
Depicting growth characteristics with computed tomography for KRAS-mutated lung adenocarcinoma
Introduction
In recent years, several non-small cell lung cancer (NSCLC) associated oncogenes have been identified (1-3), including the mutated epidermal growth factor receptor (EGFR) gene, rearranged anaplastic lymphoma kinase (ALK) gene, and mutated Kirsten rat sarcoma viral oncogene homolog (KRAS) (4). Mutations in driver genes have been shown to be significantly related to the prognosis of patients (5-7). Further, it has been estimated that 5–15% of NSCLC patients in Asian populations have the KRAS mutation (8). The KRAS is either present in the rat sarcoma (RAS)-guanosine triphosphate bound active state, or the RAS-guanosine diphosphate bound inactive state (9).
Computed tomography (CT) is the optimal modality for detecting and characterizing pulmonary tumors (10,11). In lung adenocarcinoma (LUAD), pulmonary nodules are categorized into the following three groups based on their consolidation-to-tumor (C/T) ratios, which are determined using CT images obtained within one month before surgery: the pure ground-glass opacity (pGGO) type (C/T ratio: 0); the partial-solid tumor type (0< C/T ratio <1); and the solid tumor type (C/T ratio: 1) (12). Due to it high-density resolution, lung CT scans can help to investigate the growth of LUAD.
This study analyzed 808 LUAD patients with KRAS mutations and their preoperative CT scans to evaluate the growth characteristics of LUAD in patients with KRAS mutations. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1880/rc).
Methods
Patients
This study was approved by the Ethics Review Board of the Huadong Hospital, Fudan University (No. 20230108), and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The requirement of informed consent was waived for this study due to its retrospective nature.
This study included 808 histologically confirmed LUAD patients with primary lung cancer at the Huadong Hospital, who were enrolled from January 2018 to December 2023. The patients were selected consecutively. To be eligible for inclusion in the study, the patients had to meet the following inclusion criteria: (I) have been pathologically diagnosed with LUAD; (II) have single lesions on CT scans; (III) have undergone KRAS mutation tests; and (IV) have clinical data, including age, sex, and smoking history data, available.
Of the 808 patients, 720 had preoperative CT scans, which were used to evaluate the CT features of the lesions. The medical record analysis revealed that 68 patients had no record of the size of the specimen, and 740 patients had pathological data available.
CT assessment
Preoperative chest CT scans were performed using three scanners: GE Discovery CT750 HD, 64-slice Light Speed volume CT (GE Medical Systems, GE Healthcare, Chicago, IL, USA), and Somatom Definition Flash (Siemens Healthcare, Forchheim, Germany). The following scanning parameters were used: 120 kVp, 100–200 mAs, 0.75–1.5 pitch range, and 1–1.25 mm collimation widths. A medium sharp reconstruction algorithm was used for all the imaging data and provided images of 1–1.25-mm thickness.
Interpretation of CT images
The CT images were independently reviewed by two radiologists with 10 to 12 years of experience in chest CT diagnosis. The radiologists were aware that the patients had undergone surgical resection for LUAD; however, they were blinded to clinical data, as well as gene expression and mutational status data. Both mediastinal [width =350 Hounsfield unit (HU); level =40 HU] and lung (width =1,500 HU; level =−650 HU) window settings were employed for the CT image analysis. Further, lesion location, size, and texture were retrospectively analyzed, with the long-axis diameter measured at the largest section. The lesions with 100% ground-glass opacity (GGO) were categorized as pGGO, those with 1% and 100% GGO were as classified mixed ground-glass opacity (mGGO), and those lacking GGO (0%) were classified as pure solid opacity (pSO).
Mutation analysis
To analyze the mutation status of the patients, samples were subjected to amplification refractory mutation system quantitative polymerase chain reaction analysis.
Statistical analysis
SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was employed for all the statistical analyses. The distribution differences in the categorical variables were assessed using the chi-square (χ2) test, while differences in the continuous variables were evaluated using the independent t-test. A P value of <0.05 was considered statistically significant.
