
A water cushion improved needle visualization by artificial beam steering during ultrasound-guided in-plane technique: an in vitro simulation study
Introduction
At present, bedside ultrasound has become increasingly important for the perioperative management of anesthesiologists. Ultrasound-guided vascular cannulation, nerve block, intraspinal anesthesia and other needle-based procedures have been widely used and developed in clinical practice. Excellent needle visualization is critical for these puncture procedures because inadequate visualization can cause unexpected vascular, neural or visceral injury. In-plane and out-of-plane approaches are the two major techniques used for ultrasound-guided puncture procedures. The in-plane technique can display the entire needle trajectory in real time, making it safer and easier to master. Therefore, most anesthesiologists prefer to use the in-plane technique. However, the needle may not be clearly displayed when it is inserted at a steep angle using an in-plane technique. Multiple methods, such as echogenic needles (1), tip-tracker needles (2), photoacoustic imaging (3), artificial intelligence-based needles and target detection (4), have been used to improve needle visualization, especially at steep insertion angles. Nevertheless, the evidence behind these findings is currently weak.
In this study, we introduced a simple and effective method to improve needle visualization during ultrasound-guided in-plane technique. We used a water cushion filled under the probe to steer the ultrasound beam and hypothesized that the water cushion would result in better needle visualization via artificial beam steering.
Methods
The puncture procedures was scanned under the guidance of the linear high-frequency array probe of ultrasound instrument (Navi s, Shenzhen Wisonic Medical Technology Co., Ltd., Shenzhen, China). All ultrasound scans and needle punctures were carried out by an experienced anesthesiologist.
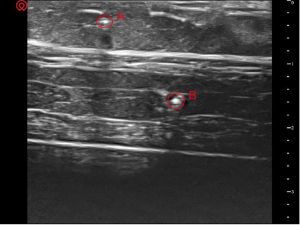
This study consisted of three phases. The first stage was to simulate the targets and create a water cushion. A piece of pork was used to simulate human soft tissue. Two rigid needles (18 G, 38 mm) were inserted into the pork to simulative blood vessels or nerves as puncture targets. The positions of the two targets were predesigned: target A was shallow and far from the operator, and target B was deep and close to the operator (Figure 1). The reason why the positions of the two targets were designed in this way was to form different inserted angles during the subsequent puncture process. A water cushion was also made beforehand: a surgical glove was filled with an appropriate amount of water and sealed hermetically.
The second stage of this study involved puncture via the conventional method (Figure 2). After confirming the ultrasound images of the two targets, the two targets were punctured via the in-plane technique. The position of the probe was adjusted to obtain the entire length of the puncture needle (Stimuplex D needle, 22 gage, 80 mm; B. Braun, Melsungen, Germany) as much as possible. Target A was punctured first, followed by target B. Then, the needle remained stationary.
The third stage of this study was to test the artificial beam steering effect of a water cushion. The water cushion filled the space between the ultrasound probe and the pork (Figure 3). The position of the probe was adjusted to obtain a complete image of the puncture needle again.
Clear ultrasound images of the needles were captured for objective visualization analysis. The angles between the ultrasound beam and the puncture needle were measured.
During the whole ultrasonic scanning process, the ultrasonic parameters, such as focus and gain, remained unchanged.
Results
In the first stage, clear ultrasound images of the soft tissue and two targets were acquired (Figure 4).
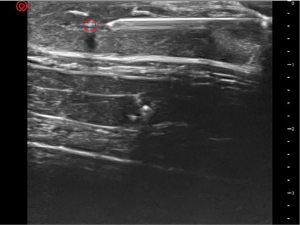
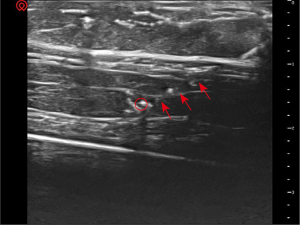
In the second stage, for target A, the puncture trajectory was shallow. The needle was clearly visible under ultrasound guidance (Figure 5). The angle between the ultrasound beam and the puncture needle was approximately 87 degrees. For target B, the puncture trajectory was steep. The needle was not clearly displayed (Figure 6). The angle between the ultrasound beam and the puncture needle was approximately 55 degrees.
