
Disrupted small-world networks in children with drug-naïve growth hormone deficiency: a DTI-based network analysis
Introduction
Growth hormone deficiency (GHD) is the most prevalent cause of short stature in children around the world, which is characterized by the decreased secretion of growth hormone (GH) from the anterior pituitary (1). Although GH is primarily known for its role in promoting linear growth, it also stimulates the production of insulin-like growth factor-1 (IGF-1) in the liver. Importantly, the GH/IGF-1 axis plays a crucial role in brain development and cognitive function (2,3), extending its influence beyond physical growth. Increasing evidence indicates that children with GHD exhibit brain structural and functional abnormalities (4,5), but its effects are not yet fully understood.
Nowadays, our pathophysiological understanding of GHD has been improved via nonconventional neuroimaging studies. According to previous resting-state functional magnetic resonance imaging (rs-fMRI) studies (6-8), abnormal spontaneous neural activity in extensive cortical regions has been found in children with GHD. A study employing dynamic regional homogeneity (ReHo) and dynamic functional connectivity methods highlighted alterations in brain networks in GHD patients, particularly in the central executive network (CEN) and cerebellar network, when compared to idiopathic short stature (ISS) (9). Structural neuroimaging has provided more insights into the cerebral alterations associated with GHD. Pediatric cases of GHD exhibit significantly decreased whole-brain gray matter volume (GMV) and cortical surface area, and thickness of bilateral hemispheres compared to children with idiopathic short stature (10), and correlated with GH level. Notably, cortical regions showing significant differences in the mean GMV and surface area are mainly distributed around the bilateral central sulci and the lateral and basal parts of the temporal lobes. In addition, pediatric GHD patients exhibit reduced volume in specific brain structures, including the splenium of the corpus callosum, right pallidum, right hippocampus, and left thalamus, indicating that deep gray matter (GM) nuclei may be more susceptible to the variations of the GH/IGF-1 axis (5).
Diffusion tensor imaging (DTI) has emerged as a valuable tool for assessing axonal and myelin integrity in developmental diseases. Studies using DTI-derived parameters have reported significantly lower fractional anisotropy (FA) in corticospinal tracts and corpus callosum of pediatric GHD patients (5,11). Further, mean diffusivity (MD) is significantly increased in bilateral corticospinal tracts in pediatric GHD (12). However, investigations into overall changes in white matter (WM) structural networks in GHD, as opposed to those observed in specific anatomical regions, are lacking. Graph-theory analysis (13) has been widely applied to study WM structural networks in various neurodevelopmental diseases, including attention deficit/hyperactivity disorder (ADHD) (14), autism spectrum disorder (ASD) (15), and Tourette syndrome (TS) (16). Given that the brain is a complex and integrative network (17), studying the brain pathophysiology of children with GHD from the perspective of the DTI-based brain network offers novel insights into understanding the effects of GHD deficiency on brain development.
In this study, we employed DTI and graph theory analysis to evaluate brain WM structural connectome in drug-naïve GHD children. Additionally, we investigated associations between altered network metrics and the behavioral symptom severity in GHD. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1927/rc).
Methods
Participants
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University (No. 2021082) and was registered in the Chinese Clinical Trial Registry (https://www.chictr.org.cn/; identifier: ChiCTR2100048109; date: July 2021). Written informed consent was provided by all participants’ guardians.
Between August 2021 and September 2023, we recruited 58 drug-naïve children with GHD and 30 typically developing controls (TDs), matched for age and gender. For the GHD group, detailed clinical data were collected from medical records, including age, gender, height, weight, serum IGF-1, adrenocorticotropic hormone (ACTH), cortisol, and thyroid-stimulating hormone (TSH). All GHD participants underwent two provocation tests to assess growth hormone secretion. Blood samples were taken at baseline time 0 and at 30, 60, 90, and 120 minutes following an intravenous injection of pyridostigmine combined with levodopa. The peak GH level after these tests was recorded. For the TD group, basic demographic information, including age, gender, height, and weight, was also documented. Behavioral problems in children with GHD were evaluated using Achenbach’s Child Behavior Check List (CBCL) (18).
