
Transverse venous sinus stenting for fulminant idiopathic intracranial hypertension during pregnancy: a report of two cases and literature review
Introduction
Idiopathic intracranial hypertension (IIH) is a condition characterized by increased intracranial pressure (ICP) without a clear primary cause, commonly seen in obese women of childbearing age (1,2). Friedman’s revised criteria state that a diagnosis of IIH requires: papilledema, normal neurologic exam except for 6th cranial nerve palsy, normal brain parenchyma on neuroimaging, normal cerebrospinal fluid (CSF) composition, and an opening pressure greater than 250 mm CSF (3).
Of those with IIH, pregnancy has a reported prevalence of between 2–12% as the body is affected by the rapid changes in cardiac output and blood volume (4). There is minimal data about the disease progression of IIH during pregnancy, with some data showing that the disease course may deteriorate significantly during pregnancy (5), while other articles oppose this to show that weight gain during pregnancy carries a lower risk of IIH (6).
Fulminant IIH (FIIH) is a rare form of IIH, marked by an acute and rapid onset of symptoms, with less than 4 weeks elapsing between initial symptoms and a significant progressive visual field loss (7). Given the progressive nature of the condition and the high risk of permanent vision loss, FIIH is considered a medical emergency requiring immediate intervention (8). Surgical options such as CSF shunting or optic nerve sheath fenestration (ONSF) are often required to mitigate vision deterioration (9).
Venous sinus stenting (VSS) has gained recognition as a promising surgical treatment option for IIH (10). This approach has become particularly relevant since venous stenosis has been identified in more than 90% of IIH patients, and accumulating data supports its significant role in the disease’s pathophysiology, although still unclear whether the stenosis is part of the cause or more of a result of the elevated ICP (11-13).
The advantage of venous stenting over other surgical options is its less invasive nature (endovascular procedure), allowing for quicker recovery as well as the potential for improvement of additional disease symptoms, such as headaches and pulsatile tinnitus (PT) (14).
While growing evidence supports the safety and efficacy of this procedure in both elective and emergent cases (15,16), there is limited data regarding its use during pregnancy (17). We would like to share two cases of young pregnant women, both in their second trimester, who presented with rapid onset of headaches, increased opening pressure and vision loss. In such cases at our institution, patients always receive an expedited workup with either computed tomography venography (CTV) or magnetic resonance venography (MRV) to exclude venous thrombosis and locate any venous stenosis. Both women were diagnosed with FIIH and were offered surgical treatment after a short course of aggressive medical therapy failed to produce improvement. Both patients opted for VSS over ventriculoperitoneal shunting (VPS), and they successfully underwent the procedure with good clinical outcomes and no obstetric implications. As there are no clear treatment recommendations for FIIH in pregnancy, we chose to share these two cases of FIIH pregnant patients treated with VSS as it may serve as a valid treatment option. We present this article in accordance with the CARE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2273/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of these two case reports and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Case #1
A 23-year-old woman with no previous medical history, G4P201, body mass index (BMI) of 33.5 kg/m2, at 16 weeks of gestation, presented to the emergency department in Soroka University Medical Center (SUMC) with 10 days of headaches, blurry vision, transient visual obscuration, and right-sided PT.
