
Endovascular treatment of total internal carotid artery occlusion caused by a carotid web: a case description and literature analysis
Introduction
Carotid web (CaW) has emerged as a critical, yet underappreciated vascular anomaly increasingly linked to ischemic strokes, especially in patients lacking traditional vascular risk factors (1). We present a case of a 56-year-old male with a significant smoking history and hypertension. The patient presented with recurrent episodes of transient right leg weakness. Initial imaging, including magnetic resonance imaging (MRI) and computed tomography angiography (CTA), revealed a subacute ischemic lesion in the left frontal lobe, along with severe stenosis of the left internal carotid artery. Despite undergoing initial medical management with dual antiplatelet therapy and statins, the patient suddenly developed motor aphasia and presented to the emergency department. Emergency transfemoral cerebral angiography (TFCA) was conducted, which revealed a completely occluded CaW. The patient underwent aspiration thrombectomy and carotid artery stenting (CAS), resulting in restored cerebral perfusion and resolution of neurological deficits. This case is reported because, unlike previously identified cases of CaW, it involved a completely occluded CaW and was successfully treated through endovascular intervention.
Case presentation
A 56-year-old male patient presented to the outpatient clinic with two episodes of transient right lower limb weakness, each lasting less than 30 minutes. The patient had a 35 pack-year smoking history and was on medication for hypertension. Neurological examination at the time of presentation revealed no signs of aphasia, unilateral weakness, or sensory abnormalities. The day before, at an external hospital, the patient underwent brain MRI/magnetic resonance angiography (MRA) and carotid ultrasound due to suspected cerebral infarction. The MRI revealed a focal high signal on diffusion weighted imaging (DWI) in the left frontal lobe that did not match the apparent diffusion coefficient (ADC), indicating a subacute ischemic lesion (Figure 1A,1B), along with reduced flow in the left carotid artery (Figure 1C). Carotid ultrasound showed a hyperechogenic plaque protruding into the lumen of the proximal left internal carotid artery (Figure 1D), causing approximately 70% stenosis.
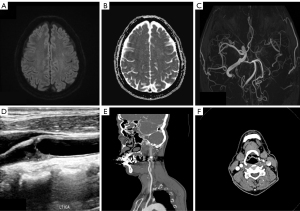
Upon further evaluation at Jeonbuk National University Hospital with brain CTA, severe stenosis extending from the proximal to the distal portion of the left internal carotid artery was confirmed, without evidence of calcification, dissection, or thrombus (Figure 1E,1F). These findings were deemed consistent with the patient’s symptoms. Therefore, CAS was recommended to the patient, but he preferred medical therapy over intervention. Due to the high risk of recurrent cerebral infarction, dual anti-platelet therapy (DAPT) consisting of aspirin 100 mg and cilostazol 200 mg was initiated. Additionally, while high-dose statin therapy was initially considered, the dose was reduced to atorvastatin 40 mg due to elevated liver enzyme levels observed in blood tests.
Five months after starting the medication, the patient presented to the emergency department with motor aphasia. Upon arrival, his vital signs were blood pressure 165/94 mmHg, pulse 79 beats per minute, respiratory rate 16 breaths per minute, and body temperature 36.6 ℃. Neurological examination revealed mild motor aphasia only, with a National Institutes of Health Stroke Scale (NIHSS) score of 1. Perfusion CTA showed occlusion of the proximal left internal carotid artery (Figure 2A) and reduced perfusion in the left frontal lobe (Figure 2B), with acute infarction evident on DWI (Figure 2C,2D). TFCA confirmed total occlusion of the proximal left internal carotid artery. Aspiration thrombectomy was performed once to remove the thrombus at the occlusion site. Follow-up digital subtraction angiography (DSA) revealed CaW formation and typical contrast stasis associated with CaW (Figure 3A-3F). To achieve definitive treatment, a PRECISE (Cordis, Miami, FL, USA) 7 mm–4 cm stent was deployed, successfully restoring blood flow in the internal carotid artery (Figure 3G,3H).
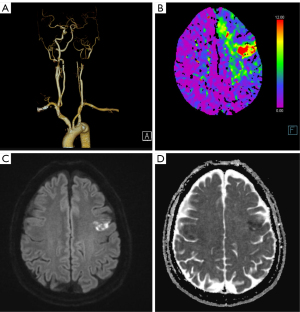
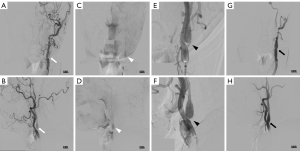
Post-procedure, the patient’s motor aphasia improved, and his NIHSS score decreased to 0. Blood tests conducted during hospitalization showed no significant abnormalities. Further evaluation, including echocardiography and 24-hour Holter monitoring, revealed no abnormalities associated with the etiology of the cerebral infarction. The patient’s antiplatelet regimen was adjusted from aspirin and cilostazol to aspirin and clopidogrel. With no neurological deficits observed during the hospital stay, the patient was discharged on the fifth day.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of the article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
This case highlights the effective use of endovascular treatment, including CAS, in managing a completely occluded CaW in a patient who initially presented with transient right lower limb weakness and later developed motor aphasia. The comprehensive management strategy, including dual antiplatelet therapy, high dose statin, and stenting, successfully restored cerebral perfusion and resolved neurological deficits. This case emphasizes the importance of early detection of CaW and timely endovascular intervention in preventing recurrent ischemic events and improving patient outcomes.
