
Bibliometric analysis of the application of artificial intelligence in orthopedic imaging
Introduction
Imaging results have become an indispensable part of clinical diagnosis and treatment for orthopedic diseases, especially for complex bone diseases, bone and joint injuries, and tumors. Currently, orthopedic and radiology physicians mainly make clinical diagnoses through manual interpretation of image results (1,2). Although doctors follow certain diagnostic principles, subjective analysis in imaging interpretation often introduces cognitive biases. This explains why even experienced physicians may have differing interpretations of the same imaging results, which can seriously impact the accuracy of orthopedic disease diagnosis (3,4). The clinical diagnostic process in orthopedic imaging has not yet been fully standardized, and differences in imaging protocols, patient populations, and clinical endpoints contribute to variations in interpretation. Therefore, finding methods to improve diagnostic efficiency and consistency remains a pressing issue.
Artificial intelligence (AI) is one of the most promising and emerging technologies, encompassing the study, development, and manufacture of systems that simulate, extend, enhance, and even surpass human intelligence, cognition, and abilities through theories, methods, techniques, applications, and self-learning evolution (5). In recent years, with the development of information technology, AI’s capabilities in data processing and its applications in the intersection of medicine and engineering have significantly improved. AI technologies, such as convolutional neural networks (CNNs) for image segmentation, recognition, data analysis, prediction, and prognosis of orthopedic imaging, have shown abilities comparable to or exceeding those of humans, offering vast potential for applications in orthopedic imaging (6-8). Given the diversity and complexity of orthopedic diseases, the use of magnetic resonance imaging (MRI), computed tomography (CT), X-ray, and ultrasound in diagnosis, treatment, and prognosis is crucial. AI’s application in orthopedic imaging can integrate past clinical experiences and a vast amount of knowledge to guide physicians in making more accurate diagnostic and therapeutic decisions, thus making the diagnosis and treatment of orthopedic diseases more efficient, standardized, and automated (9-12).
As the research on AI applications in orthopedic imaging increases, the number of publications in this field has grown explosively, and many researchers have encountered difficulties in keeping up with the latest research trends. To date, only a few reviews and meta-analyses have summarized the research on AI in orthopedic imaging, mostly focusing on a single disease overview (13,14). The overall research direction of AI in the field of orthopedic imaging, including the contributions of authors and institutions, and future research frontiers and hotspots, has not been analyzed (15). By analyzing database content and characteristics of the literature, bibliometric analysis utilizes mathematical and statistical knowledge to evaluate the development trends of related fields. It aims to predict the hotspots and key directions of future research and play a significant role in the formulation of clinical guidelines, disease treatment decisions, and related therapies. This analytical method has been widely used in the medical field (16-18).
Therefore, in order to further understand the current status of AI in orthopedic imaging and identify research hotspots, we conducted a bibliometric analysis using VOSviewer (1.6.19; Leiden University’s Centre for Science and Technology Studies, Leiden, Netherlands) and CiteSpace (6.3.R1; Drexel University, Philadelphia, PA, USA) software on the research published from 2007 to 2024 in the Web of Science (WoS) database. We aimed to elucidate the research trends of AI technology in orthopedic imaging-related fields, providing a reference for future research by relevant scholars.
Methods
Data sources and search strategy
The WoS database is one of the most comprehensive and rich interdisciplinary databases, collecting a wide range of academic journals and literature. It has been widely used as a data source for bibliometric research. The search date for this study was 14 January 2025. The search keywords included “Artificial Intelligence”, “AI”, “Machine Learning”, “Deep Learning”, “radiograph” and “Orthopedic”, connected by Boolean operators “AND” and “OR” to optimize the search scope and relevance of the results. The specific search string was:
TS=((“artificial intelligence” OR “robotic surgery” OR “feature engineering” OR “machine learning” OR “unsupervised learning” OR “semantic segmentation” OR “supervised learning” OR “deep learning” OR “neural network*” OR “artificial neural network” OR “knowledge discovery” OR “knowledge graph”) (19) AND (“radiograph” OR “CT” OR “MRI” OR “ultrasound” OR “positron emission tomography” OR “PET-CT” OR “dual-energy X-ray absorptiometry” OR “DXA” OR “bone densitometry” OR “bone density scan” OR “fluoroscopy” OR “radiology”) (20) AND (“Bone*” OR “Orthopedic” OR “Orthopaedic” OR “Spine” OR “Ribs” OR “Bone Cortex” OR “Trabeculae” OR “Bone Marrow” OR “Joints” OR “Cartilage” OR “Meniscus” OR “Bone Neoplasms”) NOT (“lung” OR “pulmonary” OR “thoracic cavity” OR “chest x-ray” OR “chest radiograph” OR “pneumonia” OR “pulmonary disease” OR “emphysema” OR “brain tumor” OR “cranial” OR “neurosurgery” OR “dental” OR “oral surgery” OR “jaw” OR “maxillofacial” OR “mandible” OR “maxilla” OR “welded joints” OR “reinforced concrete” OR “mechanical engineering” OR “mechanical property” OR “metallurgy” OR “leukemia” OR “lymphoma”)).
The language of the literature was limited to English, and the document types were limited to “article” and “review”. The time range was set from 1 January 2007 to 31 December 2024, to cover the critical period of rapid development in the field of AI applications in orthopedic imaging. After the initial literature search, the full records were derived, including information such as country/region, institution, journal, author, keywords, abstract, year, and other details. These records were then imported into CiteSpace software for merging, deduplication, and manual screening. This process was independently completed by two graduate students, Xiao Huang and Fei Han. Any discrepancies were resolved through discussion and consultation with senior researchers with associate professor titles or higher to ensure the authenticity and reliability of the results. Ultimately, a total of 3,147 documents were exported as full records in plain text format and stored as download_.txt. The collection process is shown in Figure 1.
Statistical analysis
CiteSpace (6.3.R1) was used for bibliometric and visualization analysis. This software leverages its robust data processing capabilities, intuitive network construction, and analysis functions to visually display research trends, key authors, keyword co-occurrence, and other information in specific fields. In CiteSpace, the time range was set from January 2007 to December 2024, and time slices were set to one year to examine the annual publication volume and changes in keyword information over time. The node types selected included authors, institutions, countries, and keywords, with the default clustering algorithm (such as the Louvain algorithm) applied for data processing. CiteSpace was used to construct co-citation networks, co-occurrence networks, and collaboration networks. In addition, VOSviewer (Version 1.6.19) was used to visualize the collaboration and citation relationships of countries, institutions, authors, and journals, which complement the CiteSpace analysis results.
Results
Analysis of publication trends
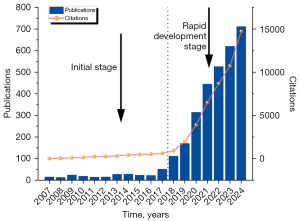
Among the final 3,147 documents, 240 (7.6%) were reviews, and 2,907 (92.4%) were articles. The overall annual publication volume showed an upward trend, although the growth was slow before 2018. This indicates that the period from 2007 to 2017 was the initial stage of research in this field, with annual publication volumes increasing slightly but consistently remaining below 100 articles per year. After 2017, the publication volume grew rapidly, reaching 711 articles in 2024, approximately 7 times the volume in 2018. Over the past seven years, the annual publication volume accounted for about 92.1% of the total volume (2,897/3,147) (Figure 2). Statistical analysis of the publication volume from 2017 to 2024 showed a high correlation between the year of publication and the number of articles, with an R2 value of 0.9898, indicating that the volume of literature in this field is likely to continue increasing in the future.
Analysis of publications by country
A total of 84 countries or regions participated in the research, with significant differences in publication volume among them. Table 1 summarizes the top 10 countries and regions in terms of publication volume over the past 18 years. China was the country with the highest publication volume (908 articles), far ahead of the United States (US), which ranked second with 790 articles. These two countries were the only ones with publication volumes exceeding 500, accounting for 28.85% and 25.10% of the total, respectively. Germany ranked third with 299 articles. However, the US ranked first in terms of centrality, total link strength (TLS), and average citations per paper, indicating its critical role in AI research in orthopedic imaging. China, although leading in publication volume (28.85%), also showed competitive performance in TLS (=279) and centrality (0.12), indicating its growing role in international collaboration and impact, though not yet dominant across all metrics. Germany and the United Kingdom (UK) demonstrated notable influence, particularly in centrality and TLS, which reflected strong global connections and consistent contributions.