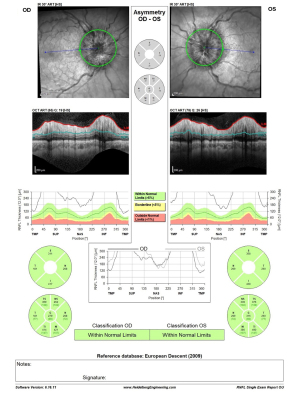
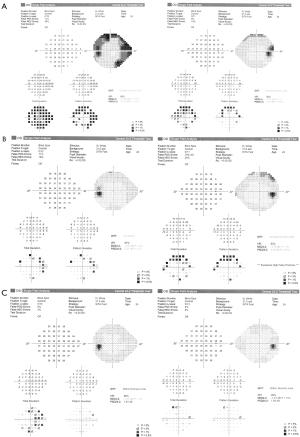
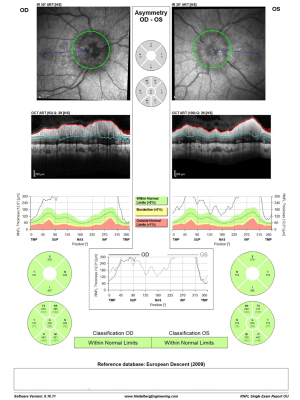
Ophthalmologic evaluation upon arrival revealed a visual acuity of 6/18 in both eyes and bilateral disc edema (Frisén scale grade of 3 in right eyes and grade 4 in left eye), with no relative afferent pupillary defect (RAPD) and color vision defect (Ishihara 12/15 plates in each eye). Optical coherence tomography (OCT) of the retinal nerve fiber layer (RNFL) demonstrated diffuse thickening: 279 µm on the right and 316 µm on the left (Figure 1). Visual field test using Humphrey visual field (HVF) Swedish Interactive Threshold Algorithm (SITA) fast 24-2 showed mild depression on the right with a mean deviation (MD) of −6.3 dB and moderate-severe depression on the left with an MD of −13.99 dB (Figure 2). The patient had no macular edema or other retinal abnormality.

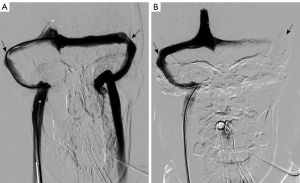
MRV of the head demonstrated signs consistent with intracranial hypertension, including flattening of posterior sclera, bilateral widening of optic nerve sheath over 6 mm, an empty Sella turcica and bilateral severe stenosis of both Transverse-Sigmoid junctions (TSJ). Lumbar puncture opening pressure (LPOP) was measured 360 mmH2O with normal CSF formula, along with the other findings met the diagnosis of IIH according to the revised Friedman’s criteria (3).
The patient was admitted to the neurology ward and was started on acetazolamide with a daily dose of 2 grams per day, 100 mg of aspirin (as a possible preparation for venous stent if needed), and 1,000 mg of intravenous (IV) methylprednisolone. The patient had difficulty with the high amount of acetazolamide treatment and experienced severe vomiting after ingesting only some of the prescribed pills, preventing her from fully adhering to the treatment. After two days of partial treatment, the OCT & HVF 24-2 testing worsened, with no improvement in headache, tinnitus, and visual obscurations (Figures 3,4).

Due to the progression of symptoms, and after a multi-disciplinary discussion (obstetrics, neuroradiology, neuro-ophthalmology, neurosurgery and neurology), she was diagnosed with FIIH and was offered surgical treatment with VPS or VSS (ONSF is not routinely performed at our institution. We usually offer patients VPS or VSS when both options are suitable according to the case. As our medical center is well known for its experience with VSS, most patients choose VSS over VPS). The patient chose VSS over CSF shunting after all the information regarding both procedures was presented to her.
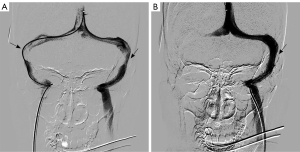
The patient was given a loading dose of 300 mg of clopidogrel, started on a daily dose of 75 mg of clopidogrel, and continued with aspirin and methylprednisolone. The patient insisted on waiting for a possible improvement with medical treatment. However, no improvement was observed during the follow-up neuro-ophthalmological assessment and therefore the procedure was performed 36 hours post medical decision making. The procedure was performed under general anesthesia with pelvis protection using a lead skirt localized under the patient to minimize radiation exposure for the fetus. IV heparin was administered during the procedure to keep activated clotting time (ACT) over 250 seconds. Severe bilateral TSJ stenosis was detected during the procedure. A pressure measurement was performed pre- and post-stenosis using a minimum of an 8-mmHg gradient as an indication for stenting. An 18-mmHg gradient was measured on the right (24 mmHg pre-stenosis/6 mmHg post-stenosis), and a 13-mmHg gradient was measured on the left (22 mmHg pre-stenosis/9 mmHg post-stenosis). A decision was made to deploy a stent on the right TSJ stenotic area as the right sinus was the dominant one and stenting on this side could theoretically provide a more effective improvement in venous drainage. Other reasons for choosing the right transverse sigmoid sinus (TSS) were the higher gradient on this side, as well as the fact that the patient was complaining of PT only on the right side, and stenting on the right side had the potential to ameliorate this complaint as well.