The CaW is an underrecognized vascular anomaly characterized by a shelf-like protrusion of the intimal layer into the lumen of the carotid artery, most commonly at the carotid bulb. This condition has been increasingly associated with ischemic strokes, particularly cryptogenic strokes in younger patients. Epidemiological data suggest a higher prevalence of CaWs in males and a potentially increased incidence among individuals of African descent, though large-scale studies remain limited (1). The prevalence of CaW is known to be less than 1%, including individuals without a history of stroke (2). Approximately 1.0–1.2% of patients who underwent CTA for suspected ischemic stroke were found to have CaW (3). Additionally, a meta-analysis found that approximately 13% of patients under the age of 60 years with cryptogenic stroke had CaW (4).
Histopathologically, CaWs represent a variant of fibromuscular dysplasia (FMD) characterized by intimal fibrosis and myxoid degeneration (5). The abnormal intimal thickening protrudes into the arterial lumen, disrupting normal blood flow and creating conditions conducive to thrombus formation, which can lead to embolic strokes (6). The precise etiology of CaWs remains unclear, with hypotheses ranging from congenital abnormalities to hormonal influences and vascular injuries (7).
The diagnosis of CaW, a vascular anomaly associated with ischemic primarily relies on advanced imaging techniques. CTA is the gold standard, offering high-resolution images that reveal the characteristic shelf-like intimal projections, clearly reveal the characteristic shelf-like intimal projections, especially when multiplanar reconstructions (MPRs) are used (2). MRA and ultrasound are also valuable alternatives, particularly for patients who cannot undergo CTA, such as those who are pregnant or have renal impairment (1). Optical coherence tomography (OCT) is an emerging tool that provides detailed images of the intimal layer, though it is predominantly used in research settings (2). Catheter angiography, though invasive, remains an essential tool, providing both definitive diagnosis and the potential for immediate therapeutic intervention (2). DSA allows for hemodynamic analysis and the detection of characteristic dynamic features such as persistent contrast stasis rostral to the web lesion (8). These imaging modalities, when used in combination, significantly enhance diagnostic accuracy and guide the management of patients with CaWs.
CaW are a significant risk factor for recurrent ischemic strokes, particularly in younger patients who lack traditional vascular risk factors (3,9,10). The CaW creates localized blood flow stagnation, and the resulting abnormal flow dynamics promote thrombus formation, which can lead to embolic strokes (3).
The stroke risk associated with CaWs appears to be influenced by the size and morphology of the web. A cross-sectional study of 86 patients with CaWs diagnosed via CTA indicated that a CaW length of ≥3 mm, an acute angle relative to the carotid wall, and occupying more than 50% of the carotid bulb were associated with an elevated risk of stroke (11). More recent studies have confirmed these findings, demonstrating a significant correlation between CaW length and volume and the occurrence of transient ischemic attacks (TIA) or strokes (11-13). The patient in this case also exhibited the characteristics described in the aforementioned study, suggesting that these are significant risk factors for recurrent cerebral infarction. Specifically, CaW in this patient was relatively large, which is believed to have contributed to in situ thrombotic occlusion.
There are currently no standardized management protocols for CaWs. According to a survey conducted by the Society of NeuroInterventional Surgery, involving 74 providers, most clinicians recommend mono- or dual-antiplatelet therapy as first-line treatment for both asymptomatic and symptomatic CaWs (14). However, the comparative efficacy of single antiplatelet therapy, dual antiplatelet therapy, or anticoagulation remains uncertain. Observational data indicate that patients with symptomatic CaWs may still face a 20% risk of stroke recurrence within 2 years, even with medical management (15,16). Nevertheless, surgical interventions, such as carotid endarterectomy and CAS, have demonstrated promising results, with patients experiencing no recurrent strokes during follow-up (3,10).
The primary treatment goal for a CaW is to flatten the web to prevent it from becoming a hemodynamic nidus for future ischemic events. CAS with a self-expanding stent is effective as it eliminates distal hemodynamic disturbances without the need for balloon angioplasty, due to the absence of flow-limiting stenosis (17).
Future research should focus on establishing standardized diagnostic criteria and treatment protocols for CaWs. Additionally, further studies on the hemodynamic implications of CaWs and the efficacy of different therapeutic interventions will be crucial for optimizing patient care. Large-scale epidemiological studies are also needed to better understand the prevalence and risk factors associated with CaWs across diverse populations.
Acknowledgments
None.