Table 1
| Rank | Countries/regions | Percentage (%) | Counts | Centrality | Total citation | Average citation per paper | TLS |
|---|---|---|---|---|---|---|---|
| 1 | China | 28.85 | 908 | 0.12 | 10,213 | 11.25 | 279 |
| 2 | USA | 25.10 | 790 | 0.28 | 20,621 | 26.10 | 567 |
| 3 | Germany | 9.50 | 299 | 0.09 | 5,531 | 18.50 | 339 |
| 4 | South Korea | 7.12 | 224 | 0.04 | 2,914 | 13.01 | 60 |
| 5 | Canada | 6.20 | 195 | 0.12 | 3,579 | 18.35 | 196 |
| 6 | United Kingdom | 5.82 | 183 | 0.16 | 3,931 | 21.48 | 214 |
| 7 | Japan | 5.15 | 162 | 0.02 | 1,929 | 11.91 | 45 |
| 8 | France | 4.99 | 157 | 0.22 | 3,359 | 21.39 | 174 |
| 9 | Switzerland | 4.96 | 156 | 0.15 | 3,836 | 24.59 | 192 |
| 10 | Italy | 4.61 | 145 | 0.12 | 3,470 | 23.93 | 136 |
TLS, total link strength.
Figure 3A shows a world map indicating that publications in this field were primarily produced by countries in North America, Europe, and East Asia, forming four main clusters centered around the US, Western Europe, China, and Iran. Figure 3B, which includes countries with a total publication volume of ≥20, illustrates the international collaboration network in AI-based orthopedic imaging research, with 31 nodes. The three countries with the strongest TLS were China (TLS =279), the US (TLS =567), and Germany (TLS =339). Additionally, the US and Canada began AI research in orthopedics earlier, with an average publication year of 2020, whereas China, as a recent emerging player, has an average publication period in 2022.

Analysis of publishing institutions and funding agencies
The study revealed that a total of 3,951 institutions had participated in research on the application of AI in orthopedic imaging. Figure 4A,4B list the top 10 institutions by citation volume and publication volume. Interestingly, the top 10 institutions by publication volume included four institutions from the US, three from China, one from Germany, one from Switzerland, and one from South Korea. In contrast, the top 10 institutions by citation volume were dominated by six institutions from the US, two from the UK, and two from Germany. The institution with the highest publication volume was Harvard Medical School (76 publications), which also ranked first in TLS (=169). Stanford University led in total citations (2,550), demonstrating its strong academic influence. Shanghai Jiao Tong University and the Chinese Academy of Sciences were notable contributors from China, showcasing their growing impact in the field. Meanwhile, European institutions such as the Technical University of Munich and the University of Zurich also demonstrated strong performance in both collaboration and citation impact.
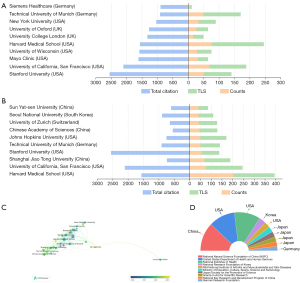
Regardless of whether it was publication volume or citation volume, the leading institutions were predominantly universities, indicating that universities are the primary hubs for AI research in orthopedic imaging. Figure 4C, which shows the co-occurrence of collaboration and publication timing among institutions, indicates that the average publication time for the top 10 institutions by publication and citation volume was around 2022, suggesting that the application of AI in orthopedic imaging has become a research hotspot in recent years.
Funding support is crucial for the importance of research. We analyzed the publication volume of funding agencies to determine the attention different countries pay to AI research in orthopedic imaging. Figure 4D summarizes the top 10 funding agencies by publication volume. The analysis revealed that three of the top six funding agencies were from the US, and three were from Japan. The National Natural Science Foundation of China (NSFC) ranked first with 322 publications, followed by the United States Department of Health and Human Services (296 publications) and the National Institutes of Health (NIH) USA (294 publications). This indicates that the high publication volume and prominence of China, the US, and Japan in this field are closely related to their strong economic support.
Analysis of journal publication volume and citation volume
The 3,147 documents were published across 600 journals, with 69 journals publishing 10 or more articles. Tables 2,3 list the top 10 journals by publication volume and citation volume, respectively. The top three journals by publication volume were Scientific Reports (152 articles), Diagnostics (69 articles), and Computer Methods and Programs in Biomedicine (61 articles). The top three journals by citation volume were Scientific Reports (2,345 citations), Medical Image Analysis (2,232 citations), and Radiology (2,213 citations).
Table 2
| Rank | Source | Counts | Citations | TLS | IF | JCR |
|---|---|---|---|---|---|---|
| 1 | Scientific Reports | 152 | 2,345 | 616 | 3.8 | Q1 |
| 2 | Diagnostics | 69 | 629 | 345 | 3 | Q1 |
| 3 | Computer Methods and Programs in Biomedicine | 61 | 1,103 | 256 | 4.9 | Q1 |
| 4 | European Radiology | 60 | 1,081 | 446 | 4.7 | Q1 |
| 5 | International Journal of Computer-Assisted Radiology and Surgery | 58 | 784 | 182 | 2.3 | Q2 |
| 6 | Medical Physics | 57 | 1,303 | 160 | 3.2 | Q1 |
| 7 | Plos One | 57 | 912 | 163 | 2.9 | Q1 |
| 8 | Computerized Medical Imaging and Graphics | 56 | 857 | 238 | 5.4 | Q1 |
| 9 | Skeletal Radiology | 56 | 617 | 414 | 1.9 | Q3 |
| 10 | Computers in Biology and Medicine | 49 | 983 | 250 | 7.0 | Q1 |
IF, impact factor; JCR, journal citation reports; TLS, total link strength.
Table 3
| Rank | Source | Citations | Counts | Average | TLS | IF | JCR |
|---|---|---|---|---|---|---|---|
| 1 | Scientific Reports | 2,345 | 152 | 15.43 | 616 | 3.8 | Q1 |
| 2 | Medical Image Analysis | 2,232 | 47 | 47.49 | 445 | 10.7 | Q1 |
| 3 | Radiology | 2,213 | 38 | 58.24 | 615 | 12.1 | Q1 |
| 4 | IEEE Transactions on Medical Imaging | 1,869 | 39 | 47.92 | 189 | 8.9 | Q1 |
| 5 | Magnetic Resonance in Medicine | 1,662 | 33 | 50.36 | 346 | 2.5 | Q2 |
| 6 | Medical Physics | 1,303 | 57 | 22.86 | 160 | 3.2 | Q1 |
| 7 | Neuroimage | 1,157 | 8 | 144.63 | 7 | 4.7 | Q1 |
| 8 | Computer Methods and Programs in Biomedicine | 1,103 | 61 | 18.08 | 256 | 4.9 | Q1 |
| 9 | European Radiology | 1,081 | 60 | 18.02 | 446 | 4.7 | Q1 |
| 10 | Computers in Biology and Medicine | 983 | 49 | 20.06 | 250 | 7 | Q1 |
IF, impact factor; JCR, journal citation reports; TLS, total link strength.
It is noteworthy that journals with the highest publication volumes did not necessarily have the highest citation volumes or impact factors (IFs). For instance, Scientific Reports, which ranked first in both publication volume (152 articles) and citation volume (2,345 citations), had a moderate (IF of 3.8). In contrast, Radiology, which ranked third in citation volume (2,213 citations), had the highest IF of 12.1 among the top-ranked journals. Similarly, Medical Image Analysis, with an impressive IF of 10.7, ranked second in citation volume despite having only 47 publications.
Among the top 10 journals by publication volume, six were classified as Q1 journals, three as Q2, and one as Q3. Meanwhile, 9 out of the top 10 journals by citation volume were Q1 journals, with Magnetic Resonance in Medicine being the only Q2 journal. Notable overlaps included Medical Physics and Computer Methods and Programs in Biomedicine, which consistently appeared in both rankings. This suggests that research in AI-based orthopedic imaging is not only widely disseminated but also achieves significant academic impact, especially through selective high-impact journals.