After the deployment of a precise 7 mm × 40 mm stent (Cordis, Parramatta, Austria) at the stenotic area on the right transverse sinus, a normal flow through the stent was demonstrated, with an improvement of drainage speed on this side. Repeated pressure gradient measurements showed a lower pre-stent pressure measurement of 15 mmHg and 11 mmHg measured post-stent, normalizing the gradient to 4 mmHg (Figure 5).
Brain computed tomography (CT) performed immediately post-procedure ruled out any hemorrhage and the patient woke up with no neurological deficits with immediate resolution of the tinnitus. The patient’s headache has improved significantly over the next couple of days with a slight improvement of papilledema (Frisén grade 3 to 2 on the right side and Frisén grade 4 to 3 on the left side). It is worth mentioning that the patient refused to take acetazolamide after the procedure due to multiple adverse effects.
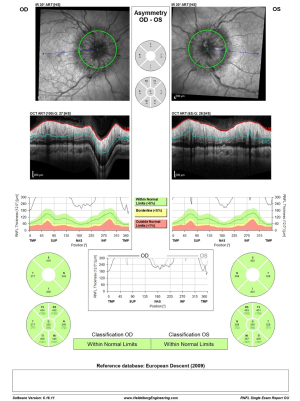
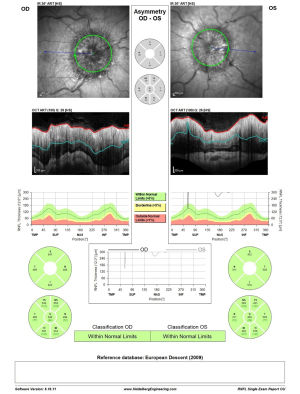
On one-week post-procedural evaluation, grade was reduced to Frisén grade 2 in both eyes, on OCT examination (RNFL thickness improved from 335 to 160 µm in the right eye and from 350 to 169 µm in the left eye; Figure 6). A week later, the exam showed a continued reduction of the papilledema until complete resolution of the disc edema at evaluation one month following the procedure. Three months post-procedure, clopidogrel treatment was discontinued after MRV approved stent patency, and aspirin was continued. The patient remained under close neurological and ophthalmological observation throughout the pregnancy time. An elective Cesarean section delivery was performed at 37 weeks. The delivery of a healthy male newborn weighing 2.6 kg was free of any complications.
OCT RNFL results are shown for pre-procedure (Figure 6) and post-procedure at one month, three months, and one month post-partum (Figure 7). HVF 24-2 post-stenting at one month, three months, and one month post-partum demonstrated complete resolution of visual field defects in both eyes (Figure 8). Visual acuity improved to 6/9 in the right eye and 6/12 in the left eye.

Currently, nine months post-procedure, treated with 100 mg aspirin only, she remains asymptomatic with normal development of her newborn male. Another MRV is planned for a twelve-month follow-up and if the stent is patent by this time and the patient continues to be symptom-free, the aspirin will be stopped. Six months of neurological and ophthalmological follow-ups will be continued according to SUMC’s protocol for post-venous stenting.
Case #2
A 29-year-old woman, without any significant past medical history, G8P401, BMI 39.5 kg/m2, at 19 weeks of gestation, presented to the emergency department with 3 weeks of headaches, blurry vision, transient visual obscurations, and left-sided tinnitus worsening in the past week.