Footnote
Funding: None.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2483/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of the article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liang S, Qin P, Xie L, Niu S, Luo J, Chen F, Chen X, Zhang J, Wang G. The carotid web: Current research status and imaging features. Front Neurosci 2023;17:1104212. [Crossref] [PubMed]
- Chen H, Colasurdo M, Costa M, Nossek E, Kan P. Carotid webs: a review of pathophysiology, diagnostic findings, and treatment options. J Neurointerv Surg 2024;16:1294-8. [Crossref] [PubMed]
- Choi PM, Singh D, Trivedi A, Qazi E, George D, Wong J, Demchuk AM, Goyal M, Hill MD, Menon BK. Carotid Webs and Recurrent Ischemic Strokes in the Era of CT Angiography. AJNR Am J Neuroradiol 2015;36:2134-9. [Crossref] [PubMed]
- Mac Grory B, Nossek E, Reznik ME, Schrag M, Jayaraman M, McTaggart R, de Havenon A, Yaghi S, Feng W, Furie K, Boyanpally A. Ipsilateral internal carotid artery web and acute ischemic stroke: A cohort study, systematic review and meta-analysis. PLoS One 2021;16:e0257697. [Crossref] [PubMed]
- Rodríguez-Castro E, Arias-Rivas S, Santamaría-Cadavid M, López-Dequidt I, Rodríguez-Yáñez M, Mosqueira AJ, Blanco Ulla M, Vázquez Herrero F, Castiñeira JA, Martínez-Sáez E, Pérez Béliz E, Mosquera N, Caicedo D, Fraga M, Pumar JM. Carotid web: the challenging diagnosis of an under-recognized entity. J Neurol 2022;269:5629-37. [Crossref] [PubMed]
- Park CC, El Sayed R, Risk BB, Haussen DC, Nogueira RG, Oshinski JN, Allen JW. Carotid webs produce greater hemodynamic disturbances than atherosclerotic disease: a DSA time-density curve study. J Neurointerv Surg 2022;14:729-33. [Crossref] [PubMed]
- Olin JW, Gornik HL, Bacharach JM, Biller J, Fine LJ, Gray BH, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation 2014;129:1048-78. [Crossref] [PubMed]
- Olindo S, Marnat G, Chausson N, Turpinat C, Smadja D, Gaillard N. Carotid webs associated with ischemic stroke. Updated general review and research directions. Rev Neurol (Paris) 2021;177:627-38. [Crossref] [PubMed]
- Joux J, Boulanger M, Jeannin S, Chausson N, Hennequin JL, Molinié V, Smadja D, Touzé E, Olindo S. Association Between Carotid Bulb Diaphragm and Ischemic Stroke in Young Afro-Caribbean Patients: A Population-Based Case-Control Study. Stroke 2016;47:2641-4. [Crossref] [PubMed]
- Joux J, Chausson N, Jeannin S, Saint-Vil M, Mejdoubi M, Hennequin JL, Deschamps L, Smadja D, Olindo S. Carotid-bulb atypical fibromuscular dysplasia in young Afro-Caribbean patients with stroke. Stroke 2014;45:3711-3. [Crossref] [PubMed]
- Tabibian BE, Parr M, Salehani A, Mahavadi A, Rahm S, Kaur M, Howell S, Jones JG, Liptrap E, Harrigan MR. Morphological characteristics of symptomatic and asymptomatic carotid webs. J Neurosurg 2022;137:1727-32. [Crossref] [PubMed]
- Haussen DC, Grossberg JA, Bouslama M, Pradilla G, Belagaje S, Bianchi N, Allen JW, Frankel M, Nogueira RG. Carotid Web (Intimal Fibromuscular Dysplasia) Has High Stroke Recurrence Risk and Is Amenable to Stenting. Stroke 2017;48:3134-7. [Crossref] [PubMed]
- Compagne KCJ, Dilba K, Postema EJ, van Es ACGM, Emmer BJ, Majoie CBLM, van Zwam WH, Dippel DWJ, Wentzel JJ, van der Lugt A, Gijsen FJH. Flow Patterns in Carotid Webs: A Patient-Based Computational Fluid Dynamics Study. AJNR Am J Neuroradiol 2019;40:703-8. [Crossref] [PubMed]
- Wojcik K, Milburn J, Vidal G, Tarsia J, Steven A. Survey of Current Management Practices for Carotid Webs. Ochsner J 2019;19:296-302. [Crossref] [PubMed]
- Guglielmi V, Compagne KCJ, Sarrami AH, Sluis WM, van den Berg LA, van der Sluijs PM, Mandell DM, van der Lugt A, Roos YBWEM, Majoie CBLM, Dippel DWJ, Emmer BJ, van Es ACGM, Coutinho JM. MR CLEAN trial and MR CLEAN Registry Investigators. Assessment of Recurrent Stroke Risk in Patients With a Carotid Web. JAMA Neurol 2021;78:826-33. [Crossref] [PubMed]
- Olindo S, Chausson N, Signate A, Mecharles S, Hennequin JL, Saint-Vil M, Edimonana-Kaptue M, Jeannin S, Landais A, Cabre P, Sibon I, Smadja D, Joux J. Stroke Recurrence in First-Ever Symptomatic Carotid Web: A Cohort Study. J Stroke 2021;23:253-62. [Crossref] [PubMed]
- Brinjikji W, Agid R, Pereira VM. Carotid Stenting for Treatment of Symptomatic Carotid Webs: A Single-Center Case Series. Interv Neurol 2018;7:233-40. [Crossref] [PubMed]