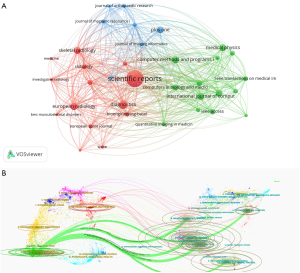
As shown in Figure 5A, the top three journals by TLS were Scientific Reports, Radiology (third in citation volume), and European Radiology. Figure 5B presents a dual-map overlay of journal disciplines in AI research in orthopedic imaging, clearly demonstrating the knowledge flow between different disciplines and the research frontiers or hotspots. On the left are the citing journals, whereas on the right are the cited journals (21). The analysis results reveal that articles published in medicine medical and clinical-related journals frequently cite articles from molecular, computing, computer, biology, nursing, health, medicine, and sports-related journals. Conversely, articles published in molecular biology and immunology-related journals often cite literature from molecular biology, genetics, and clinical medicine-related journals. This shows that AI research in orthopedic imaging spans multiple disciplines, which are research hotspots in this field.
Author analysis
A total of 16,422 authors had participated in research on the application of AI in orthopedic imaging. Figure 6A,6B illustrate the productivity and citation trends of the top authors in AI-based orthopedic imaging. Majumdar, Sharmila (33 publications) and Pedoia, Valentina (32 publications) were the most productive authors, ranking high in terms of both publication volume and citations. However, although Kijowski, Richard (16 publications) and Liu, Fang (12 publications) did not have the highest publication volume, their citation counts were among the top in the field, highlighting the significant impact of their work. Notably, Sconfienza, Luca Maria had a higher TLS (=49), suggesting a stronger influence and wider collaboration in the field.
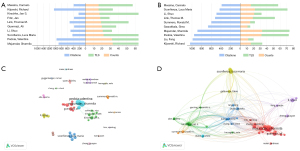
Regarding citation volume, Kijowski, Richard ranked first with 1,039 citations despite publishing only 16 papers, demonstrating his substantial influence in the field. Although his TLS was lower at 17 compared to other authors, this does not imply weak influence within academic networks. Instead, his high citation volume reflects the significant impact of his research, even though his academic collaborations may be more limited.
In summary, authors with higher TLS values (such as Luca Maria Sconfienza) tended to have stronger influence within academic collaboration networks, whereas highly productive authors such as Majumdar, Sharmila and Pedoia, Valentina also demonstrated considerable academic impact, with a TLS of 41, showing their important role in academic collaborations. This suggests that, although highly productive authors may have wider networks, TLS serves as a key indicator of academic collaboration and influence in the field. Figure 6C displays three major collaborative groups centered around Majumdar, Sharmila, Pedoia, Valentina, and Guermazi, Ali, who are key authors connecting different research clusters, demonstrating their significant roles in this field. This aligns with the ranking of co-cited authors.
Figure 6D shows the collaboration network of authors with more than 10 publications, revealing limited collaboration among them. This further suggests that researchers in this field need to deepen their cooperation. The author collaboration network reveals distinct clusters of researchers contributing to the field of AI in orthopedic imaging. Notably, Sharmila Majumdar and Ali Guermazi are central figures in the network, forming a significant cluster with other closely related researchers. Additionally, Luca Maria Sconfienza and Fabio Galbusera represent another influential group, demonstrating robust collaborative ties within their respective research domains. This visualization highlights the interconnected nature of leading researchers, showcasing both the diversity and concentration of expertise in this area.
Reference and co-cited reference analysis
Table 4 summarizes the top 10 most co-cited documents. According to the citation analysis, the top three most cited papers explored the application of deep CNNs in medical imaging. Krizhevsky et al. introduced the ImageNet classification method, which laid the foundation for medical image classification tasks and became a key reference in the field. Ronneberger et al. proposed the U-Net architecture, focusing on medical image segmentation, and is widely used in various medical image processing tasks. Litjens et al. [2017] provided a comprehensive review of DL applications in medical image analysis, offering a theoretical framework and technical guidelines for subsequent research (22-24).
Table 4
| Rank | Title | First authors | Journal | Publication year | Total citations |
|---|---|---|---|---|---|
| 1 | ImageNet classification with deep convolutional neural networks | Alex Krizhevsky | Communications of the ACM | 2017 | 142 |
| 2 | U-Net: Convolutional Networks for Biomedical Image Segmentation | Olaf Ronneberger | Medical Image Computing and Computer-Assisted Intervention | 2015 | 132 |
| 3 | A survey on deep learning in medical image analysis | Geert Litjens | Medical Image Analysis | 2017 | 114 |
| 4 | nnU-Net: A self-configuring method for deep learning-based biomedical image segmentation | Fabian Isensee | Nature Method | 2021 | 107 |
| 5 | Deep Residual Learning for Image Recognition | Kaiming He | 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR) | 2016 | 95 |
| 6 | Deep-learning-assisted diagnosis for knee magnetic resonance imaging: Development and retrospective validation of MRNet | Nicholas Bien | PloS Medicine | 2018 | 85 |
| 7 | Automated segmentation of knee bone and cartilage combining statistical shape knowledge and convolutional neural networks: Data from the Osteoarthritis Initiative | Felix Ambellan | Medical Image Analysis | 2019 | 80 |
| 8 | Deep convolutional neural network and 3D deformable approach for tissue segmentation in musculoskeletal magnetic resonance imaging | Fang Liu | Magnetic Resonance in Medicine | 2018 | 77 |
| 9 | Densely Connected Convolutional Networks | Gao Huang | 2017 IEEE Conference on Computer Vision and Pattern Recognition | 2017 | 77 |
| 10 | Use of 2D U-Net Convolutional Neural Networks for Automated Cartilage and Meniscus Segmentation of Knee MR Imaging Data to Determine Relaxometry and Morphometry | Berk Norman | Radiology | 2018 | 75 |
Figure 7A presents the timeline of co-cited references in AI-based orthopedic imaging research, showing good clustering effect and network homogeneity with a Q value of 0.8451 and an S value of 0.9045. Based on the reference clustering analysis, AI in orthopedic imaging research can be divided into three main categories: First, automation and DL applications, including automatic segmentation (#0), DL reconstruction (#5), and segmentation methods (#3), focusing on image segmentation and reconstruction techniques. Second, orthopedic diseases and pathology, including research on knee osteoarthritis (#1) and opportunistic osteoporosis (#4). Lastly, specific anatomical structure studies, such as human patellar cartilage (#9). These clusters highlight the broad application of DL technologies and their significance in disease diagnosis within the field of orthopedic imaging.

Figure 7B summarizes the top 20 references with the strongest citation bursts. Based on the citation burst analysis, the most significant reference bursts began in 2017, with Ronneberger’s 2015 paper on U-Net for biomedical image segmentation being the most influential in terms of burst strength (25). This paper’s citation burst has remained the strongest, continuing into 2020, highlighting its sustained impact on AI-based orthopedic imaging (26). This suggests that the future research hotspot in AI-based orthopedic imaging will focus on further optimizing computer algorithms for image segmentation and classification in orthopedic imaging.
Keyword co-occurrence and overlay analysis
The 3,147 articles contain a total of 6,005 keywords, with 49 keywords appearing 25 times or more. Figure 8A summarizes the top 20 most frequent keywords, with “deep learning”, “artificial intelligence”, and “machine learning” being the most common. This perfectly aligns with our research theme and previous findings, indicating the current research hotspots and frontiers in AI-based orthopedic imaging. Additionally, MRI was the most frequently studied imaging modality, ranking high among the keywords. Analyzing these and other keywords suggests that the segmentation and classification of MRI images are significant research hotspots in this field.
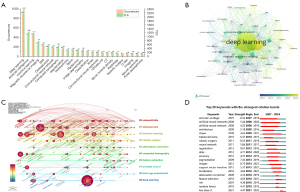
Figure 8B displays the occurrence time and frequency of different keywords, where the closer to yellow, the more recent the keyword, and the larger the circle radius, the higher the frequency (27). Notably, “deep learning” is at the center of the entire network. Keywords such as “magnetic resonance imaging”, “convolutional neural network”, and “artificial intelligence” have emerged since 2021, indicating that these directions are current research advancements and likely to remain long-term research hotspots.
