
In vitro evaluation of second-generation peripheral hydrogel coils: packing density comparison with fibered and bare coils and assessment of hydrogel dislodgment
Introduction
Embolization coils are among the most widely used embolic agents (1). These coils, mainly made of metal alloys such as platinum-tungsten or stainless steel, are typically inserted into the target vessel through an angiographic catheter or a microcatheter. Once in place, they reduce blood flow through mechanical blockage and serve as a thrombogenic scaffold for clot formation, ultimately leading to vessel occlusion (1). Since the introduction of coil embolization in the 1970s, it has become a standard treatment approach for various vascular diseases, such as aneurysm, arteriovenous malformation, endoleak, and hemorrhage (2-5).
Hydrogel coils are embolization coils that feature a cross-linked polymer that expands upon contact with blood (6). Initially developed to reduce the recurrence of cerebral aneurysms after coil embolization, these coils are now also used to treat a wide range of peripheral arterial and venous diseases (7-9). The first-generation peripheral hydrogel coils (AZUR HydroCoil; Terumo, Tokyo, Japan) are coated with the polymer on their surface and can expand up to four times their original size. However, the widespread adoption of these coils has been hindered by their stiffness, which makes achieving tight packing technically challenging (9,10). Additionally, there are concerns about the potential risk of hydrogel dislodgment, which could lead to embolization of the non-target vessels downstream (11).
Recently, the second-generation peripheral hydrogel coils (AZUR CX; Terumo) were introduced. To decrease the stiffness of the coil and prevent hydrogel dislodgment, the polymer is now encased inside the primary wind of the coil, rather than being coated on the surface. Therefore, unlike the first-generation hydrogel coils, which are recommended by the manufacturer only for filling the space, the second-generation hydrogel coils are recommended for both establishing the base and filling the space. However, it remains unclear how effectively these coil pack compare to fibered and bare coils, and whether they are prone to hydrogel dislodgment.
The purpose of this in vitro study was to compare the packing density of second-generation peripheral hydrogel coils with fibered and bare coils and to determine their susceptibility to hydrogel dislodgment.
Methods
Study design
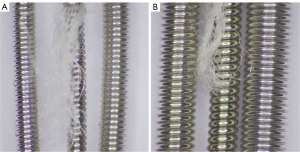
This study does not require Institutional Review Board approval. In this study, we used three types of embolization coils: 12 detachable second-generation peripheral hydrogel coils (AZUR CX; Terumo), 12 detachable fibered coils (Interlock; Boston Scientific, Natick, MA, USA), and 12 pushable bare coils (Alpha Interventional Medicine, Guangzhou, China). The hydrogel and bare coils were provided by their manufacturers at no cost, while the fibered coils were purchased locally. All these coils are made of platinum-tungsten alloy and are available in 0.018-inch and 0.035-inch platforms. For the 0.018-inch coils, we used three with a 4-mm diameter and three with a 6-mm diameter, while for the 0.035-inch coils, we used three with a 6-mm diameter and three with an 8-mm diameter. These diameter coils were selected due to the similarity in length of their primary wind (Table 1). The three types of coils used in this study vary in their primary wind diameter (Figure 1). The fibered coils have the smallest diameter, resulting in the lowest coil volumes. The hydrogel coils have a slightly larger diameter and volumes than the fibered coils. In terms of shape, the hydrogel coils feature a complex three-dimensional configuration (helical-shaped second-generation peripheral hydrogel coils are not produced by the manufacture), while the fibered and bare coils have a helical shape. All coils were inserted into a flow model under fluoroscopy, after which the packing densities of the three different coil types were calculated and compared for each specification. Additionally, hydrogel dislodgment in the flow model was analyzed over a 28-day period for the hydrogel coils.