Participants with GHD were included if they met the following criteria: short stature, defined as height below the third percentile or less than 2 standard deviations below the mean for their age-matched population (19); peak serum GH levels below 10 µg/L after at least two provocation tests; absence of ACTH deficiency, hypoglycemia, thyroid-related disorders, familial genetic conditions, or metabolic diseases; and right-handedness. Participants were excluded if they had other psychiatric or personality disorders, dependence on psychotropic drugs, difficulty cooperating during magnetic resonance imaging (MRI) examinations or poor image quality, a history of organic or metabolic brain diseases, or other MRI contraindications.
Image acquisition
A coronal T2-weighted sequence was collected to rule out any cranial organic lesion. MRI examinations of all participants were executed on a 3.0T scanner (SIGNA Pioneer GE Healthcare, Milwaukee, WI, USA) using 32-channel head coils. Sagittal three-dimensional T1-weighted fast spoiled gradient echo-based sequence (T1-FSPGR) with 1.00 mm isotropic resolution and DTI scan were performed for each participant. DTI data were acquired using a single-shot echo planar imaging sequence [repetition time/echo time (TR/TE) =10,000/88.6 ms; field of view (FOV) =256×256 mm2; matrix size =128×128; voxel size =2 mm3; number of excitations =1; number of directions =32; b value =1,000 s/mm2]. Moreover, images were acquired axially parallel to the anterior commissure-posterior commissure (AC-PC) to cover the entire brain.
Data processing and WM network construction
Data preprocessing and brain WM construction were performed using PANDA software (https://www.nitrc.org/projects/panda/), a pipeline designed for analyzing brain diffusion MRI data (20). The preprocessing steps included skull stripping with the brain extraction tool (BET), eddy current correction, diffusion tensor (DT) fitting to generate fractional anisotropy maps (DTIFIT), and registration to Montreal Neurological Institute (MNI) space with a voxel size of 2×2×2 mm3. Deterministic fiber tractography was then applied to reconstruct whole-brain WM tracts using the Fiber Assignment by Continuous Tracking (FACT) algorithm (21). Tractography terminated when either the fiber angle exceeded 45° or FA values fell below 0.2, which was appropriate for the current analysis.
The automated anatomical labeling (AAL) atlas was used to define network nodes, dividing the brain into 90 cortical and subcortical regions (22). Each T1-weighted image was co-registered to individual b0 images in the original diffusion space using a linear transformation and subsequently nonlinearly mapped to MNI space. Transformation parameters were inverted to warp the AAL atlas from MNI space back to the diffusion space while preserving discrete labeling values through nearest-neighbor interpolation. Network edges were defined by calculating the mean FA values of connecting fibers. Finally, each participant’s data was represented as a weighted, undirected, symmetrical 90×90 anatomical connectivity matrix.
Network analysis
Network properties were computed using the GRETNA toolbox (http://www.nitrc.org/projects/gretna/) in MATLAB (23). For each network metric, the area under the curve (AUC) was calculated over the sparsity range from S1=0.01 to Sn=0.40 with an interval of ΔS=0.01. This approach provides a summary scalar for the topological characterization of brain networks, independent of single-threshold selection. This method minimizes the impact of discrepancies in overall correlation strength between groups and allows for the exploration of differences in relative network organization (21). Graph theoretical analysis was applied to each participant’s WM network. Global network profiles included small-world parameters such as characteristic path length (Lp), clustering coefficient (Cp), normalized shortest pathlength (λ), normalized clustering coefficient (γ), and small-worldness (σ = γ/λ). Network efficiency parameters, including local efficiency (Eloc) and global efficiency (Eglob), were also examined. Nodal properties analyzed in this study included nodal degree, nodal betweenness, and nodal efficiency. Finally, between-group differences in nodal profiles were visualized using the BrainNet Viewer toolbox (https://www.nitrc.org/projects/bnv/).
Statistical analysis
Statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). The Shapiro-Wilk test was used to assess the normality of continuous variables. Quantitative variables were compared using independent-sample t-tests, whereas qualitative variables were analyzed using chi-square tests. Statistical significance was defined as P<0.05.
Between-group comparisons of the AUC for each network metric were conducted using nonparametric permutation tests (24), with group as a fixed factor and age and gender included as covariates. To account for multiple comparisons, the Benjamini-Hochberg false discovery rate (BHFDR) approach was applied to control the error rate. Finally, partial correlation analyses were performed to evaluate the relationships between topological metrics and clinical symptom severity, adjusting for age and gender as confounding factors. The significance level was set at P<0.05.