Figure 8C shows the timeline of keyword clusters, forming 10 clusters. Themes such as 0 machine learning, 1 osteoporosis, 2 osteoarthritis, and 3 DL have remained prominent from 2007 to the present. Based on the keyword clustering analysis, AI-based orthopedic imaging research can be divided into three main areas. First, AI and machine learning technologies (such as machine learning, DL, and AI) have become central to research, driving the development and optimization of algorithms in image analysis. Second, orthopedic diseases and treatments, particularly osteoporosis, osteoarthritis, and total knee arthroplasty (TKA), highlight the importance of AI in disease diagnosis and treatment, especially in personalized treatment planning. Finally, medical imaging applications, such as robotic surgery and body composition analysis, demonstrate the potential of AI in enhancing image analysis accuracy and assisting in surgeries. These research trends indicate that AI is profoundly transforming diagnostic and treatment approaches in orthopedics, providing vast prospects for future development.
Figure 8D presents the top 20 keywords with the strongest citation bursts. Based on the citation burst analysis of the top 20 keywords from 2007 to 2024, several key trends in the application of AI in orthopedic imaging are evident. Artificial neural networks and machine learning technologies have triggered significant citation bursts starting in 2008 and 2011, driving the development of computational methods in this field. Image segmentation, as an important research direction, has been continuously emphasized since 2009, highlighting its significance in the automation of diagnostic processes in orthopedic imaging. Furthermore, robotic surgery, with a citation burst starting in 2011, indicates the growing application of AI in enhancing surgical precision. Keywords such as osteoporosis and osteoarthritis displayed sustained interest, indicating that AI applications in the diagnosis and treatment of these conditions remain a major research focus. Emerging topics such as low-dose CT and random forest reflect the ongoing integration of advanced imaging techniques and machine learning methods. This citation burst analysis reveals the dynamic evolution of AI applications in orthopedic imaging, particularly in the continuous advancements in image analysis and treatment optimization.
Discussion
With the rapid development of the internet, we have entered an era of information explosion. It is crucial to grasp the research hotspots and the latest research achievements to maintain a leading position in the research field amidst the massive and complex information influx. Different from systematic reviews and meta-analyses, bibliometric analysis, using software such as CiteSpace and VOSviewer, organizes and analyzes vast amounts of information from scientific literature. This process yields knowledge maps of specific research fields, reflecting the knowledge structure, scientific frontiers, and research hotspots, thus guiding subsequent researchers in their future studies (28,29). This study summarizes the application and development of AI in the field of orthopedic imaging through bibliometric analysis, revealing the development trends over the past 18 years and predicting future research hotspots in this field.
The attention of AI in the field of orthopedic imaging is increasing annually
On the whole, the number of scientific publications is one of the important indicators to prove whether a research field is prosperous (30). This study shows that in the 18 years from 2007 to 2024, the number of published papers based on the application of AI in orthopedic imaging has been steadily increasing, especially in the past seven years, which account for over 90% of the total publications. This trend is closely linked to the deepening research on computer algorithms. After 2018, the surge in publication volume was strongly associated with the introduction, application, and promotion of new DL models, such as deep residual networks and spatially-constrained CNNs (31-33). From this point of view, AI-based research in orthopedic imaging has become an important area in clinical practice with extremely broad application prospects.
According to the ranking of countries/regions, China had the highest number of published papers, accounting for 28.85% of the total, but its national centrality is only 0.12. In contrast, the US ranked second in publication volume, contributing 25.10% of the total, with a centrality of 0.28. This disparity suggests that although China has made significant contributions to the field, some publications may be of relatively low quality. Nevertheless, China’s crucial role in AI-based orthopedic imaging research cannot be denied. As shown in Figure 3B, China entered the research field later than the US and several Western European countries. However, in recent years, China has demonstrated considerable vitality in both publication volume and timing, establishing itself as a key player in this field now and in the future.
In terms of international collaboration, as illustrated in Figure 3A,3B, the US and China serve as the central hubs, maintaining strong research connections with countries such as Germany, the UK, Canada, and Australia. However, these collaborations are primarily concentrated among China, the US, and Western European countries, with limited contributions from other regions. Therefore, strengthening international cooperation is essential for advancing this field. Developing countries/regions, in particular, should enhance cross-border collaboration to achieve success in AI-based orthopedic imaging research.
From the perspective of institutional contributions to AI research in orthopedic imaging, the top 10 most productive institutions were predominantly from the US and China, underscoring their central roles in driving advancements in this field. Among institutions ranked by citation count, six were from the US, two from China, and two from Western Europe, highlighting the significant contributions of these regions. Notably, the top five most-cited institutions were all based in the US, further cementing its leadership in cutting-edge research.
Harvard University, with the highest number of publications, and Stanford University, ranking first in citation count despite having only 50 publications, demonstrated exceptional research quality and influence. These findings position both institutions as global leaders in AI-based orthopedic imaging research. Strengthening collaboration with these leading institutions is recommended to foster innovation and elevate the overall impact of research in this field.
Among the top 10 institutions by publication volume, four were from the US and three were from China. Notably, Harvard University, the University of California, and Stanford University were also among the top 10 most-cited institutions, underscoring the US’ leading position in AI research within orthopedic imaging. These institutions serve as benchmarks for high-quality research in the field. In contrast, although three Chinese institutions ranked among the top 10 in publication volume, their citation rankings were relatively low, potentially due to their later entry into the field (as shown in Figure 4B,4C). This suggests that although Chinese institutions contributed significantly in quantity, there is room for improvement in research quality. As illustrated in Figure 4D, one driving factor behind the large publication output from institutions in both China and the US is their substantial economic investment in this field. This finding highlights the critical role of strong financial support in enabling countries and institutions to actively participate and lead in AI-based orthopedic imaging research.
Identifying suitable journals and journal classifications provides valuable and reliable reference information for researchers. It usually helps them to understand the current landscape and choose the most appropriate target journals for publishing their research. Total citations, IF, and journal citation reports (JCR) category are commonly used to evaluate the academic standing of journals (34-36). Tables 2,3 compare the top 10 journals ranked by publication and citation counts. Most of these journals are multidisciplinary, with IFs ranging from 1.9 to 7.0 for the top 10 cited journals. This indicates that AI-related orthopedic imaging research is still in its early stages, with significant gaps remaining to be addressed. Scientific Reports led in publication volume, reflecting a prevalence of articles with moderate innovation. Researchers in this field are encouraged to enhance the originality of their studies to drive substantial advancements.
Analyzing the top authors and co-cited authors revealed that scholars such as Majumdar, Sharmila, Pedovia, Valentina, Sconfienza, Luca Mara, and Li, Shuo ranked among the top 10 in both publication volume and citation counts. This underscores their expertise in AI applications for orthopedic imaging and highlights opportunities for future collaboration. However, as shown in Figure 6C, collaboration between these authors remains limited, with only a few exhibiting extensive cooperative networks. Strengthening interdisciplinary and cross-team collaboration is essential to accelerate progress in this field. Furthermore, Figure 6C identifies Majumdar, Sharmila, Pedovia, Valentina, and Guermazi, Ali as key connecting nodes among co-cited authors, emphasizing their influential roles. Notably, the Pedovia, Valentina team has made substantial contributions to joint and musculoskeletal tissue segmentation research, establishing foundational methodologies for orthopedic imaging AI (37,38). Majumdar’s work has expanded the application scope of AI in disease imaging, demonstrating its potential in broader clinical contexts (39-41). These findings suggest that these teams are likely to produce groundbreaking research in the future, making collaboration with them a strategic priority for researchers seeking impactful outcomes in AI-based orthopedic imaging.
Through the analysis of citations and co-citations of literature on AI in orthopedic imaging, we can identify important literature in this field, evaluate the evolution of related research, analyze current research frontiers, and predict future research hotspots. This is one of the critical methods of bibliometric research (42,43). Table 3 lists the top 10 most cited literature, with the top four papers all cited over 100 times, indicating the significant global importance of AI in orthopedic imaging research. The most cited document is a guideline about using micro-CT to scan bone microstructure published in the Journal of Bone and Mineral Research by Bouxsein, Mary in 2010. This guideline specifies the requirements for image acquisition and processing to assess trabecular and cortical bone morphology, setting a standard for the application of AI in imaging (21). In 2013, Shotton, Jamie published an article in Communications of the ACM, which has an IF of 22.7. Specifically, his team developed a method for predicting human postures using a single image in this study, advancing the development of AI systems for predicting and reconstructing 3D structures from images (23). From this perspective, the application of AI in the field of orthopedic imaging has broad prospects, and more research teams will enter this field.