Results
Patient demographics
The study included 808 patients, 341 men and 467 women, who had an average age of 61.4 years (range, 16–86 years). Among the patients, 40 (4.9%) had KRAS mutations, and 65 had a history of smoking.
Correlation between KRAS status and clinical factors
The results of the statistical analysis (Table 1) indicated that the incidence of the KRAS mutation was higher in the male patients than the female patients [8.5% (29/341) vs. 2.4% (11/467), P<0.0001], and in the older patients than the younger patients [6.5% (29/448) vs. 3.1% (11/360), P=0.02].
Table 1
| Groups | KRAS mutation | KRAS wild type | P value |
|---|---|---|---|
| Sex, n | <0.0001 | ||
| Male | 29 | 312 | |
| Female | 11 | 456 | |
| Age (years), n | 0.02 | ||
| ≤60 | 11 | 349 | |
| >60 | 29 | 419 | |
| Smoking, n | |||
| Yes | 7 | 65 | |
| No | 33 | 703 | 0.05 |
| No¶ | 29 | 312 | 0.74† |
| Yes‡ | 3 | 35 | 0.90§ |
| #T stage, n | |||
| T1a | 11 | 198 | 0.59 |
| ≥T1b | 23 | 508 | |
| #N stage, n | |||
| N0 | 32 | 675 | 0.68 |
| ≥ N1 | 2 | 31 |
†, only includes males; ‡, smoking index >400; §, between males with a “never-smoking status” and those with a smoking index >400; ¶, only including males; #, 68 of 808 patients had no corresponding records. KRAS, Kirsten rat sarcoma viral oncogene homolog.
The correlation analysis revealed a marginally statistically significant association between the KRAS mutation and smoking in the overall cohort [9.7% (7/72) vs. 4.5% (33/736), P=0.05]. However, when excluding the female cohort, which had no smokers, no significant difference was observed between the smokers and non-smokers. The smoker group included patients with a smoking index of >400, and this group was compared with the never-smoking group to assess the incidence of the KRAS mutation, but no statistically significant difference was found.
The LUAD patients were also classified into T1a and ≥T1b stages, but no correlation was found between the KRAS mutation and T stage. Moreover, no correlation was found between lymph node status and the KRAS mutation in the LUAD patients.
Comparison of CT texture nodules between the patients with the KRAS mutation and KRAS wild-type lesions
Of the 720 patients with CT scans, 34 had KRAS mutations. The results indicated that the incidence of the KRAS mutation was statistically lower in the mGGO group than the pGGO group [1.3% (3/232) vs. 6.7% (15/225), P=0.0032 females] and pSO group [1.3% (3/232) vs. 6.1% (16/263), P=0.0056]. However, no statistically significant difference was found between the pGGO and pSO groups in terms of the KRAS mutation (P=0.79) (Table 2 and Figures 1,2).
Table 2
| CT texture | KRAS mutation | KRAS wild type | P value |
|---|---|---|---|
| pGGO, n | 15 | 210 | 0.0032†, 0.0056‡, 0.79§ |
| mGGO, n | 3 | 229 | |
| pSO, n | 16 | 247 |
†, mGGO vs. pGGO; ‡, mGGO vs. pSO; §, pGGO vs. pSO. CT, computed tomography; KRAS, Kirsten rat sarcoma viral oncogene homolog; LUAD, lung adenocarcinoma; mGGO, mixed ground-glass opacity; pGGO, pure ground-glass opacity; pSO, pure solid opacity.
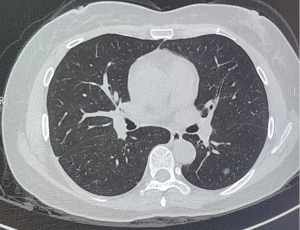
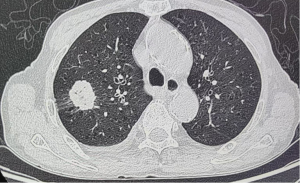
Comparison of pGGO and pSO rates in different sizes of lesions between the KRAS mutation and KRAS wild-type lesions
The pGGO and pSO rates of lesions ≤1 cm were compared. The frequency of ≤1 cm pGGO was higher than that of ≤1 cm pSO, but the level of statistical significance was marginal [12.0% (11/92) vs. 0 (0/27), P=0.05) (Table 3).