Results
Participant characteristics
This study initially enrolled 78 children with GHD; ultimately, 58 patients with GHD were included in the study. The other participants were excluded for the following reasons: (I) head movements (11 children with GHD); and (II) poor image quality (9 children with GHD). There were no significant differences in age, sex distribution between the GHD and TD groups (all P>0.05). Detailed demographic and clinical characteristics of the study participants are summarized in Table 1.
Table 1
| Characteristics | GHD group (n=58) | Control group (n=30) | P value |
|---|---|---|---|
| Age (years) | 9.44±2.57 | 9.52±2.52 | 0.898 |
| Sex, male | 38 (66%) | 19 (63%) | 0.839 |
| Weight (kg) | 24.55±6.88 | 33.23±9.12 | <0.001* |
| Height (cm) | 123.63±13.62 | 137.89±13.98 | <0.001* |
| Weight SDS | −1.71±0.75 | 0.13±0.25 | <0.001* |
| Height SDS | −2.34±0.43 | 0.06±0.31 | <0.001* |
| Peak GH (µg/L) | 5.39±2.15 | NA | – |
| IGF-1 (ng/mL) | 179.33±74.92 | NA | – |
| ACTH (pmol/L) | 6.07±3.15 | NA | – |
| Cortisol (μg/dL) | 10.28±3.49 | NA | – |
| TSH (µIU/mL) | 3.18±3.42 | NA | – |
Data are represented as the mean ± SD or n (%). For comparisons of demographics, P values are obtained using two sample t-test or chi-square test; *, P<0.05 was considered significant. ACTH, adrenocorticotropic hormone; GH, growth hormone; IGF-1, insulin-like growth factor-1; GHD, growth hormone deficiency; NA, not available; SD, standard deviation; SDS, standard deviation score; TDs, typically developing controls; TSH, thyroid stimulating hormone.
Alterations in global properties of the WM networks
Both the GHD and TD groups exhibited typical features of small-world topology in the WM morphological network (σ>1) across the defined threshold range. However, significant differences in global network properties were observed between the groups. The GHD group showed significantly decreased Eglob (P<0.001) and Eloc (P<0.001). The GHD group also showed significantly increased Lp (P<0.001), higher λ (P<0.001), decreased Cp (P<0.001) and reduced σ (P=0.012) (Figure 1). No significant difference was found in γ (P=0.300).

Alterations in nodal properties of the WM networks
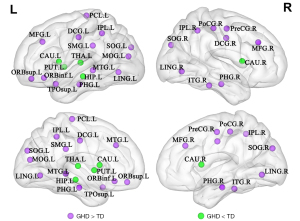
Analysis of nodal properties based on the AAL-90 atlas revealed significant differences between GHD patients and TD controls across several brain networks: (I) the default-mode network (DMN) that included increased nodal parameters involving right precentral gyrus (PreCG.R), bilateral median cingulate and paracingulate gyri (DCG), bilateral parahippocampal gyrus (PHG), bilateral inferior parietal lobule (IPL), left supramarginal gyrus (SMG.L), and left middle temporal gyrus (MTG.L), and decreased nodal parameters involving left hippocampus (HIP.L); (II) the CEN that included increased nodal parameters involving left orbital part of superior frontal gyrus (ORBsup.L), bilateral middle frontal gyrus (MFG), left orbital part of Inferior frontal gyrus (ORBinf.L), and right inferior temporal gyrus (ITG.R); (III) the visual network (VN) that included increased nodal parameters involving bilateral lingual gyrus (LING), bilateral superior occipital gyrus (SOG) and left middle occipital gyrus (MOG.L); (IV) the basal ganglia (mainly in corpus striatum) that included decreased nodal parameters involving bilateral caudate nucleus (CAU) and left putamen (PUT.L) and decreased nodal parameters in left thalamus (THA). Increased nodal parameters involving the salience network (SN)-related left temporal pole (TPOsup.L), sensorimotor network (SMN)-related right postcentral gyrus (PoCG.R), and left paracentral lobule (PCL.L) were also observed in GHD (Table 2 and Figure 2).