The application of AI in orthopedic imaging research has shown a rapidly growing trend. Studies utilizing CNNs and multimodal fusion models are predominantly focused on the diagnosis of orthopedic conditions, including the detection of spinal deformities (44), the development and validation of arthritis diagnostic models (45), bone age assessment (46), surgical outcome prediction and evaluation (47), as well as cancer prognosis prediction (48). For instance, Ma et al. developed a method combining 3D orthopedic data with CNNs to assess balance during walking, aiming to prevent fall-related risks and the exacerbation of related diseases. This resulted in a quick and accurate gait assessment method (49). Additionally, Hao and collaborators developed a hypergraph neural network (Hyper-GNN) specifically tailored for orthopedic motion recognition. By extracting both local and global structural information, this network overcame the interference from irrelevant joints, achieving more accurate performance compared to existing AI systems (50). Evidently, the current and future applications of AI in orthopedic imaging hold promising potential.
The hot topic of AI application in the orthopedic imaging field
The burst detection function in CiteSpace can identify the sudden increase in the popularity of cited references or keywords during specific periods, which is an effective method for recognizing and analyzing research themes or hot topics (51,52). Through clustering analysis, we identified 10 major categories of co-cited literature, as shown in Figure 7A. These categories primarily include image segmentation, disease-specific applications (such as knee osteoarthritis, osteoporosis, bone tumors, and spinal stenosis), and algorithm development. This indicates that AI research in orthopedic imaging is largely focused on advancing image segmentation, developing applications for specific diseases, and upgrading algorithms. However, many orthopedic diseases remain underexplored, presenting opportunities for expanding AI applications. Additionally, algorithm optimization and image recognition remain foundational tasks in this field. Future researchers could enhance algorithm and segmentation performance by extending AI applications to other orthopedic diseases or collaborating with software developers, thereby accelerating AI integration into orthopedic imaging. As shown in Figure 7B, the first citation burst occurred in 2017, driven by Krizhevsky’s development of a deep CNN for high-resolution image classification and the introduction of the “dropout” regularization method. These innovations significantly improved algorithmic performance and sparked a surge in AI research in orthopedic imaging (23). This underscores the pivotal role of algorithmic evolution in advancing AI applications in this domain. Continued investment in algorithm research is essential for fostering transformative progress in this field (53). In recent years, new highlights have emerged, with CNNs becoming a research focal point. For example, in 2018, Yang et al. proposed a dynamic skeleton model, spatio-temporal graph convolutional networks (GCN), which enhanced AI’s learning capabilities (54). In 2020, Sezer addressed limitations of traditional convolutional networks, such as high computational complexity and inflexible spatiotemporal receptive fields, by introducing advanced methods for action recognition using skeletal data (55). These developments have further propelled AI-based human posture recognition, a continuing research hotspot (54,55). Thus, the study of CNN algorithms based on the human skeleton for recognizing human posture is likely to be a significant future research direction.
In summary, advancing algorithm research and enhancing image segmentation performance are essential for driving progress in AI-based orthopedic imaging. Additionally, expanding AI applications to underexplored orthopedic diseases presents significant opportunities for future research and innovation.
Through keyword co-occurrence analysis, we identified key trends in current research topics, unveiled changes in the field, and highlighted major research hotspots. This widely utilized approach in bibliometric studies offers a comprehensive perspective on the knowledge structure and developmental trajectory of the domain (56,57). As illustrated in Figure 8A, “deep learning”, “artificial intelligence”, “machine learning”, and “magnetic resonance imaging” are among the most frequently occurring keywords, underscoring the focus of AI research in orthopedic imaging on algorithm development and imaging data analysis. Among imaging modalities, “MRI” and “CT” dominate, reflecting their pivotal role in orthopedic diagnostics and aligning closely with the theme of this study.
Subsequently, we conducted cluster analysis on the keywords included in the literature and found that all hot keywords can be divided into 10 major categories: machine learning, osteoporosis, osteoarthritis, DL, image segmentation, AI, total knee replacement surgery, robotic surgery, body composition, and intramedullary nailing. Further analysis revealed that AI in orthopedic imaging research can be divided into three major categories: research and development of computer bone algorithms in orthopedic imaging, application research in some diseases, and completion of robot-assisted surgery. These categories reflect that current and future hotspots in the field of orthopedic imaging are mostly concentrated in the above three areas. The timeline analysis of AI in orthopedic imaging clearly demonstrates the relationship between technological evolution and different research directions in the field. From 2007 to 2010, the research mainly focused on developing foundational algorithms such as feature extraction, classification models, and texture analysis, laying the groundwork for image segmentation and recognition. This phase primarily concentrated on algorithmic exploration with limited clinical translation. Meanwhile, studies on osteoporosis centered on measuring bone mineral density (BMD) and analyzing the microstructure of cancellous bone, providing a basis for subsequent development of disease risk assessment models.
Between 2010 and 2015, AI technologies began to transition into clinical applications. Support vector machines (SVM) and neural network techniques were widely applied to tasks such as spinal segmentation and bone age assessment. Concurrently, research on osteoarthritis leveraged MRI to perform cartilage segmentation, focusing on anatomical details such as the knee joint and anterior cruciate ligament. In the field of TKA, advancements in 3D reconstruction and automatic measurement technologies promoted the initial stages of intelligent surgical planning, signaling an expansion of AI applications from single imaging tasks to multi-task integration. The period from 2015 to 2020 marked a pivotal phase for AI technologies, with the widespread adoption of CNN significantly advancing image segmentation, reconstruction, and diagnostic techniques. For example, Sapthagirivasan et al. developed algorithms using image processing and simulation techniques to study the microstructure and mechanical stiffness of bones, which performed well in research on osteoarthritis and osteoporosis (58). In osteoporosis, quantitative computed tomography (QCT) combined with finite element analysis was employed to improve fracture risk assessment accuracy. In osteoarthritis research, the integration of synovitis, inflammatory biomarkers, and imaging techniques further refined knee joint inflammation evaluation. In TKA, the introduction of automatic segmentation techniques and DL algorithms enhanced the recognition of complex anatomical structures and improved intraoperative navigation precision.
Since 2020, research hotspots have increasingly focused on integrating emerging technologies with clinical needs. Keywords such as “3D display”, “task analysis”, and “graph convolutional networks” have emerged, indicating that recent research hotspots include 3D imaging and the development of CNN systems. Techniques such as GCN, multimodal fusion, and 3D image reconstruction have demonstrated significant advancements across multiple domains. For instance, in TKA research, the application of feature fusion and computer vision technologies has driven rapid development in intraoperative navigation systems. In osteoarthritis, keywords such as “biomarkers” and “inflammation” highlight the importance of integrating imaging analysis with clinical pathology. With the continuous development of AI and the increasing investment in preliminary research on DL algorithms, CNNs are currently an important algorithm for processing medical images and have been widely applied in the field of orthopedic imaging (59-62). Notably, the concept of multimodal fusion has gained traction, combining imaging data, biomarkers, and clinical information to optimize diagnosis and treatment strategies.
In summary, the combination of timeline and keyword cluster analyses reveals the research hotspots and evolutionary trends of AI technologies in different domains of orthopedic imaging. This approach avoids the subjectivity of manually defined stratification standards and provides a more comprehensive and objective panorama of the field. Furthermore, the progression of these technologies underscores the need for future research to optimize algorithm performance (e.g., GCN and multimodal fusion models), validate their applications in clinical scenarios, and extend AI utilization to a broader range of orthopedic diseases, thereby promoting intelligent and precise advancements in the field.
However, the skepticism of clinicians and radiologists towards AI-generated results hinders the widespread clinical application of AI models (63-65). Therefore, improving the accuracy of DL algorithms is a research direction for the development of AI based on DL in clinical environments. In addition, the era of diagnosing orthopedic diseases solely through a single examination method is over. Many studies have shown that multimodal fusion methods that integrate imaging examinations, clinical history, lifestyle habits, and patient basic information provide more accurate diagnosis, prognosis evaluation, and treatment strategy selection for orthopedic diseases. Therefore, the development and research of multimodal fusion models may also be one of the hotspots in AI application research in the field of orthopedic imaging (66-68).