Table 3
| CT textures and size | KRAS mutation | KRAS wild type | P value |
|---|---|---|---|
| pGGO ≤1 cm | 11 (12.0) | 81 | 0.05† |
| pSO ≤1 cm | 0 (0.0) | 27 |
†, Fisher’s exact test of probability. The number in parentheses is the percentage of the corresponding lesions in a total of the same column. KRAS, Kirsten rat sarcoma viral oncogene homolog; LUAD, lung adenocarcinoma; pGGO, pure ground-glass opacity; pSO, pure solid opacity.
Discussion
The literature suggests that approximately 5–15% of NSCLC patients in Asian populations carry KRAS mutations (8), which is consistent with the results of this study, which had a KRAS mutation rate of 4.9%. Further, this study showed that KRAS mutations were more common in male and older LUAD patients, which is also in line with the findings of previous studies (13-15). For example, one study (16) reported that 275 of 3,829 LUAD patients had KRAS mutations. KRAS mutations have also found to be correlated with a male gender but not age (17-22). Conversely, other studies have reported that the KRAS mutation is not associated with sex or age (13-23). These conflicting results can be attributed to the scarcity of KRAS mutations and ethnic differences; thus, further research needs to be conducted.
A few studies (13,23) have reported that smoking is not associated with the KRAS mutation; however, the most studies, including one meta-analysis (24), have reported that the KRAS mutation is correlated with smoking (14,15,17-19,21,24-27). The present study found a marginal correlation between smoking and the KRAS mutation when both females and males were included in the cohort (P=0.05). However, this correlation disappeared when the females were excluded from the cohort, which is significant, as most females in China are non-smokers. Further, the comparison of the incidence of the KRAS mutation between heavy smokers (those with a smoking index >400) and never smokers revealed no significant difference. Zhu et al. (16) studied 275 patients with the KRAS mutation, and found that the KRAS mutation was more common in smokers before propensity score matching, but found no correlation after propensity score matching. In general, the prevalence of smoking is higher in men than women worldwide (28-33). A meta-analysis of 68,868 lung cancer cases reported that the prevalence of smoking in men ranged from 11.7% to 97.5%, while in women, it ranged from 5.9% to 67.7% (34). However, previous studies did not distinguish between males and females when evaluating the effect of smoking on KRAS mutations. Thus, further research needs to be conducted to determine whether the correlation between the KRAS mutation and smoking is due to the prevalence of smoking or ethnic differences.
In this study, no correlation was found between the T and N stages, and the KRAS mutation, which is consistent with the findings of previous studies (16,21,35).
Research on CT texture features is scarce. Some studies have found no significant association between KRAS mutations and the presence of any GGO (36-38) or the GGO ratio (35); however, these studies cannot be compared with the present study, as they did not distinguish between pGGO and mGGO. Notably, Wang et al. (39) revealed that KRAS mutations were more common in lesions with a lower GGO proportion, while Park et al. (40) found that KRAS mutations were associated with solid tumors. However, these studies are not comparable with the present study, as the sample size of Wang et al.’s (39) study was relatively small, and the Park et al.’s (40) study only included advanced LUAD patients.
This study found that the KRAS mutation lesions had a CT texture feature that could be described as “more at the two ends and less in the middle” (i.e., a dumbbell shape). Further, the frequency of mGGOs was significantly lower than that of pGGOs and pSOs. The incidence rates of pGGOs, mGGOs, and pSOs in LUAD have been reported to range from 17.1–39.2%, 28.5–28.8%, and 32.0–54.3%, respectively (16,26,39), while in the present study, the rates were 31.3%, 32.2%, and 36.4%, respectively. These results fall within the range of previous findings, suggesting that they were not influenced by interpretive bias.