Table 2
| Brain regions | Category | P value | ||
|---|---|---|---|---|
| Nodal degree | Nodal efficiency | Nodal betweenness | ||
| GHD > TD | ||||
| PreCG.R | DMN | 0.020** | <0.001** | 0.040* |
| ORBsup.L | CEN | 0.002** | <0.001** | 0.013* |
| MFG.L | CEN | 0.062 | <0.001** | 0.045** |
| MFG.R | CEN | 0.035** | <0.001** | 0.015* |
| ORBinf.L | CEN | <0.001** | <0.001** | 0.008* |
| DCG.L | DMN | 0.386 | 0.001** | 0.014** |
| DCG.R | DMN | 0.151 | 0.005** | 0.033* |
| PHG.L | DMN | <0.001** | <0.001** | 0.034* |
| PHG.R | DMN | 0.020** | 0.001** | 0.008* |
| LING.L | VN | <0.001** | <0.001** | 0.029* |
| LING.R | VN | 0.144 | 0.007** | 0.036** |
| SOG.L | VN | 0.004** | <0.001** | 0.040* |
| SOG.R | VN | <0.001** | <0.001** | 0.019* |
| MOG.L | VN | 0.880 | <0.001** | 0.017** |
| PoCG.R | SMN | <0.001** | <0.001** | 0.026* |
| IPL.L | DMN | <0.001** | <0.001** | 0.022* |
| IPL.R | DMN | <0.001** | <0.001** | <0.001** |
| SMG.L | DMN | 0.003** | 0.001** | 0.017** |
| PCL.L | SMN | <0.001** | 0.037** | 0.028** |
| TPOsup.L | SN | 0.003** | <0.001** | 0.045** |
| MTG.L | DMN | 0.654 | 0.001** | <0.001** |
| ITG.R | CEN | 0.039** | <0.001** | 0.045** |
| GHD < TD | ||||
| HIP.L | DMN | <0.001** | <0.001** | <0.001** |
| CAU.L | Striatum | <0.001** | <0.001** | 0.042** |
| CAU.R | Striatum | <0.001** | <0.001** | 0.015* |
| PUT.L | Striatum | <0.001** | <0.001** | 0.013* |
| THA.L | Thalamus | <0.001** | <0.001** | 0.047* |
Twenty-seven regions with PFDR <0.05 in at least two node profile were included. *, uncorrected P<0.05; **, PFDR <0.05. All P values were obtained using a permutation test. The brain regions were defined by AAL. AAL, automated anatomical labeling; CAU, caudate nucleus; CEN, central executive network; DCG, median cingulate and paracingulate gyri; DMN, default-mode network; FDR, false discovery rate; GHD, growth hormone deficiency; HIP, hippocampus; IPL, inferior parietal, but supramarginal and angular gyri; ITG, inferior temporal gyrus; L, left; LING, lingual gyrus; MFG, middle frontal gyrus; MTG, middle temporal gyrus; MOG, middle occipital gyrus; ORBsup, superior frontal gyrus, orbital part; ORBinf, inferior frontal gyrus, orbital part; PCL, paracentral lobule; PreCG, precentral gyrus; PHG, parahippocampal gyrus; PUT, lenticular nucleus, putamen; SOG, superior occipital gyrus; PoCG, postcentral gyrus; R, right; SMG, supramarginal gyrus; SN, salience network; SMN, sensorimotor network; TD, typically developing; TPOsup, temporal pole: superior temporal gyrus; THA, thalamus; VN, visual network.
Relationships between network metrics with serum markers and behavior scores
As for behavioral problems (Figure 3A-3C), total scores of Achenbach’s CBCL were positively correlated with nodal efficiency of the left middle occipital gyrus (r=0.275; P=0.048, uncorrected), right inferior parietal lobe (r=0.293; P=0.035, uncorrected), and MTG.L (r=0.295; P=0.034, uncorrected).