Despite the expansive prospects of AI in orthopedic imaging, several limitations remain in the practical development of AI-based models, as these models inherently rely on large data sets. For instance, some studies are limited by insufficient data, which may compromise AI’s accuracy. Liu et al. developed an AI model using data from 3,352 malignant tumor patients, manually delineating 14,972 bone lesions to classify benign and malignant tumors. Although the predictive model achieved area under the curve (AUC) scores exceeding 0.85, gathering more cases could further enhance its diagnostic utility (69). Additionally, multimodal integration often requires subjective indicators, which are inherently challenging to standardize, potentially leading to biases in model prediction and diagnostic capabilities (70). Lastly, different AI algorithms may produce varying predictive outcomes on the same dataset, highlighting the current lack of widely applicable AI technologies in orthopedic imaging (71,72). Therefore, although AI has shown great potential in orthopedic imaging research, combined with the summary and analysis of this study, future research may need to continue to focus on the development of more high-performance and applicable algorithms, and expand the application of AI in other diseases in the field of imaging, with the aim of enhancing the accuracy and excellence of clinical operations.
Limitations
We must acknowledge several limitations in this study. First, we selected only the Web of Science Core Collection (WoSCC) as the data source, which may lead to incomplete coverage of literature in the related research fields (73,74). The main reason for choosing WoS as the data source was its high compatibility with the bibliometric analysis tools used in this study, which supports efficient data processing and analysis (75,76). Although other databases, such as PubMed, contain a large number of medical-related articles, the articles retrieved from PubMed largely overlap with those in WoS. Moreover, the articles not included in WoS are often of lower quality or exhibit bias, and excluding these articles helped to improve the reliability of the study. Additionally, non-English literature is relatively sparse in the field of orthopedic imaging, so we opted to include only English-language articles to ensure consistency and comparability in the analysis. The reasons for selecting WoS were explained in detail in the methods section. Second, due to the limitations of bibliometric analysis software, merging literature from different databases is challenging, adding complexity to the process of screening articles from multiple sources. Finally, as databases are continuously updated, the latest high-quality studies may not have been included in this study (77,78). However, given that we performed a thorough screening of WoS literature, and considering that WoS tends to offer more stable, high-quality publications, we believe that this choice does not significantly affect the conclusions of the study. Nonetheless, we recognize that future work could further enhance the study by integrating more data across multiple databases.
Conclusions
This study included 3,147 documents published between 2007 and 2024 for bibliometric analysis, using CiteSpace and VOSviewer as the main analysis tools, providing a comprehensive analysis of AI applications in orthopedic imaging research. The study found that AI has been widely applied and researched in orthopedic imaging and it is still a hot research area that is rapidly developing. This suggests that AI research in orthopedic imaging will continue to increase in the future. At present, the US dominates in this field, but China is an emerging power. Research institutions, countries, and teams should strengthen cooperation, especially international cooperation, which is crucial for new research teams. In addition, developing DL algorithms (especially CNNs), expanding the application of AI in processing image data related to orthopedic diseases (segmentation, classification, and feature extraction), and applying AI in surgery are likely to be key research hotspots in the future application of AI in orthopedic imaging. At the same time, the development of AI in the field of orthopedic imaging also faces challenges such as verifying the accuracy of results, compatibility, and security of data from different institutions. However, with the continuous improvement of algorithm performance and the expansion of application scope, AI will show greater application prospects for orthopedic imaging management.
Acknowledgments
None.
Footnote
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1384/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jackowski JR, Wellings EP, Cancio-Bello A, Nieboer MJ, Barlow JD, Hidden KA, Yuan BJ. Computed tomography provides effective detection of traumatic arthrotomy of the elbow. J Shoulder Elbow Surg 2023;32:1280-4. [Crossref] [PubMed]
- Ghasemi A, Ahlawat S, Fayad LM. Magnetic Resonance Imaging Biomarkers of Bone and Soft Tissue Tumors. Semin Musculoskelet Radiol 2024;28:39-48. [Crossref] [PubMed]
- Hofbauer LC, Busse B, Eastell R, Ferrari S, Frost M, Müller R, Burden AM, Rivadeneira F, Napoli N, Rauner M. Bone fragility in diabetes: novel concepts and clinical implications. Lancet Diabetes Endocrinol 2022;10:207-20. [Crossref] [PubMed]
- Maksymowych WP, Lambert RG, Østergaard M, Baraliakos X. Response to: ‘Correspondence on ‘MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group’’ by Jibri et al. Ann Rheum Dis 2023;82:e122. [Crossref] [PubMed]
- Myers TG, Ramkumar PN, Ricciardi BF, Urish KL, Kipper J, Ketonis C. Artificial Intelligence and Orthopaedics: An Introduction for Clinicians. J Bone Joint Surg Am 2020;102:830-40. [Crossref] [PubMed]
- Martin BI, Bono CM. Artificial intelligence and spine: rise of the machines. Spine J 2021;21:1604-5. [Crossref] [PubMed]
- Shiraishi J, Li Q, Appelbaum D, Doi K. Computer-aided diagnosis and artificial intelligence in clinical imaging. Semin Nucl Med 2011;41:449-62. [Crossref] [PubMed]
- Bird A, Oakden-Rayner L, McMaster C, Smith LA, Zeng M, Wechalekar MD, Ray S, Proudman S, Palmer LJ. Artificial intelligence and the future of radiographic scoring in rheumatoid arthritis: a viewpoint. Arthritis Res Ther 2022;24:268. [Crossref] [PubMed]
- Zech JR, Jaramillo D, Altosaar J, Popkin CA, Wong TT. Artificial intelligence to identify fractures on pediatric and young adult upper extremity radiographs. Pediatr Radiol 2023;53:2386-97. [Crossref] [PubMed]
- Stadler CB, Lindvall M, Lundström C, Bodén A, Lindman K, Rose J, Treanor D, Blomma J, Stacke K, Pinchaud N, Hedlund M, Landgren F, Woisetschläger M, Forsberg D. Proactive Construction of an Annotated Imaging Database for Artificial Intelligence Training. J Digit Imaging 2021;34:105-15. [Crossref] [PubMed]
- Kluck DG, Makarov MR, Kanaan Y, Jo CH, Birch JG. Comparison of “Human” and Artificial Intelligence Hand-and-Wrist Skeletal Age Estimation in an Epiphysiodesis Cohort. J Bone Joint Surg Am 2023;105:202-6. [Crossref] [PubMed]
- Si L, Zhong J, Huo J, Xuan K, Zhuang Z, Hu Y, Wang Q, Zhang H, Yao W. Deep learning in knee imaging: a systematic review utilizing a Checklist for Artificial Intelligence in Medical Imaging (CLAIM). Eur Radiol 2022;32:1353-61. [Crossref] [PubMed]
- Teng Z, Li L, Xin Z, Xiang D, Huang J, Zhou H, Shi F, Zhu W, Cai J, Peng T, Chen X. A literature review of artificial intelligence (AI) for medical image segmentation: from AI and explainable AI to trustworthy AI. Quant Imaging Med Surg 2024;14:9620-52. [Crossref] [PubMed]
- Federer SJ, Jones GG. Artificial intelligence in orthopaedics: A scoping review. PLoS One 2021;16:e0260471. [Crossref] [PubMed]
- Korneev A, Lipina M, Lychagin A, Timashev P, Kon E, Telyshev D, Goncharuk Y, Vyazankin I, Elizarov M, Murdalov E, Pogosyan D, Zhidkov S, Bindeeva A, Liang XJ, Lasovskiy V, Grinin V, Anosov A, Kalinsky E. Systematic review of artificial intelligence tack in preventive orthopaedics: is the land coming soon? Int Orthop 2023;47:393-403. [Crossref] [PubMed]
- Wu H, Zhou Y, Xu L, Tong L, Wang Y, Liu B, Yan H, Sun Z. Mapping Knowledge Structure and Research Frontiers of Ultrasound-Induced Blood-Brain Barrier Opening: A Scientometric Study. Front Neurosci 2021;15:706105. [Crossref] [PubMed]
- Li C, Ojeda-Thies C, Renz N, Margaryan D, Perka C, Trampuz A. The global state of clinical research and trends in periprosthetic joint infection: A bibliometric analysis. Int J Infect Dis 2020;96:696-709. [Crossref] [PubMed]
- Ahmad T, Baig M, Othman SS, Malibary H, Ahmad S, Rasheed SM, Al Bataineh MT, Al-Omari B. Bibliometric Analysis and Visualization Mapping of Anthrax Vaccine Publications from 1991 through 2021. Vaccines (Basel) 2022;10:1007. [Crossref] [PubMed]
- Shen Z, Hu J, Wu H, Chen Z, Wu W, Lin J, Xu Z, Kong J, Lin T. Global research trends and foci of artificial intelligence-based tumor pathology: a scientometric study. J Transl Med 2022;20:409. [Crossref] [PubMed]
- Gračanin AG, Ćurić J, Lončarević J, Morović-Vergles J. Magnetic resonance imaging in the diagnosis and follow-up of giant cell arteritis: case report and review of literature. Eur J Rheumatol 2015;2:125-8. [Crossref] [PubMed]
- Liu S, Sun YP, Gao XL, Sui Y. Knowledge domain and emerging trends in Alzheimer’s disease: a scientometric review based on CiteSpace analysis. Neural Regen Res 2019;14:1643-50. [Crossref] [PubMed]
- Krizhevsky A, Sutskever I, Hinton GE. ImageNet Classification with Deep Convolutional Neural Networks. Communications of the ACM 2017;60:84-90.