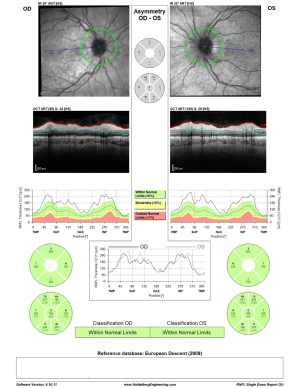
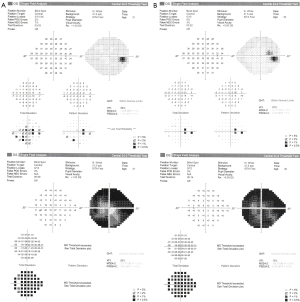
Initial ophthalmologic evaluation demonstrated a visual acuity of 6/9 in the right eye and hand motion in the left eye. RAPD demonstrated on the left, and color vision by Ishihara color plates of 10/15 on the right eye and 1/15 on the left eye. Ophthalmoscopy revealed significant bilateral disc edema with a Frisén score of 4 on both eyes. OCT RNFL demonstrated diffuse thickening of the RNFL in both eyes: right eye RNFL thickness 540 µm, 450 µm in the left eye (Figure 9), and the HVF 24-2 showed a severe peripheral defect in the right eye with a preserved central and superior temporal visual field (MD) of −12.9 dB, but total obscuration of the left eye visual field (MD) of −34.1 dB (Figure 10). The patient had no macular edema or other retinal abnormality.

Obstetrics and gynecology (OBGYN) was consulted and gave clearance to perform CTV rather than wait for MRI availability in order to emergently rule out cerebral venous thrombosis. The CTV revealed bilateral transverse sinus stenosis and a bilateral increase of the optic nerve sheath diameter over 6 mm without demonstration of an empty Sella turcica. Lumbar puncture revealed an opening pressure of 400 mmH2O with normal CSF contents, meeting the revised Friedman’s criteria for the diagnosis of IIH (3).
She was admitted to the neurology ward for further examination, including daily neuro-ophthalmological evaluation with OCT and HVF. On the first day of admission, she was started on 2 grams of acetazolamide with an increasing dosage up to 3 grams the next day, aspirin 100 mg (as a possible preparation for venous stent), and 1,000 mg IV methylprednisolone, yet her symptoms continued to worsen after a week of treatment.
Due to the urgency of the case and after a multi-disciplinary council (obstetrics, neuroradiology, neuro-ophthalmology, neurosurgery and neurology), she was diagnosed with FIIH and subsequently offered surgical intervention with either VSS or CSF shunting. Similarly to the first case, this patient remained hesitant regarding any procedural intervention and decided on medical treatment with the decision that if further deterioration or no improvement occurred within the next few days she would agree to go through VSS. Clopidogrel 75 mg once daily was added to the patient’s medical treatment regimen. After 4 days, a further decline in her ophthalmologic evaluation was noted and the patient agreed to go through with VSS.
The patient underwent the procedure under general anesthesia with radiation protection of the pelvic area. IV heparin was administered during the procedure to keep her ACT over 250 seconds. During the procedure, a severe bilateral TSJ stenosis was demonstrated on injection from the superior sagittal sinus. Extremely high-pressure gradients were measured: on the right, the gradient was 29 mmHg (49 pre-stenosis/20 mmHg post-stenosis) and on the left the gradient was 28 mmHg (49 pre-stenosis/21 post-stenosis). A precise stent 7 mm × 40 mm (Cordis, Parramatta, Austria) was deployed within the stenotic part of the left TSJ. The decision to stent the left TSJ was made as the patient complained of left PT pre-procedure and the pressure gradients were similar on both sides (Figure 11).
Following the stent placement, repeat pressure gradient measurements improved significantly with good flow through the stent, resulting in a pressure gradient of 4 mmHg (34 mmHg pre-stenosis and 30 mmHg post-stenosis). CT imaging immediately post-procedure ruled out any hemorrhage and the patient woke up with no neurological deficits. The patient reported an immediate resolution of the PT post-procedure with gradual improvement in headache.