As for nodal profiles (Figure 3D-3F), in GHD patients, serum IGF-1 concentration was negatively correlated with nodal betweenness in the left CAU (r=−0.277; P=0.047, uncorrected), and negatively correlated with nodal betweenness of the ITG.R (r=−0.350; P=0.011, uncorrected) and nodal degree of the ITG.R (r=−0.307; P=0.027, uncorrected). For whole-brain organization level (Figure 3G,3H), GH peak level of GHD patients was negatively correlated with Eglob (r=−0.345; P=0.010, uncorrected), and positively correlated with Lp (r=0.363; P=0.008, uncorrected). No significant correlations were found between clinical variables and any other global or nodal metrics (P>0.05).
Discussion
Main findings
The present study investigated the topological organization of WM structural networks in GHD patients. The main findings are as follows: (I) at the global level, GHD showed decreased integration in the organization of the white matter networks, characterized by a shift towards a “weaker small-worldness” pattern; (II) at the nodal level, altered nodal profiles were primarily observed in regions associated with the basal ganglia (mainly corpus striatum), DMN, and CEN regions, which are known to be involved in GHD; (III) the nodal efficiency of MOG.L, IPL.R, and MTG.L was positively correlated with CBCL scores in GHD. These results provide insights into the altered topological organization of WM structural networks in pediatric GHD, suggesting widespread disruptions in brain connectivity that may underlie the behavioral symptoms associated with this condition.
Global properties of WM networks in GHD
Our analysis revealed that children with GHD display characteristics of a “weaker small-worldness” pattern in their WM structural networks. Small-worldness, characterized by a combination of high clustering and short average path length, supports the fundamental organizational principles of integration and segregation in structural brain networks (25). The increased Lp observed in GHD patients compared to TD groups, suggests a disruption in the efficiency of information transfer across the brain. This could be attributed to alterations in the integrity of WM fiber bundles. Concurrently, the decreased global efficiency in the GHD group further supports this interpretation, indicating a reduced capacity for parallel information processing and global integration of brain functions. These global topological alterations suggest a disturbance in the balance between functional separation and integration of structural brain networks in GHD. Such changes may underlie the distributed information processing deficits observed in GHD and represent a shift towards a more regularized network structure (26). This pattern of altered global network properties has been observed in other neurodevelopmental disorders and may reflect a common pathway of disrupted brain organization in conditions affecting early brain development.
Nodal profiles of WM networks in GHD
Our study revealed both increased and decreased nodal profiles across various brain regions in children with GHD. Notably, reduced nodal profiles were observed primarily in the corpus striatum, thalamus, and hippocampus, whereas increased nodal profiles were found in DMN, CEN, VN, SMN, and SN regions. The GH/IGF-1 axis plays an important role in brain development and maintenance of cognitive function (27). Previous research has shown that the corpus striatum (putamen, caudate) expresses a high density of GH and IGF-1 receptor (28). Our findings of altered nodal properties in these regions are consistent with an earlier morphologic study that demonstrated decreased GMV in the striatum, thalamus, and hippocampus (5) of GHD patients. Importantly, our results extend beyond structural volume changes, revealing alterations in node properties within the WM structural network. This suggests that GHD affects not only the GMV of deep brain structures but also their connectivity patterns and integration within larger-scale brain networks. The observed increases in nodal profiles across multiple cortical networks (DMN, CEN, VN, SMN, SN) align with previous functional MRI studies reporting abnormal cortical activity in these networks in children with GHD (7,8,29). These widespread alterations suggest that GHD impacts the regulation of large-scale WM neural networks, affecting both deep GM structures, and cortical regions. Collectively, these findings point to abnormalities in the cortico-striato-thalamo-cortical (CSTC) circuit as a potential key factor underlying the behavioral symptoms observed in GHD. The CSTC circuit is critical for various cognitive and motor functions, and its disruption could explain the diverse range of symptoms seen in GHD patients.
Relationships between nodal network profiles and clinical variables
Our correlation analyses revealed significant relationships between network properties and clinical variables in GHD patients. Notably, nodal betweenness, degree of the right ITG and left caudate were negatively correlated with IGF-1 levels. The ITG is involved in higher cognitive functions, including visual recognition, language comprehensions, and emotion regulation (30). These correlations suggest that lower IGF-1 levels may be associated with altered connectivity and information flow through these critical brain regions. Additionally, we found positive correlations between nodal efficiency of the MOG, MTG, and IPL and CBCL’s scores. The IPL, particularly in the right hemisphere, is a key component of the ventral attention network and plays a crucial role in attention redirection (31,32). These correlations indicate that increased efficiency in these regions is associated with more pronounced behavioral problems in GHD children, possibly reflecting compensatory mechanisms or altered network dynamics. These brain-behavior relationships are consistent with a previous report that GH deficiency hinders WM development and impairs cognitive performance in children (33). Furthermore, animal research has shown that GH treatment during or post hypoxic insult can enhance myelination and promote functional recovery (34). This suggests that the GH/IGF-1 axis serves as an important systemic mechanism for maintaining WM integrity and brain structure.