- Rong Y, Xiang D, Zhu W, Shi F, Gao E, Fan Z, Chen X. Deriving external forces via convolutional neural networks for biomedical image segmentation. Biomed Opt Express 2019;10:3800-14. [Crossref] [PubMed]
- Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, Ghafoorian M, van der Laak JAWM, van Ginneken B, Sánchez CI. A survey on deep learning in medical image analysis. Med Image Anal 2017;42:60-88. [Crossref] [PubMed]
- Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. Medical Image Computing and Computer-Assisted Intervention-MICCAI 2015:234-41.
- Zunair H, Ben Hamza A. Sharp U-Net: Depthwise convolutional network for biomedical image segmentation. Comput Biol Med 2021;136:104699. [Crossref] [PubMed]
- Wei N, Xu Y, Li Y, Shi J, Zhang X, You Y, Sun Q, Zhai H, Hu Y. A bibliometric analysis of T cell and atherosclerosis. Front Immunol 2022;13:948314. [Crossref] [PubMed]
- Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A 2004;101:5303-10. [Crossref] [PubMed]
- Wilson M, Sampson M, Barrowman N, Doja A. Bibliometric Analysis of Neurology Articles Published in General Medicine Journals. JAMA Netw Open 2021;4:e215840. [Crossref] [PubMed]
- Liu Z, Wang S, Zhang Y, Feng Y, Liu J, Zhu H. Artificial Intelligence in Food Safety: A Decade Review and Bibliometric Analysis. 2023;12:1242.
- Liu Y, Liu W, Chen H, Xie S, Wang C, Liang T, Yu Y, Liu X. Artificial intelligence versus radiologist in the accuracy of fracture detection based on computed tomography images: a multi-dimensional, multi-region analysis. Quant Imaging Med Surg 2023;13:6424-33. [Crossref] [PubMed]
- Guo M, Sun Y, Zhu Y, Han M, Dou G, Wen S. Pruning and quantization algorithm with applications in memristor-based convolutional neural network. Cogn Neurodyn 2024;18:233-45. [Crossref] [PubMed]
- Soffer S, Ben-Cohen A, Shimon O, Amitai MM, Greenspan H, Klang E. Convolutional Neural Networks for Radiologic Images: A Radiologist’s Guide. Radiology 2019;290:590-606. [Crossref] [PubMed]
- Garfield E. The history and meaning of the journal impact factor. JAMA 2006;295:90-3. [Crossref] [PubMed]
- Sánchez-Gómez MB, Duarte-Clíments G, Gómez-Salgado J, González-Pacheco ME, de Castro-Peraza ME, Novo-Muñoz MM, Rodríguez-Gómez JÁ, Martínez-Riera JR, Pessoa-Moreira R, Martins MDR, Echevarría-Pérez P, Bonilla-Calero AI. Research, Reading, and Publication Habits of Nurses and Nursing Students Applied to Impact Journals: International Multicentre Study. Int J Environ Res Public Health 2023;20:4697. [Crossref] [PubMed]
- Wu H, Cheng K, Guo Q, Yang W, Tong L, Wang Y, Sun Z. Mapping Knowledge Structure and Themes Trends of Osteoporosis in Rheumatoid Arthritis: A Bibliometric Analysis. Front Med (Lausanne) 2021;8:787228. [Crossref] [PubMed]
- Pedoia V, Majumdar S, Link TM. Segmentation of joint and musculoskeletal tissue in the study of arthritis. MAGMA 2016;29:207-21. [Crossref] [PubMed]
- Pedoia V, Haefeli J, Morioka K, Teng HL, Nardo L, Souza RB, Ferguson AR, Majumdar S. MRI and biomechanics multidimensional data analysis reveals R(2) -R(1ρ) as an early predictor of cartilage lesion progression in knee osteoarthritis. J Magn Reson Imaging 2018;47:78-90. [Crossref] [PubMed]
- Iriondo C, Pedoia V, Majumdar S. Lumbar intervertebral disc characterization through quantitative MRI analysis: An automatic voxel-based relaxometry approach. Magn Reson Med 2020;84:1376-90. [Crossref] [PubMed]
- Tibrewala R, Ozhinsky E, Shah R, Flament I, Crossley K, Srinivasan R, Souza R, Link TM, Pedoia V, Majumdar S. Computer-Aided Detection AI Reduces Interreader Variability in Grading Hip Abnormalities With MRI. J Magn Reson Imaging 2020;52:1163-72. [Crossref] [PubMed]
- Namiri NK, Lee J, Astuto B, Liu F, Shah R, Majumdar S, Pedoia V. Deep learning for large scale MRI-based morphological phenotyping of osteoarthritis. Sci Rep 2021;11:10915. [Crossref] [PubMed]
- Shi J, Wei S, Gao Y, Mei F, Tian J, Zhao Y, Li Z. Global output on artificial intelligence in the field of nursing: A bibliometric analysis and science mapping. J Nurs Scholarsh 2023;55:853-63. [Crossref] [PubMed]
- Guo Y, Sun L, Zhong W, Zhang N, Zhao Z, Tian W. Artificial intelligence-assisted repair of peripheral nerve injury: a new research hotspot and associated challenges. Neural Regen Res 2024;19:663-70. [Crossref] [PubMed]
- Tian Z, Wang D, Sun X, Fan Y, Guan Y, Zhang N, Zhou M, Zeng X, Yuan Y, Bu H, Wang H. Current status and trends of artificial intelligence research on the four traditional Chinese medicine diagnostic methods: a scientometric study. Ann Transl Med 2023;11:145. [Crossref] [PubMed]
- Chen K, Asada T, Ienaga N, Miura K, Sakashita K, Sunami T, Kadone H, Yamazaki M, Kuroda Y. Two-stage video-based convolutional neural networks for adult spinal deformity classification. Front Neurosci 2023;17:1278584. [Crossref] [PubMed]
- Lee KH, Lee RW, Lee KH, Park W, Kwon SR, Lim MJ. The Development and Validation of an AI Diagnostic Model for Sacroiliitis: A Deep-Learning Approach. Diagnostics (Basel) 2023;13:3643. [Crossref] [PubMed]
- Fan F, Liu H, Dai X, Liu G, Liu J, Deng X, Peng Z, Wang C, Zhang K, Chen H, Yin C, Zhan M, Deng Z. Automated bone age assessment from knee joint by integrating deep learning and MRI-based radiomics. Int J Legal Med 2024;138:927-38. [Crossref] [PubMed]
- Gurung B, Liu P, Harris PDR, Sagi A, Field RE, Sochart DH, Tucker K, Asopa V. Artificial intelligence for image analysis in total hip and total knee arthroplasty: a scoping review. Bone Joint J 2022;104-B:929-37. [Crossref] [PubMed]
- Ma X, Zeng B, Xing Y. Combining 3D skeleton data and deep convolutional neural network for balance assessment during walking. Front Bioeng Biotechnol 2023;11:1191868. [Crossref] [PubMed]
- Hao X, Li J, Guo Y, Jiang T, Yu M. Hypergraph Neural Network for Skeleton-Based Action Recognition. IEEE Trans Image Process 2021;30:2263-75. [Crossref] [PubMed]
- Xu D, Liu B, Wang J, Zhang Z. Bibliometric analysis of artificial intelligence for biotechnology and applied microbiology: Exploring research hotspots and frontiers. Front Bioeng Biotechnol 2022;10:998298. [Crossref] [PubMed]
