di Franco A, Rong LQ, Piccini J, Mack M. Postoperative atrial fibrillation: from mechanisms to treatment. Eur Heart J 2023;44:1020-39. [Crossref] [PubMed]
- Boons J, Van Biesen S, Fivez T, de Velde MV, Al Tmimi L. Mechanisms, Prevention, and Treatment of Atrial Fibrillation After Cardiac Surgery: A Narrative Review. J Cardiothorac Vasc Anesth 2021;35:3394-403. [Crossref] [PubMed]
- Goyal P, Kim M, Krishnan U, Mccullough SA, Cheung JW, Kim LK, Pandey A, Borlaug BA, Horn EM, Safford MM, Kamel H. Post-operative atrial fibrillation and risk of heart failure hospitalization. Eur Heart J 2022;43:2971-80. [Crossref] [PubMed]
- Qiu D, Peng L, Ghista DN, Wong KKL. Left Atrial Remodeling Mechanisms Associated with Atrial Fibrillation. Cardiovasc Eng Technol 2021;12:361-72. [Crossref] [PubMed]
- Fan K, Chen L, Liu F, Ding X, Yan P, Gao M, Yu W, Liu H, Yu Y. Predicting New-Onset Postoperative Atrial Fibrillation Following Isolated Coronary Artery Bypass Grafting: Development and Validation of a Novel Nomogram. Int J Gen Med 2022;15:937-48. [Crossref] [PubMed]
- Bagherinejad Somesarayi SA, Faridi L, Mohammadi K, Kazemi Arbat B, Rahimi M, Parizad R, Toufan Tabrizi M. Conventional and two-dimensional strain echocardiography in predicting postoperative atrial fibrillation after coronary artery bypass grafting surgery. Caspian J Intern Med 2023;14:60-8. [PubMed]
- Zhang H, Qiao H, Yang B, Lu Y, Bai T, Xue J, Liu Y. Development and validation of a diagnostic model based on left atrial diameter to predict postoperative atrial fibrillation after off-pump coronary artery bypass grafting. J Thorac Dis 2023;15:3708-25. [PubMed]
- Sánchez FJ, Pueyo E, Diez ER. Strain Echocardiography to Predict Postoperative Atrial Fibrillation. Int J Mol Sci 2022;23:1355. [Crossref] [PubMed]
- Mandoli GE, Pastore MC, Benfari G, Bisleri G, Maccherini M, Lisi G, Cameli P, Lisi M, Dokollari A, Carrucola C, Vigna M, Montesi G, Valente S, Mondillo S, Cameli M. Left atrial strain as a pre-operative prognostic marker for patients with severe mitral regurgitation. Int J Cardiol 2021;324:139-45. [Crossref] [PubMed]
- Molnár AÁ, Merkely B. The Added Value of Atrial Strain Assessment in Clinical Practice. Diagnostics (Basel) 2022;12:982. [Crossref] [PubMed]
- Chen C, Yang Y, Ma W, Qi L, Zhang B, Zhang Y. Left atrial phasic function remodeling during its enlargement: a two-dimensional speckle-tracking echocardiography study. BMC Cardiovasc Disord 2022;22:231. [Crossref] [PubMed]
- Abdelrazek G, Mandour K, Osama M, Elkhashab K. Strain and strain rate echocardiographic imaging predict occurrence of atrial fibrillation in post-coronary artery bypass grafting patients. Egypt Heart J 2021;73:62. [Crossref] [PubMed]
- Pastore MC, Degiovanni A, Grisafi L, Renda G, Sozzani M, Giordano A, Salvatici C, Lorenz V, Pierfelice F, Cappelli C, De Donno F, Focardi M, Ricci F, Benedetto U, Gallina S, Cameli M, Patti G. Left Atrial Strain to Predict Postoperative Atrial Fibrillation in Patients Undergoing Coronary Artery Bypass Grafting. Circ Cardiovasc Imaging 2024;17:e015969. [Crossref] [PubMed]
- Sreedhar SP, Gupta A, Chitiboi T, Pradella M, Elbaz MSM. MRI Quantification of Left Atrial Circumferential Strain in Mitral Regurgitation: A Feasibility and Reproducibility Study. J Magn Reson Imaging 2024;60:988-98. [Crossref] [PubMed]
- Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA, Velazquez EJ. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019;32:1-64. [Crossref] [PubMed]
- Mariscalco G, Biancari F, Zanobini M, Cottini M, Piffaretti G, Saccocci M, Banach M, Beghi C, Angelini GD. Bedside tool for predicting the risk of postoperative atrial fibrillation after cardiac surgery: the POAF score. J Am Heart Assoc 2014;3:e000752. [Crossref] [PubMed]
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263-72. [Crossref] [PubMed]
- El-Chami MF, Kilgo PD, Elfstrom KM, Halkos M, Thourani V, Lattouf OM, Delurgio DB, Guyton RA, Leon AR, Puskas JD. Prediction of new onset atrial fibrillation after cardiac revascularization surgery. Am J Cardiol 2012;110:649-54. [Crossref] [PubMed]
- Yang H, Yuan C, Yang J, Xiang H, Lan W, Tang Y. A novel predictive model for new-onset atrial fibrillation in patients after isolated cardiac valve surgery. Front Cardiovasc Med 2022;9:949259. [Crossref] [PubMed]
- Pivatto Júnior F, Teixeira Filho GF, Sant’anna JR, Py PM, Prates PR, Nesralla IA, Kalil RA. Advanced age and incidence of atrial fibrillation in the postoperative period of aortic valve replacement. Rev Bras Cir Cardiovasc 2014;29:45-50. [Crossref] [PubMed]
- Keepa J, Wensley C, Jull A. Incidence and predictors of new-onset atrial fibrillation after cardiac surgery at Auckland City Hospital. N Z Med J 2023;136:13-23. [PubMed]
- Hernández-Leiva E, Alvarado P, Dennis RJ. Postoperative Atrial Fibrillation: Evaluation of its Economic Impact on the Costs of Cardiac Surgery. Braz J Cardiovasc Surg 2019;34:179-86. [Crossref] [PubMed]
- LaPar DJ, Speir AM, Crosby IK, Fonner E Jr, Brown M, Rich JB, Quader M, Kern JA, Kron IL, Ailawadi GInvestigators for the Virginia Cardiac Surgery Quality Initiative. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg 2014;98:527-33; discussion 533. [Crossref] [PubMed]
- Melduni RM, Schaff HV, Bailey KR, Cha SS, Ammash NM, Seward JB, Gersh BJ. Implications of new-onset atrial fibrillation after cardiac surgery on long-term prognosis: a community-based study. Am Heart J 2015;170:659-68. [Crossref] [PubMed]
- Donal E, Lip GY, Galderisi M, Goette A, Shah D, Marwan M, et al. EACVI/EHRA Expert Consensus Document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur Heart J Cardiovasc Imaging 2016;17:355-83. [Crossref] [PubMed]
- López-Galvez R, Rivera-Caravaca JM, Roldán V, Orenes-Piñero E, Esteve-Pastor MA, López-García C, Saura D, González J, Lip GYH, Marín F. Imaging in atrial fibrillation: A way to assess atrial fibrosis and remodeling to assist decision-making. Am Heart J 2023;258:1-16. [Crossref] [PubMed]
- Hung LT, Alshareef A, Al-Ahdal TMA, Anh PTT, Huan DQ. Do Van Trang, Zia S, Van Sy H, Huy NT. Predicting atrial fibrillation after cardiac surgery using a simplified risk index. J Electrocardiol 2021;67:45-9. [Crossref] [PubMed]
- Iwataki M, Takeuchi M, Otani K, Kuwaki H, Haruki N, Yoshitani H, Tamura M, Abe H, Otsuji Y. Measurement of left atrial volume from transthoracic three-dimensional echocardiographic datasets using the biplane Simpson’s technique. J Am Soc Echocardiogr 2012;25:1319-26. [Crossref] [PubMed]