Mechanism of GHD impact on WM and myelin development
GHD can significantly impact WM and myelin development through multiple mechanisms. Firstly, GH and its downstream effector IGF-1 play crucial roles in promoting the proliferation and differentiation of oligodendrocyte progenitor cells (OPCs). In GHD, the reduced levels of GH and IGF-1 lead to a decreased pool of OPCs available for myelination (34,35). Myelination is a complex process that highly depends on the proper functioning of OPCs, which give rise to mature oligodendrocytes that produce myelin sheaths around axons. Without sufficient OPCs, the development of myelin is hampered. Secondly, axonal–glial signaling is essential for the proper formation of myelin. GH and IGF-1 are involved in modulating this signaling pathway. In normal conditions, axons release signals that interact with glial cells, including oligodendrocytes, to initiate and regulate myelin wrapping. In GHD, the altered levels of GH and IGF-1 disrupt this communication. Lastly, GHD can also affect neurotransmitter systems that are associated with myelin development. Dopamine influences oligodendrocyte function and myelin production. GHD may disrupt the normal regulation of dopamine-related pathways. The dysregulation of dopamine-related genes leads to a decrease in myelin-specific protein expression, thereby affecting myelin development in the WM.
In conclusion, GHD impacts myelin development through various means, mainly involving the interference with OPCs, axonal-glial signaling, and neurotransmitter-related processes, all of which are crucial for normal WM myelination.
Limitations
Future studies should address some of the limitations of the current work. Longitudinal designs examining changes in brain structural networks before and after GH replacement therapy are needed to elucidate the effects of treatment on brain development. Additionally, stratifying GHD patients into subgroups (e.g., complete vs. partial GHD) could provide more nuanced insights into the relationship between hormone levels and brain network organization. Although there are templates specifically designed for pediatric populations (36,37), their widespread applicability and high-quality validation remain limited. Particularly concerning variations across different age groups and standardization compared to adult templates, there is currently no consensus on established standards. To ensure that our findings could be effectively compared and validated against a substantial body of existing literature, we opted for the widely accepted adult MNI template and AAL atlas. This choice not only helps maintain consistency in our research but also facilitates reference and replication by other researchers. A small sample (36) indicated that the adult template was similarly applicable to the pediatric population, with no significant deviations observed. These results suggest that, within the context of our current study, using an adult template represents a reasonable alternative approach. Although we have adopted adult templates as a provisional measure in this study, we acknowledge the importance of pediatric-specific templates. We are committed to addressing this limitation in future projects.