It may be that the CT texture features of pGGO, mGGO, and pSO present in a dumbbell shape because the initial increase in the size of the GGO nodule is followed by the appearance and gradual enlargement of a solid portion within the lesion (41). Lesions transitioning from pGGO to mGGO, or from mGGO to pSO have shown a rapid increase in size (10,42,43). Overall, it can be inferred that the mGGO associated with the KRAS mutation is a distinct type. If pGGO lesions of this type transition to mGGO, the solid components in the mGGO increase rapidly, resulting in a rapid change to pSO. Therefore, in LUAD, the mGGO of the KRAS period is very short, resulting in reduced frequency of visible mGGOs.
To validate this hypothesis, the frequency of ≤1 cm pGGO and pSO LUAD were compared between the KRAS mutation and KRAS wild-type lesions. The results showed that the frequency of pSO was less than that of pGGO [0% (0/27) vs. 12.0% (11/92), P=0.05], but the P value was only marginally significant. Studies have found that the presence of a solid portion (an mGGO or pSO) is a risk factor for a significant size increase (10,44). Thus, it can be inferred that most KRAS mutation lesions change into pSOs at a size of at least >1 cm, but as stated above, the statistical significance was marginal. This showed that the speed at which pGGO changes into pSO in KRAS mutation lesions was slower than that of KRAS wild-type lesions in LUAD.
Limitations
This study had a number of limitations. First, the sample size was relatively small; therefore, the stratification of smokers by KRAS status was not possible. Second, the majority of the Chinese female patients had no history of smoking due to cultural factors. Therefore, it was not appropriate to compare smoking with the KRAS mutation in this group. Third, only patients with single lesions were included in the study, and patients with multiple lesions were not included in the study. Fourth, due to the inherent limitations of a retrospective analysis, this study only provides preliminary data on the CT texture features of LUAD for the KRAS mutation. Fifth, as the patients lacked CT scans, an analysis of the progression characteristics of LUAD with and without the KRAS mutation could not be conducted; therefore, the progression characteristics are speculative, which weakens our hypothesis. Further studies need to be conducted to gather evidence in support of our hypothesis. Sixth, the identification of the progression feature of LUAD with the KRAS mutation was based on a comparison between LUAD with or without the KRAS mutation, and no comparisons with other kinds of mutations (e.g., EGFR and ALK) were made. In the future, we intend to conduct analyses of other types of mutations in LUAD.
Conclusions
The results showed that the incidence of the KRAS mutation was higher in male and older LUAD patients. Further, smoking was not found to be associated with the KRAS mutation. Moreover, in KRAS-mutant LUAD, mGGO was observed less frequently than pGGO and pSO, and most lesions that transitioned from pGGO to pSO were at least >1 cm in size. Further large-scale studies and investigations need to be conducted to verify these findings, especially for the marginally statistically significant results.
Acknowledgments
The authors would like to thank all the reviewers who reviewed the article and MJEditor (www.mjeditor.com) for their linguistic assistance during the preparation of this manuscript.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1880/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1880/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was approved by the Ethics Review Board of the Huadong Hospital, Fudan University (No. 20230108), and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The requirement for informed consent was waived for this study due to its retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosen JC, Sacher A, Tsao MS. Direct GDP-KRAS(G12C) inhibitors and mechanisms of resistance: the tip of the iceberg. Ther Adv Med Oncol 2023;15:17588359231160141. [Crossref] [PubMed]