- Herz C, Vergnet N, Tian S, Aly AH, Jolley MA, Tran N, Arenas G, Lasso A, Schwartz N, O’Neill KE, Yushkevich PA, Pouch AM. Open-source graphical user interface for the creation of synthetic skeletons for medical image analysis. J Med Imaging (Bellingham) 2024;11:036001. [Crossref] [PubMed]
- Wu Z, Sun P, Chen X, Tang K, Xu T, Zou L, Wang X, Tan M, Cheng F, Weise T. SelfGCN: Graph Convolution Network With Self-Attention for Skeleton-Based Action Recognition. IEEE Trans Image Process 2024;33:4391-403. [Crossref] [PubMed]
- Yang Y, Yan LF, Zhang X, Han Y, Nan HY, Hu YC, Hu B, Yan SL, Zhang J, Cheng DL, Ge XW, Cui GB, Zhao D, Wang W. Glioma Grading on Conventional MR Images: A Deep Learning Study With Transfer Learning. Front Neurosci 2018;12:804. [Crossref] [PubMed]
- Sezer A, Sezer HB. Deep Convolutional Neural Network-Based Automatic Classification of Neonatal Hip Ultrasound Images: A Novel Data Augmentation Approach with Speckle Noise Reduction. Ultrasound Med Biol 2020;46:735-49. [Crossref] [PubMed]
- Feng H, Chen J, Zhang Z, Lou Y, Zhang S, Yang W. A bibliometric analysis of artificial intelligence applications in macular edema: exploring research hotspots and Frontiers. Front Cell Dev Biol 2023;11:1174936. [Crossref] [PubMed]
- Xie Y, Li X, Chen F, Wen R, Jing Y, Liu C, Wang J. Artificial intelligence diagnostic model for multi-site fracture X-ray images of extremities based on deep convolutional neural networks. Quant Imaging Med Surg 2024;14:1930-43. [Crossref] [PubMed]
- Sapthagirivasan V, Anburajan M, Janarthanam S. Extraction of 3D Femur Neck Trabecular Bone Architecture from Clinical CT Images in Osteoporotic Evaluation: a Novel Framework. J Med Syst 2015;39:81. [Crossref] [PubMed]
- Rus D, Tolley MT. Design, fabrication and control of soft robots. Nature 2015;521:467-75. [Crossref] [PubMed]
- Akgundogdu A, Jennane R, Aufort G, Benhamou CL, Ucan ON. 3D image analysis and artificial intelligence for bone disease classification. J Med Syst 2010;34:815-28. [Crossref] [PubMed]
- Li M, Hsu W, Xie X, Cong J, Gao W SACNN. Self-Attention Convolutional Neural Network for Low-Dose CT Denoising With Self-Supervised Perceptual Loss Network. IEEE Trans Med Imaging 2020;39:2289-301. [Crossref] [PubMed]
- Zhang J, Gong W, Ye L, Wang F, Shangguan Z, Cheng Y. A Review of deep learning methods for denoising of medical low-dose CT images. Comput Biol Med 2024;171:108112. [Crossref] [PubMed]
- Rak M, Steffen J, Meyer A, Hansen C, Tönnies KD. Combining convolutional neural networks and star convex cuts for fast whole spine vertebra segmentation in MRI. Comput Methods Programs Biomed 2019;177:47-56. [Crossref] [PubMed]
- Hallinan JTPD, Zhu L, Yang K, Makmur A, Algazwi DAR, Thian YL, Lau S, Choo YS, Eide SE, Yap QV, Chan YH, Tan JH, Kumar N, Ooi BC, Yoshioka H, Quek ST. Deep Learning Model for Automated Detection and Classification of Central Canal, Lateral Recess, and Neural Foraminal Stenosis at Lumbar Spine MRI. Radiology 2021;300:130-8. [Crossref] [PubMed]
- Rudin C. Stop Explaining Black Box Machine Learning Models for High Stakes Decisions and Use Interpretable Models Instead. Nat Mach Intell 2019;1:206-15. [Crossref] [PubMed]
- Rasheed K, Qayyum A, Ghaly M, Al-Fuqaha A, Razi A, Qadir J. Explainable, trustworthy, and ethical machine learning for healthcare: A survey. Comput Biol Med 2022;149:106043. [Crossref] [PubMed]
- Karim MR, Islam T, Shajalal M, Beyan O, Lange C, Cochez M, Rebholz-Schuhmann D, Decker S. Explainable AI for Bioinformatics: Methods, Tools and Applications. Brief Bioinform 2023;24:bbad236. [Crossref] [PubMed]
- Schwarz GM, Simon S, Mitterer JA, Frank BJH, Aichmair A, Dominkus M, Hofstaetter JG. Artificial intelligence enables reliable and standardized measurements of implant alignment in long leg radiographs with total knee arthroplasties. Knee Surg Sports Traumatol Arthrosc 2022;30:2538-47. [Crossref] [PubMed]
- Liu Y, Yang P, Pi Y, Jiang L, Zhong X, Cheng J, Xiang Y, Wei J, Li L, Yi Z, Cai H, Zhao Z. Automatic identification of suspicious bone metastatic lesions in bone scintigraphy using convolutional neural network. BMC Med Imaging 2021;21:131. [Crossref] [PubMed]
- Karnuta JM, Murphy MP, Luu BC, Ryan MJ, Haeberle HS, Brown NM, Iorio R, Chen AF, Ramkumar PN. Artificial Intelligence for Automated Implant Identification in Total Hip Arthroplasty: A Multicenter External Validation Study Exceeding Two Million Plain Radiographs. J Arthroplasty 2023;38:1998-2003.e1. [Crossref] [PubMed]
- Hao X, Li J, Guo Y, Jiang T, Yu M. Hypergraph Neural Network for Skeleton-Based Action Recognition. IEEE Trans Image Process 2021;30:2263-75. [Crossref] [PubMed]
- Tan JR, Gao Y, Raghuraman R, Ting D, Wong KM, Cheng LT, Oh HC, Goh SH, Yan YY. Application of deep learning algorithms in classification and localization of implant cutout for the postoperative hip. Skeletal Radiol 2025;54:67-75. [Crossref] [PubMed]
- Xu T, An D, Jia Y, Yue Y A. Review: Point Cloud-Based 3D Human Joints Estimation. Sensors (Basel) 2021;21:1684. [Crossref] [PubMed]
- Lee BD, Lee MS. Automated Bone Age Assessment Using Artificial Intelligence: The Future of Bone Age Assessment. Korean J Radiol 2021;22:792-800. [Crossref] [PubMed]
- Yeoh PSQ, Lai KW, Goh SL, Hasikin K, Hum YC, Tee YK, Dhanalakshmi S. Emergence of Deep Learning in Knee Osteoarthritis Diagnosis. Comput Intell Neurosci 2021;2021:4931437. [Crossref] [PubMed]
- Huang P, Feng Z, Shu X, Wu A, Wang Z, Hu T, Cao Y, Tu Y, Li Z. A bibliometric and visual analysis of publications on artificial intelligence in colorectal cancer (2002-2022). Front Oncol 2023;13:1077539. [Crossref] [PubMed]
- Cheek J, Garnham B, Quan J. What’s in a number? Issues in providing evidence of impact and quality of research(ers). Qual Health Res 2006;16:423-35. [Crossref] [PubMed]
- Cutcliffe JR, Harder HG. The perpetual search for parsimony: enhancing the epistemological and practical utility of qualitative research findings. Int J Nurs Stud 2009;46:1401-10. [Crossref] [PubMed]