Conclusions
By integrating clinical symptoms, structural network analysis, and hormone levels, we offer a comprehensive exploration of the mechanisms by which GHD affects structural brain networks in the developing brain. Our results presented altered topological organization of WM structural networks in pediatric GHD, characterized by a shift towards “weaker small-worldness” pattern, suggesting widespread disruptions in brain connectivity that may underlie the behavioral symptoms associated with this condition. Our findings underscore the importance of considering large-scale brain network alterations in understanding the neurobiology of GHD.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1927/rc
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1927/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University (No. 2021082). Written informed consent was provided by all participants’ guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lim HH, Kim YM, Lee GM, Yu J, Han HS, Yu J. Growth Responses During 3 Years of Growth Hormone Treatment in Children and Adolescents With Growth Hormone Deficiency: Comparison Between Idiopathic, Organic and Isolated Growth Hormone Deficiency, and Multiple Pituitary Hormone Deficiency. J Korean Med Sci 2022;37:e90. [Crossref] [PubMed]
- Arwert LI, Veltman DJ, Deijen JB, van Dam PS, Delemarre-van deWaal HA, Drent ML. Growth hormone deficiency and memory functioning in adults visualized by functional magnetic resonance imaging. Neuroendocrinology 2005;82:32-40. [Crossref] [PubMed]
- Gunnell D, Miller LL, Rogers I, Holly JM. Association of insulin-like growth factor I and insulin-like growth factor-binding protein-3 with intelligence quotient among 8- to 9-year-old children in the Avon Longitudinal Study of Parents and Children. Pediatrics 2005;116:e681-6. [Crossref] [PubMed]
- Yuan T, Ying J, Jin L, Li C, Gui S, Li Z, Wang R, Zuo Z, Zhang Y. The role of serum growth hormone and insulin-like growth factor-1 in adult humans brain morphology. Aging (Albany NY) 2020;12:1377-96. [Crossref] [PubMed]
- Webb EA, O'Reilly MA, Clayden JD, Seunarine KK, Chong WK, Dale N, Salt A, Clark CA, Dattani MT. Effect of growth hormone deficiency on brain structure, motor function and cognition. Brain 2012;135:216-27. [Crossref] [PubMed]
- Ding JR, Liu Y, Chen Q, Feng C, Tang Z, Zhang H, Hua B, Ding X, Wang M, Ding Z. Frequency Dependent Changes of Regional Homogeneity in Children with Growth Hormone Deficiency. Neuroscience 2023;530:183-91. [Crossref] [PubMed]
- Zhang F, Hua B, Wang T, Wang M, Ding ZX, Ding JR. Abnormal amplitude of spontaneous low-frequency fluctuation in children with growth hormone deficiency: A resting-state functional magnetic resonance imaging study. Neurosci Lett 2021;742:135546. [Crossref] [PubMed]
- Zhang F, Hua B, Wang M, Wang T, Ding Z, Ding JR. Regional homogeneity abnormalities of resting state brain activities in children with growth hormone deficiency. Sci Rep 2021;11:334. [Crossref] [PubMed]
- Tang J, Xia Y, Liu N, Li L, Zou P, Zhu P, Shan X, Lui S, Lu Y, Yan Z. Growth hormone deficiency interferes with dynamic brain networks in short children. Psychoneuroendocrinology 2022;142:105786. [Crossref] [PubMed]
- Zhang Z, Wang Y, Gao Y, Li Z, Zhang S, Lin X, Hou Z, Yu Q, Wang X, Liu S. Morphological changes of the cerebral cortex between children with isolated growth hormone deficiency and idiopathic short stature. Brain Res 2020;1748:147081. [Crossref] [PubMed]
- Zhou Z, Luo Y, Li K, Zhong S, Zhu Y, Yang H, Wang L, Chen S, Duan L, Gong F, Gong G, Zhu H, Pan H. Brain white matter alterations in young adult male patients with childhood-onset growth hormone deficiency: a diffusion tensor imaging study. Endocrine 2024;83:724-32. [Crossref] [PubMed]
- Zhou Z, Luo Y, Gao X, Zhu Y, Bai X, Yang H, Bi Q, Chen S, Duan L, Wang L, Gong F, Feng F, Gong G, Zhu H, Pan H. Alterations in brain structure and function associated with pediatric growth hormone deficiency: A multi-modal magnetic resonance imaging study. Front Neurosci 2022;16:1043857. [Crossref] [PubMed]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52:1059-69. [Crossref] [PubMed]
- Wu L, Su S, Dai Y, Qiu H, Lin L, Zou M, Qian L, Liu M, Zhang H, Chen Y, Yang Z. Disrupted Small-World Networks in Children with Drug-Naïve Attention-Deficit/Hyperactivity Disorder: A DTI-Based Network Analysis. Dev Neurosci 2024;46:201-9. [Crossref] [PubMed]
- Qian L, Li Y, Wang Y, Wang Y, Cheng X, Li C, Cui X, Jiao G, Ke X. Shared and Distinct Topologically Structural Connectivity Patterns in Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder. Front Neurosci 2021;15:664363. [Crossref] [PubMed]
- Wen H, Liu Y, Rekik I, Wang S, Zhang J, Zhang Y, Peng Y, He H. Disrupted topological organization of structural networks revealed by probabilistic diffusion tractography in Tourette syndrome children. Hum Brain Mapp 2017;38:3988-4008. [Crossref] [PubMed]
- Sporns O. The human connectome: a complex network. Ann N Y Acad Sci 2011;1224:109-25. [Crossref] [PubMed]
- Achenbach TM, Edelbrock C. Manual for the child behavior checklist and revised child behavior profile. Burlington: University of Vermont, Department of Psychiatry; 1983.