- Fan F, Zhang H. A case description of pulmonary enteric adenocarcinoma with KRAS G12D mutation. Quant Imaging Med Surg 2023;13:8787-92. [Crossref] [PubMed]
- Gower A, Wang Y, Giaccone G. Oncogenic drivers, targeted therapies, and acquired resistance in non-small-cell lung cancer. J Mol Med (Berl) 2014;92:697-707. [Crossref] [PubMed]
- Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS, Squire J, Thunnissen E, Ladanyi MCollege of American Pathologists International Association for the Study of Lung Cancer and Association for Molecular Pathology. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Mol Diagn 2013;15:415-53. Erratum in: J Mol Diagn 2013;15:730. [Crossref] [PubMed]
- Meng D, Yuan M, Li X, Chen L, Yang J, Zhao X, Ma W, Xin J. Prognostic value of K-RAS mutations in patients with non-small cell lung cancer: a systematic review with meta-analysis. Lung Cancer 2013;81:1-10. [Crossref] [PubMed]
- Dong Y, Li Y, Jin B, Zhang J, Shao J, Peng H, Tu S, Han B. Pathologic subtype-defined prognosis is dependent on both tumor stage and status of oncogenic driver mutations in lung adenocarcinoma. Oncotarget 2017;8:82244-55. [Crossref] [PubMed]
- Kosaka T, Yatabe Y, Onozato R, Kuwano H, Mitsudomi T. Prognostic implication of EGFR, KRAS, and TP53 gene mutations in a large cohort of Japanese patients with surgically treated lung adenocarcinoma. J Thorac Oncol 2009;4:22-9. [Crossref] [PubMed]
- Del Re M, Rofi E, Restante G, Crucitta S, Arrigoni E, Fogli S, Di Maio M, Petrini I, Danesi R. Implications of KRAS mutations in acquired resistance to treatment in NSCLC. Oncotarget 2018;9:6630-43. [Crossref] [PubMed]
- Wu MY, Zhang EW, Strickland MR, Mendoza DP, Lipkin L, Lennerz JK, Gainor JF, Heist RS, Digumarthy SR. Clinical and Imaging Features of Non-Small Cell Lung Cancer with G12C KRAS Mutation. Cancers (Basel) 2021;13:3572. [Crossref] [PubMed]
- Lee SW, Leem CS, Kim TJ, Lee KW, Chung JH, Jheon S, Lee JH, Lee CT. The long-term course of ground-glass opacities detected on thin-section computed tomography. Respir Med 2013;107:904-10. [Crossref] [PubMed]
- Kim T, Cha YJ, Chang YS. Correlation of PD-L1 Expression Tested by 22C3 and SP263 in Non-Small Cell Lung Cancer and Its Prognostic Effect on EGFR Mutation-Positive Lung Adenocarcinoma. Tuberc Respir Dis (Seoul) 2020;83:51-60. [Crossref] [PubMed]
- Kinoshita F, Toyokawa G, Matsubara T, Kozuma Y, Haratake N, Takamori S, Akamine T, Hirai F, Takenaka T, Tagawa T, Maehara Y. Prognosis of Early-stage Part-solid and Pure-solid Lung Adenocarcinomas. Anticancer Res 2019;39:2665-70. [Crossref] [PubMed]
- Riely GJ, Kris MG, Rosenbaum D, Marks J, Li A, Chitale DA, Nafa K, Riedel ER, Hsu M, Pao W, Miller VA, Ladanyi M. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res 2008;14:5731-4. [Crossref] [PubMed]
- Liu X, Jiang G, Sun X, Su G, Zhang X, Shen D, Yan N. Relationship between driver gene mutations and clinical pathological characteristics in older lung adenocarcinoma. Front Oncol 2023;13:1275575. [Crossref] [PubMed]
- Zhao M, Zhan C, Li M, Yang X, Yang X, Zhang Y, Lin M, Xia Y, Feng M, Wang Q. Aberrant status and clinicopathologic characteristic associations of 11 target genes in 1,321 Chinese patients with lung adenocarcinoma. J Thorac Dis 2018;10:398-407. [Crossref] [PubMed]
- Zhu W, Han H, Ma Z, Cao H, Yan Y, Zhao Y, Deng C, Xu H, Fu F, Fan F, Zhang Y, Chen H. Prognostic value of KRAS G12V mutation in lung adenocarcinoma stratified by stages and radiological features. J Thorac Cardiovasc Surg 2024;168:1525-1537.e6. [Crossref] [PubMed]