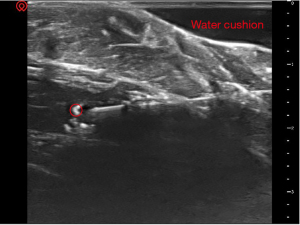
In the third stage, a triangular liquid dark area formed by the water cushion can be seen between the ultrasound probe and the soft tissue. The ultrasound beam was no longer perpendicular to the soft tissue surface. Instead, the ultrasound entered the soft tissue obliquely. A clear image of the needle was obtained (Figure 7). The angle between the ultrasound beam and the puncture needle was approximately 85 degrees.
Discussion
Our simulation study demonstrated that artificial beam steering generated with a water cushion improved needle visualization. Filling a water cushion under an ultrasound probe is a simple and effective way to enhance needle visualization.
Excellent needle visualization is important for the safety and success of ultrasound-guided puncture procedures. The use of ultrasound has not eliminated failures and complications for needle-based procedures. One of the most common errors is advancing the needle under inadequate visualization of the puncture trajectory.
Ultrasonic physics determines that needle visualization becomes worse as the insertion angle of the needle increases due to the loss of the echo signal. A previous study revealed that when the insertion angle exceeded 30 degrees (the angle between the ultrasound beam and the puncture needle was less than 60 degrees), needle visualization significantly worsened (5). This was similar to the angle we measured. Due to the instability of the force applied to the probe, the angle measurement may change, but this change is very slight.
A variety of technologies, including electronic beam steering (6), help to increase the reflected ultrasonic waves and obtain a clear view of the needle. Electron beam steering is a technology that changes the angle of the incident ultrasound beam to make it more perpendicular to the puncture needle. By forcing the incident ultrasonic beam perpendicular to the puncture needle, the reflected ultrasound beam is also more perpendicular to the puncture needle. Therefore, electronic beam steering increases the amount of reflected ultrasound beam back to the ultrasonic probe and improves the visualization of the needle. However, the need for additional specialized and expensive equipment is the greatest limitation to the widespread adoption of this technology.
In this study, we filled a water cushion between the ultrasound probe and the surface of the pork and artificially changed the angle of the incident ultrasound beam. The water cushion served the same purpose as electron beam steering. The effect of artificial beam steering generated with a water cushion made the ultrasound beam more perpendicular to the needle. It is convenient to make a water cushion using surgical gloves in the operating room. This beam steering was achieved with little additional equipment. Artificial beam steering generated with a water cushion has the advantages of high efficiency and easy implementation.
Our study has several limitations. First, we did not compare the effects of different angular beam steering generated by different sizes of water cushions. The appropriate steering angle needs further exploration. Second, we did not compare artificial beam steering with other existing needle visualization enhancement technologies. Third, we did not evaluate whether artificial beam steering led to the degradation of image quality. Finally, this procedure has not been performed by multiple operators with different levels of experience, and whether it is suitable for living human tissues needs further verification.
Conclusions
Needle visualization is vital for ultrasound-guided puncture procedures. This study revealed that excellent needle visualization at a steep insertion angle can be achieved via artificial beam steering generated with a water cushion during the ultrasound-guided in-plane technique.
Acknowledgments
None.
Footnote
Funding: None.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1995/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethics approval is not applicable.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fuzier R, Casalprim J, Bataille B, Harper I, Magues JP. The echogenicity of nerve blockade needles. Anaesthesia 2015;70:462-6. [Crossref] [PubMed]
- McLeod GA. Novel approaches to needle tracking and visualisation. Anaesthesia 2021;76:160-70. [Crossref] [PubMed]
- Hui X, Rajendran P, Ling T, Dai X, Xing L, Pramanik M. Ultrasound-guided needle tracking with deep learning: A novel approach with photoacoustic ground truth. Photoacoustics 2023;34:100575. [Crossref] [PubMed]
- Arapi V, Hardt-Stremayr A, Weiss S, Steinbrener J. Bridging the simulation-to-real gap for AI-based needle and target detection in robot-assisted ultrasound-guided interventions. Eur Radiol Exp 2023;7:30. [Crossref] [PubMed]
- Liu Y, Sun X, Qian W, Liu W, Mei W. Enhanced needle visibility by microbubbles generated with negative pressure using an in-plane technique. Reg Anesth Pain Med 2019;rapm-2019-100570. Epub ahead of print. [Crossref] [PubMed]
- Prabhakar C, Uppal V, Sondekoppam RV. Effect of Beam Steering on Echogenic and Nonechogenic Needle Visibility at 40°, 50°, and 60° Needle Insertion Angles. Anesth Analg 2018;126:1926-9. [Crossref] [PubMed]