- Cook DM, Rose SR. A review of guidelines for use of growth hormone in pediatric and transition patients. Pituitary 2012;15:301-10. [Crossref] [PubMed]
- Cui Z, Zhong S, Xu P, He Y, Gong G. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci 2013;7:42. [Crossref] [PubMed]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex 2009;19:524-36. [Crossref] [PubMed]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273-89. [Crossref] [PubMed]
- Wang J, Wang X, Xia M, Liao X, Evans A, He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci 2015;9:386. [PubMed]
- Zhang J, Wang J, Wu Q, Kuang W, Huang X, He Y, Gong Q. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry 2011;70:334-42. [Crossref] [PubMed]
- Watts DJ, Strogatz SH. Collective dynamics of 'small-world' networks. Nature 1998;393:440-2. [Crossref] [PubMed]
- Su S, Chen Y, Qian L, Dai Y, Yan Z, Lin L, Zhang H, Liu M, Zhao J, Yang Z. Evaluation of individual-based morphological brain network alterations in children with attention-deficit/hyperactivity disorder: a multi-method investigation. Eur Child Adolesc Psychiatry 2023;32:2281-9. [Crossref] [PubMed]
- Annenkov A. The insulin-like growth factor (IGF) receptor type 1 (IGF1R) as an essential component of the signalling network regulating neurogenesis. Mol Neurobiol 2009;40:195-215. [Crossref] [PubMed]
- Araujo DM, Lapchak PA, Collier B, Chabot JG, Quirion R. Insulin-like growth factor-1 (somatomedin-C) receptors in the rat brain: distribution and interaction with the hippocampal cholinergic system. Brain Res 1989;484:130-8. [Crossref] [PubMed]
- Hu Y, Liu X, Chen X, Chen T, Ye P, Jiang L, Fu Y, Xie X, Shan X, Yan Z. Differences in the functional connectivity density of the brain between individuals with growth hormone deficiency and idiopathic short stature. Psychoneuroendocrinology 2019;103:67-75. [Crossref] [PubMed]
- Lin YH, Young IM, Conner AK, Glenn CA, Chakraborty AR, Nix CE, Bai MY, Dhanaraj V, Fonseka RD, Hormovas J, Tanglay O, Briggs RG, Sughrue ME. Anatomy and White Matter Connections of the Inferior Temporal Gyrus. World Neurosurg 2020;143:e656-66. [Crossref] [PubMed]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002;3:201-15. [Crossref] [PubMed]
- Wang J, Zhang J, Rong M, Wei X, Zheng D, Fox PT, Eickhoff SB, Jiang T. Functional topography of the right inferior parietal lobule structured by anatomical connectivity profiles. Hum Brain Mapp 2016;37:4316-32. [Crossref] [PubMed]
- van Dam PS, de Winter CF, de Vries R, van der Grond J, Drent ML, Lijffijt M, Kenemans JL, Aleman A, de Haan EH, Koppeschaar HP. Childhood-onset growth hormone deficiency, cognitive function and brain N-acetylaspartate. Psychoneuroendocrinology 2005;30:357-63. [Crossref] [PubMed]
- Ren SY, Xia Y, Yu B, Lei QJ, Hou PF, Guo S, et al. Growth hormone promotes myelin repair after chronic hypoxia via triggering pericyte-dependent angiogenesis. Neuron 2024;112:2177-2196.e6. [Crossref] [PubMed]
- Hua K, Forbes ME, Lichtenwalner RJ, Sonntag WE, Riddle DR. Adult-onset deficiency in growth hormone and insulin-like growth factor-I alters oligodendrocyte turnover in the corpus callosum. Glia 2009;57:1062-71. [Crossref] [PubMed]
- Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DLBrain Development Cooperative Group. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 2011;54:313-27. [Crossref] [PubMed]
- Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Hum Brain Mapp 2002;17:48-60. [Crossref] [PubMed]