- Brcic L, Jakopovic M, Misic M, Seiwerth F, Kern I, Smojver-Jezek S, Quehenberger F, Samarzija M, Seiwerth S. Analysis of the frequency of EGFR, KRAS and ALK mutations in patients with lung adenocarcinoma in Croatia. Diagn Pathol 2016;11:90. [Crossref] [PubMed]
- Zheng D, Wang R, Zhang Y, Pan Y, Cheng X, Cheng C, Zheng S, Li H, Gong R, Li Y, Shen X, Sun Y, Chen H. The prevalence and prognostic significance of KRAS mutation subtypes in lung adenocarcinomas from Chinese populations. Onco Targets Ther 2016;9:833-43. [Crossref] [PubMed]
- Lu S, Guo A, Hu H, Ying X, Li Y, Huang Z, Xu W, Tao S, Hu X, Yan N, Zhang X, Shen D, Sasaki T, Arulananda S, Onodera K, He Z. Correlation analysis between driver gene mutation and clinicopathological features in lung adenocarcinoma based on real-world cumulative clinical data. Transl Lung Cancer Res 2024;13:1296-306. [Crossref] [PubMed]
- Ma Z, Zhang Y, Deng C, Fu F, Deng L, Li Y, Chen H. The prognostic value of Kirsten rat sarcoma viral oncogene homolog mutations in resected lung adenocarcinoma differs according to clinical features. J Thorac Cardiovasc Surg 2022;163:e73-85. [Crossref] [PubMed]
- Kaseda K, Asakura K, Kazama A, Ozawa Y. Clinicopathological and prognostic features of surgically resected pathological stage I lung adenocarcinoma harboring epidermal growth factor receptor and K-ras mutation. Thorac Cancer 2017;8:229-37. [Crossref] [PubMed]
- Takamochi K, Oh S, Suzuki K. Differences in EGFR and KRAS mutation spectra in lung adenocarcinoma of never and heavy smokers. Oncol Lett 2013;6:1207-12. [Crossref] [PubMed]
- Kim HR, Ahn JR, Lee JG, Bang DH, Ha SJ, Hong YK, Kim SM, Nam KC, Rha SY, Soo RA, Riely GJ, Kim JH, Cho BC. The impact of cigarette smoking on the frequency of and qualitative differences in KRAS mutations in Korean patients with lung adenocarcinoma. Yonsei Med J 2013;54:865-74. [Crossref] [PubMed]
- Mao C, Qiu LX, Liao RY, Du FB, Ding H, Yang WC, Li J, Chen Q. KRAS mutations and resistance to EGFR-TKIs treatment in patients with non-small cell lung cancer: a meta-analysis of 22 studies. Lung Cancer 2010;69:272-8. [Crossref] [PubMed]
- Porta M, Crous-Bou M, Wark PA, Vineis P, Real FX, Malats N, Kampman E. Cigarette smoking and K-ras mutations in pancreas, lung and colorectal adenocarcinomas: etiopathogenic similarities, differences and paradoxes. Mutat Res 2009;682:83-93. [Crossref] [PubMed]
- Yang Y, Gao Y, Lu F, Wang E, Liu H. Correlation of CT features of lung adenocarcinoma with sex and age. Sci Rep 2024;14:13414. [Crossref] [PubMed]
- Ahrendt SA, Decker PA, Alawi EA, Zhu Yr YR, Sanchez-Cespedes M, Yang SC, Haasler GB, Kajdacsy-Balla A, Demeure MJ, Sidransky D. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer 2001;92:1525-30. [Crossref] [PubMed]
- Jayakumar N, Chaiton M, Zhang B, Selby P, Schwartz R. Sex Differences in Use of Smoking Cessation Services and Resources: A Real-World Study. Tob Use Insights 2020;13:1179173X20901500.
- Kopra J, Härkänen T, Tolonen H, Jousilahti P, Kuulasmaa K, Reinikainen J, Karvanen J. Adjusting for selective non-participation with re-contact data in the FINRISK 2012 survey. Scand J Public Health 2018;46:758-66. [Crossref] [PubMed]
- Xavier MO, Del-Ponte B, Santos IS. Epidemiology of smoking in the rural area of a medium-sized city in Southern Brazil. Rev Saude Publica 2018;52:10s. [Crossref] [PubMed]
- Laux TS, Bert PJ, González M, Unruh M, Aragon A, Lacourt CT. Prevalence of obesity, tobacco use, and alcohol consumption by socioeconomic status among six communities in Nicaragua. Rev Panam Salud Publica 2012;32:217-25. [Crossref] [PubMed]
- Clair C, De Kleijn MJ, Jaunin-Stalder N, Cornuz J. Gender and disparities: the example of tobacco smoking. Rev Med Suisse 2015;11:1298-303.