On formal evaluation, two days post-procedure, visual acuity testing demonstrated significant improvement from hand motion to 6/12 in the left eye and no change to the right eye at 6/9. Color vision by Ishihara color plates improved to 13/15 in the right eye and to 4/15 in the left eye. The degree of papilledema decreased from Frisén grade 4 to grade 3 on both sides with an improved OCT examination (RNFL thickness decreased from 540 to 348 µm in the right eye and from 450 to 307 µm in the left eye; Figure 12). One week later, there was a further reduction of RNFL thickness (Figure 13), and the disc edema in the right eye was Frisén score of 2 and left eye Frisén score of 3. A complete resolution of the disc edema was assessed one month following the stenting procedure, and at this point, acetazolamide treatment was discontinued. A follow-up MRI/MRV was then performed, approving stent patency and allowing the patient to discontinue the clopidogrel while continuing the aspirin. The patient remained under close medical observation until her delivery by cesarean section performed electively on the 37th week of pregnancy. The delivery went through without any complications with a healthy newborn delivered.
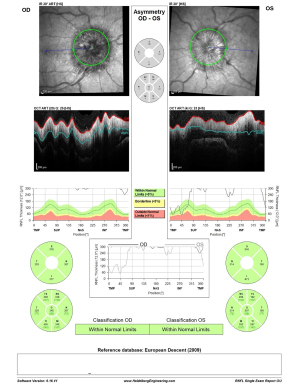
OCT RNFL results are shown for pre-procedure (Figure 9) and post-procedure at two days (Figure 12), one week (Figure 13), three months, and one month post-partum (Figure 14). HVF 24-2 was done pre- and post-stenting at one and three months, as well as one-month post-partum, demonstrating a complete resolution of visual field defects of the right eye MD of −2.1 dB and slight improvement of the severe damage on the left eye visual field MD of −29.3 dB (Figure 15). Visual acuity had also improved to 6/6 in the right eye and 6/9 in the left eye.
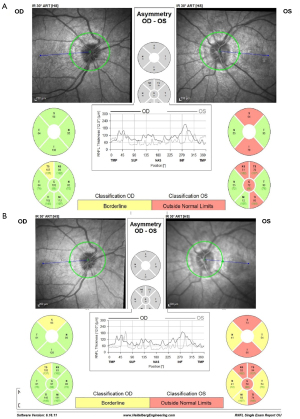
Currently 9 months post-procedure, the patient remains asymptomatic with left optic nerve atrophy and severe damage to the left visual field as shown in Figure 15, with normal development of her newborn. Treatment and follow-up will be continued according to SUMC’s protocol as detailed in case #1.
Discussion
FIIH is a rare condition that can potentially lead to vision loss. Given its rare presentation during pregnancy, both medical and surgical interventions lack sufficient research and their impact on the developing fetus remains unclear. Selecting the optimal treatment modality for both mother and fetus is a complex challenge (8,18).
Weight reduction, which is considered first-line treatment for IIH, is not a suitable option during pregnancy. Regarding the use of acetazolamide during pregnancy, to date, there is no compelling evidence of an adverse effect of acetazolamide on human pregnancies and it is categorized as a class C drug (18).
The rapid progression of FIIH necessitates immediate intervention to prevent complete and irreversible vision loss. Often medical management alone is insufficient, as in these cases where both women were offered surgical interventions due to progressive deterioration of vision despite medical treatment. Future cases may also consider using a lumbar drain to help rapidly reduce ICP during the acute period to potentially give the conservative treatments more time to take effect before committing to an invasive procedure.
The choices among surgical interventions in FIIH remain controversial as no specific procedure is superior and they mostly depend on local protocols and availability. The treatment of FIIH should be selected on a case-by-case basis after multidisciplinary discussions (17). CSF diversion techniques are usually the first surgical intervention for medically refractory patients with IIH (19), providing a rapid and effective reduction in intracranial pressure. However, this procedure carries a significant risk of failure, with several long-term retrospective studies finding the revision rate was higher than 50%, of which more than 30% required multiple revisions (20,21). ONSF is a reasonable option if the primary focus is solely on preventing vision loss, however, it doesn’t solve headaches or tinnitus and has an extensive list of possible complications (22). Considering the primarily young patient population, the potential complications and need for repeated surgeries should be carefully evaluated.