- Hidayet Ş, Yağmur J, Bayramoğlu A, Taşolar MH, Kurtoğlu E, Özyalın F. Prediction of postoperative atrial fibrillation with left atrial mechanical functions and NT-pro ANP levels after coronary artery bypass surgery: A three-dimensional echocardiography study. Echocardiography 2018;35:661-6. [Crossref] [PubMed]
- Boyd AC, Thomas L. Left atrial volumes: two-dimensional, three-dimensional, cardiac magnetic resonance and computed tomography measurements. Curr Opin Cardiol 2014;29:408-16. [Crossref] [PubMed]
- Hirose T, Kawasaki M, Tanaka R, Ono K, Watanabe T, Iwama M, Noda T, Watanabe S, Takemura G, Minatoguchi S. Left atrial function assessed by speckle tracking echocardiography as a predictor of new-onset non-valvular atrial fibrillation: results from a prospective study in 580 adults. Eur Heart J Cardiovasc Imaging 2012;13:243-50. [Crossref] [PubMed]
- Imanishi J, Tanaka H, Sawa T, Motoji Y, Miyoshi T, Mochizuki Y, Fukuda Y, Tatsumi K, Matsumoto K, Okita Y, Hirata K. Left atrial booster-pump function as a predictive parameter for new-onset postoperative atrial fibrillation in patients with severe aortic stenosis. Int J Cardiovasc Imaging 2014;30:295-304. [Crossref] [PubMed]
- Darweesh RM, Baghdady YK, El Hossary H, Khaled M. Importance of left atrial mechanical function as a predictor of atrial fibrillation risk following cardiac surgery. Int J Cardiovasc Imaging 2021;37:1863-72. [Crossref] [PubMed]
- Chen L, Zhang C, Wang J, Guo L, Wang X, Liu F, Li X, Zhao Y. Left atrial strain measured by 4D Auto LAQ echocardiography is significantly correlated with high risk of thromboembolism in patients with non-valvular atrial fibrillation. Quant Imaging Med Surg 2021;11:3920-31. [Crossref] [PubMed]
- Liu M, Sun M, Li L, Li P, Hou S, Li Z, Sun X, Hua S. Left atrial function in young strength athletes: four-dimensional automatic quantitation study. Int J Cardiovasc Imaging 2022;38:1929-37. [Crossref] [PubMed]
- Levy F, Debry N, Labescat AL, Meimoun P, Malaquin D, Marechaux S, Rusinaru D, Jeu A, Ennezat PV, Castel AL, Tribouilloy C. Echocardiographic prediction of postoperative atrial fibrillation after aortic valve replacement for aortic stenosis: a two-dimensional speckle tracking left ventricular longitudinal strain multicentre pilot study. Arch Cardiovasc Dis 2012;105:499-506. [Crossref] [PubMed]
- Albabtain MA, Almathami EA, Alghosoon H, Alsubaie FF, Abdelaal IM, Ismail H, Adam AI, Arafat AA. Scores predicting atrial fibrillation after mitral valve surgery: Do we need a more specific score? J Arrhythm 2024;40:342-8. [Crossref] [PubMed]
- Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ Res 2017;120:1501-17. [Crossref] [PubMed]
- Bessissow A, Khan J, Devereaux PJ, Alvarez-Garcia J, Alonso-Coello P. Postoperative atrial fibrillation in non-cardiac and cardiac surgery: an overview. J Thromb Haemost 2015;13:S304-12. [Crossref] [PubMed]
- Zhu S, Che H, Fan Y, Jiang S. Prediction of new onset postoperative atrial fibrillation using a simple Nomogram. J Cardiothorac Surg 2023;18:139. [PubMed]
- Koch CG, Li L, Van Wagoner DR, Duncan AI, Gillinov AM, Blackstone EH. Red cell transfusion is associated with an increased risk for postoperative atrial fibrillation. Ann Thorac Surg 2006;82:1747-56. [Crossref] [PubMed]