- Khattab A, Javaid A, Iraqi G, Alzaabi A, Ben Kheder A, Koniski ML, Shahrour N, Taright S, Idrees M, Polatli M, Rashid N, El Hasnaoui ABREATHE Study Group. Smoking habits in the Middle East and North Africa: results of the BREATHE study. Respir Med 2012;106:S16-24. [Crossref] [PubMed]
- Yu Y, Liu H, Zheng S, Ding Z, Chen Z, Jin W, Wang L, Wang Z, Fei Y, Zhang S, Ying K, Zhang R. Gender susceptibility for cigarette smoking-attributable lung cancer: a systematic review and meta-analysis. Lung Cancer 2014;85:351-60. [Crossref] [PubMed]
- Sugano M, Shimizu K, Nakano T, Kakegawa S, Miyamae Y, Kaira K, Araki T, Kamiyoshihara M, Kawashima O, Takeyoshi I. Correlation between computed tomography findings and epidermal growth factor receptor and KRAS gene mutations in patients with pulmonary adenocarcinoma. Oncol Rep 2011;26:1205-11. [Crossref] [PubMed]
- Rizzo S, Petrella F, Buscarino V, De Maria F, Raimondi S, Barberis M, Fumagalli C, Spitaleri G, Rampinelli C, De Marinis F, Spaggiari L, Bellomi M. CT Radiogenomic Characterization of EGFR, K-RAS, and ALK Mutations in Non-Small Cell Lung Cancer. Eur Radiol 2016;26:32-42. [Crossref] [PubMed]
- Glynn C, Zakowski MF, Ginsberg MS. Are there imaging characteristics associated with epidermal growth factor receptor and KRAS mutations in patients with adenocarcinoma of the lung with bronchioloalveolar features? J Thorac Oncol 2010;5:344-8. [Crossref] [PubMed]
- Wang H, Schabath MB, Liu Y, Stringfield O, Balagurunathan Y, Heine JJ, Eschrich SA, Ye Z, Gillies RJ. Association Between Computed Tomographic Features and Kirsten Rat Sarcoma Viral Oncogene Mutations in Patients With Stage I Lung Adenocarcinoma and Their Prognostic Value. Clin Lung Cancer 2016;17:271-8. [Crossref] [PubMed]
- Wang T, Zhang T, Han X, Liu XI, Zhou N, Liu Y. Impact of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification of stage IA adenocarcinoma of the lung: Correlation between computed tomography images and EGFR and KRAS gene mutations. Exp Ther Med 2015;9:2095-103. [Crossref] [PubMed]
- Park J, Kobayashi Y, Urayama KY, Yamaura H, Yatabe Y, Hida T. Imaging Characteristics of Driver Mutations in EGFR, KRAS, and ALK among Treatment-Naïve Patients with Advanced Lung Adenocarcinoma. PLoS One 2016;11:e0161081. [Crossref] [PubMed]
- Takashima S, Maruyama Y, Hasegawa M, Yamanda T, Honda T, Kadoya M, Sone S. CT findings and progression of small peripheral lung neoplasms having a replacement growth pattern. AJR Am J Roentgenol 2003;180:817-26. [Crossref] [PubMed]
- Zhang Z, Zhou L, Min X, Li H, Qi Q, Sun C, Sun K, Yang F, Li X. Long-term follow-up of persistent pulmonary subsolid nodules: Natural course of pure, heterogeneous, and real part-solid ground-glass nodules. Thorac Cancer 2023;14:1059-70. [Crossref] [PubMed]
- Chang B, Hwang JH, Choi YH, Chung MP, Kim H, Kwon OJ, Lee HY, Lee KS, Shim YM, Han J, Um SW. Natural history of pure ground-glass opacity lung nodules detected by low-dose CT scan. Chest 2013;143:172-8. [Crossref] [PubMed]
- Kim BG, Um SW. A narrative review of the clinical approach to subsolid pulmonary nodules. Ann Transl Med 2023;11:217. [Crossref] [PubMed]