VSS has demonstrated high technical and clinical success rates with a low complication profile in carefully selected patients (15,16). VSS has also been reported to improve odds of headache recurrence compared with VPS (23). However, the literature still lacks clear treatment recommendations for FIIH during pregnancy, with no studies to suggest that one surgical procedure is better than another (2,18). It is also important to consider that pregnant patients experience increases in blood volume, cardiac output, and changes in uterine size that compress the abdomen. These physiologic changes of pregnancy could impact the ICP and venous flow dynamics, as well as potentially affect the accuracy of the pressure gradient measurements or stent placement during the procedure. The necessity of intraprocedural anticoagulation and dual antiplatelet therapy further complicates performing VSS during pregnancy, as there is not much literature on the subject. Patel et al. published the only other case we found where VSS was performed during pregnancy. In that case, which also reported favorable outcomes, a different antiplatelet regimen was used, consisting of aspirin and ticagrelor (17). In both of our cases, aspirin and clopidogrel were used as the antithrombotic regimen with no related complications for the mother or fetus. Using dual-antiplatelet therapy during pregnancy is still not well-studied, but available literature on the subject supports our choice of therapy by demonstrating several cases with positive outcomes (24). However, this remains a topic that requires further research.
Following the procedure, both patients demonstrated a significant reduction in symptoms related to elevated ICP. The tinnitus resolved within a few days, headache within a week, and disc edema within a month. The first patient demonstrated visual field improvement in both eyes, with a complete resolution of the defects. The second patient, who presented with a significant visual field loss, exhibited a complete recovery in one eye and showed improvement with a remaining defect in the other eye. It is possible that the irreversible damage to the visual field in one eye of the second patient may be attributed to patient’s decision to delay treatment and may have been avoided if earlier action was taken. Both patients delivered healthy children via a cesarean section.
Conclusions
Two cases of FIIH in pregnancy treated successfully with VSS were described. Both cases show major improvement in signs and symptoms while remaining safe for both mother and fetus. This contributes additional data to the small growing body of evidence regarding FIIH treatment in pregnancy, as well as offering VSS as a possible treatment option. Further studies are needed to establish an evidence-based treatment algorithm.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-2273/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2273/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of these two case reports and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Srivastava O, Micieli JA. Resolution of Fulminant Idiopathic Intracranial Hypertension Treated with Acetazolamide. Case Rep Neurol 2022;14:424-8. [Crossref] [PubMed]
- Thurtell MJ. Idiopathic Intracranial Hypertension. Continuum (Minneap Minn) 2019;25:1289-309. [Crossref] [PubMed]
- Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013;81:1159-65. [Crossref] [PubMed]
- Hallan DR, Lin AC, Tankam CS, Madden D, Rizk E. Pregnancy and Childbirth in Women With Idiopathic Intracranial Hypertension. Cureus 2022;14:e30420. [Crossref] [PubMed]
- Thaller M, Homer V, Mollan SP, Sinclair AJ. Disease Course and Long-term Outcomes in Pregnant Women With Idiopathic Intracranial Hypertension: The IIH Prospective Maternal Health Study. Neurology 2023;100:e1598-610. [Crossref] [PubMed]
- Lambert-Cheatham NA, Nagia L, Pasmanter NR, Pellizzari R, Lee B, Miller NJ, Kaufman DI. Impact of Pregnancy on Papilledema and Vision Loss in Idiopathic Intracranial Hypertension Patients: A Chart Review and Case Series of 13 Patients. J Neuroophthalmol 2024;44:206-11. [Crossref] [PubMed]
- Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology 2007;68:229-32. [Crossref] [PubMed]
- Espino Barros Palau A, Morgan ML, Yalamanchili S, Lee AG. Fulminant idiopathic intracranial hypertension managed with oral acetazolamide. Can J Ophthalmol 2016;51:e121-5. [Crossref] [PubMed]
- Byth LA, Lust K, Jeffree RL, Paine M, Voldanova L, Craven AM. Management of idiopathic intracranial hypertension in pregnancy. Obstet Med 2022;15:160-7. [Crossref] [PubMed]
- National Trends of Cerebral Venous Sinus Stenting for the Treatment of Idiopathic Intracranial Hypertension. Neurology 2024;e208069. Erratum for: Neurology 2023;101:402-6. [PubMed]
- Zhao K, Gu W, Liu C, Kong D, Zheng C, Chen W, Li X, Liang Y, Zhou H. Advances in the Understanding of the Complex Role of Venous Sinus Stenosis in Idiopathic Intracranial Hypertension. J Magn Reson Imaging 2022;56:645-54. [Crossref] [PubMed]
- Fargen KM. Idiopathic intracranial hypertension is not idiopathic: proposal for a new nomenclature and patient classification. J Neurointerv Surg 2020;12:110-4. [Crossref] [PubMed]
- West JL, Greeneway GP, Garner RM, Aschenbrenner CA, Singh J, Wolfe SQ, Fargen KM. Correlation between angiographic stenosis and physiologic venous sinus outflow obstruction in idiopathic intracranial hypertension. J Neurointerv Surg 2019;11:90-4. [Crossref] [PubMed]
- Townsend RK, Fargen KM. Intracranial Venous Hypertension and Venous Sinus Stenting in the Modern Management of Idiopathic Intracranial Hypertension. Life (Basel) 2021;11:508. [Crossref] [PubMed]
- Horev A, Ben-Arie G, Walter E, Tsumi E, Regev T, Aloni E, Biederko R, Zlotnik Y, Lebowitz Z, Shelef I, Honig A. Emergent cerebral venous stenting: A valid treatment option for fulminant idiopathic intracranial hypertension. J Neurol Sci 2023;452:120761. [Crossref] [PubMed]
- Azzam AY, Mortezaei A, Morsy MM, Essibayi MA, Ghozy S, Elamin O, Azab MA, Elswedy A, Altschul D, Kadirvel R, Brinjikji W, Kallmes DF. Venous sinus stenting for idiopathic intracranial hypertension: An updated Meta-analysis. J Neurol Sci 2024;459:122948. [Crossref] [PubMed]
- Patel MS, Akhter AS, Rocco MT, Akhter A, Nimjee SM. A Novel Case of Transverse Sinus Stenting and Ticagrelor Use During Pregnancy for Idiopathic Intracranial Hypertension. Stroke: Vascular and Interventional Neurology 2023; [Crossref]
- Tyndel F, Steriade C, Gallo A, Wennberg R, Radovanovic I. Fulminant Idiopathic Intracranial Hypertension in Pregnancy. Case Rep Neurol 2022;14:251-5. [Crossref] [PubMed]
- Satti SR, Leishangthem L, Spiotta A, Chaudry MI. Dural venous sinus stenting for medically and surgically refractory idiopathic intracranial hypertension. Interv Neuroradiol 2017;23:186-93. [Crossref] [PubMed]
- Sinclair AJ, Kuruvath S, Sen D, Nightingale PG, Burdon MA, Flint G. Is cerebrospinal fluid shunting in idiopathic intracranial hypertension worthwhile? A 10-year review. Cephalalgia 2011;31:1627-33. [Crossref] [PubMed]
- Rosenberg ML, Corbett JJ, Smith C, Goodwin J, Sergott R, Savino P, Schatz N. Cerebrospinal fluid diversion procedures in pseudotumor cerebri. Neurology 1993;43:1071-2. [Crossref] [PubMed]
- Gilbert AL, Chwalisz B, Mallery R. Complications of Optic Nerve Sheath Fenestration as a Treatment for Idiopathic Intracranial Hypertension. Semin Ophthalmol 2018;33:36-41. [Crossref] [PubMed]
- Hilvert AM, Gauhar F, Longo M, Grimaudo H, Dugan J, Mummareddy N, Chitale R, Froehler MT, Fusco MR. Venous sinus stenting versus ventriculoperitoneal shunting: comparing clinical outcomes for idiopathic intracranial hypertension. J Neurointerv Surg 2024;16:1264-7. [Crossref] [PubMed]
- Antonijevic N, Gosnjic N, Marjanovic M, Antonijevic J, Culafic M, Starcevic J, Plavsic M, Mostic Stanisic D, Uscumlic A, Lekovic Z, Matic D. Antiplatelet Drugs Use in Pregnancy-Review of the Current Practice and Future Implications. J Pers Med 2024;14:560. [Crossref] [PubMed]