Table 1
| Coil type | Coil specification | Primary wind | Coil volume (mm3) | |
|---|---|---|---|---|
| Diameter (inch) | Length (cm) | |||
| Hydrogel | 0.018-inch | |||
| 4-mm diameter | 0.015 | 13 | 14.82 | |
| 6-mm diameter | 0.015 | 20 | 22.80 | |
| 0.035-inch | ||||
| 6-mm diameter | 0.029 | 17 | 72.44 | |
| 8-mm diameter | 0.029 | 24 | 102.27 | |
| Fibered | 0.018-inch | |||
| 4-mm diameter | 0.012 | 15 | 10.94 | |
| 6-mm diameter | 0.012 | 20 | 14.59 | |
| 0.035-inch | ||||
| 6-mm diameter | 0.021 | 20 | 44.69 | |
| 8-mm diameter | 0.021 | 20 | 44.69 | |
| Bare | 0.018-inch | |||
| 4-mm diameter | 0.018 | 12 | 19.70 | |
| 6-mm diameter | 0.018 | 16 | 26.27 | |
| 0.035-inch | ||||
| 6-mm diameter | 0.034 | 14 | 82.01 | |
| 8-mm diameter | 0.034 | 20 | 117.15 | |
Flow model construction
The flow model employed in this study was constructed to simulate a physiological arterial system (Figure 2). This model utilized 36 transparent silicone tubes (Longer Precision Pump, Baoding, China) with an internal diameter of either 3.2, 4.8, or 6.4 mm. A single embolization coil was deployed in each of the 36 tubes. Coils with a diameter of 4 mm were inserted into the 3.2-mm tubes, 6-mm diameter coils into the 4.8-mm tubes, and 8-mm diameter coils into the 6.4-mm tubes. These tubes were selected because the coils to be inserted are precisely 25% larger in diameter. Each tube individually constituted a closed-circuit system, connected to a thermostatic bath (WT-10; Biocomma, Shenzhen, China) filled with phosphate-buffered saline (pH 7.4), maintained at a constant 37 ℃ to replicate the human body’s temperature. Peristaltic pumps (WT600-2J-A; Longer Precision Pump) were used to produce a pulsatile flow pattern in each tube, simulating the cardiac cycle at a rate of 80 revolutions per minute. A mesh filter, capable of filtering out particles larger than 20 microns, was installed at the downstream end of each tube.
Embolization coil insertion
All embolization coils were inserted by an interventional radiologist with over 12 years of experience and skilled in the use of hydrogel, fibered, and bare coils. A 5-Fr introducer sheath (Radifocus; Terumo) was installed into the silicone tube of the flow model. From this sheath, a 5-Fr Cobra-2 angiographic catheter (Radifocus; Terumo) was advanced approximately 30 cm into the tube, and the 0.035-inch coils were inserted into the tube through this catheter under fluoroscopic guidance (Innova 3100; GE, Waukesha, WI, USA). For the 0.018-inch coils, insertion was performed using a 2.8-Fr microcatheter (Progreat; Terumo), which was advanced approximately 15 cm into the tube through the angiographic catheter. The detachable coils were inserted following the instructions in the device manual, and the pushable coils were inserted using either a 0.035-inch (Glidewire; Terumo) or a 0.021-inch (Progreat; Terumo) hydrophilic guidewire. Consistent efforts were made to insert all coils at a similar speed. The detachable fibered coils allowed for repositioning at any time, whereas the detachable hydrogel coils were subject to a repositioning timeframe (up to 30 minutes for the 0.018-inch coils and 20 minutes for the 0.035-inch coils). Considering these differences, repositioning was limited to a maximum of three attempts for each coil, regardless of coil type.
Packing density calculation
The packing density of the embolization coil in the silicone tube was calculated using the same method employed by AngioCalc (http://www.angiocalc.com), an open-source packing density calculator endorsed by the manufacturers of the hydrogel and fibered coils used in this study. The packing density was determined using the following equation: (V1 ÷ V2) × 100, where V1 is the volume of the coil, and V2 is the volume of the embolized segment of the silicone tube. The volume of the coil (V1) was calculated with the equation V1 = (π × D2 × L) ÷ 4, where D is the diameter of the coil’s primary wind, and L is the length of the coil’s primary wind. The volume of the embolized segment of the silicone tube (V2) was calculated with the equation V2 = (π × D2 × L) ÷ 4, where D is the internal diameter of the silicone tube, and L is the embolization length. The embolization length, which was defined as the length of the silicone tube occupied by the coil, was measured using a digital caliper.
Hydrogel dislodgment analysis
The mesh filter, situated at the downstream end of each silicone tube, was inspected and replaced at set intervals: 30 minutes after coil insertion, and then on days 3, 7, 14, and 28. During the 28-day study period, the thermostatic bath containing phosphate-buffered saline consistently maintained a temperature of 37 ℃, while the pulsatile pump operated at 80 revolutions per minute. The phosphate-buffered saline was replaced every 3 days to ensure optimal conditions were maintained throughout the study. Upon each inspection, the filter was examined using a digital microscope equipped with measuring software (TX5300; Seepack, Shenzhen, China). After the study period, the silicone tubes were carefully cut open to extract the hydrogel coils, which were then inspected using the same digital microscope to identify any hydrogel fragments on their surfaces. Hydrogel fragments found on the surface of the coils and those captured by the filter were both subjected to examination using a scanning electron microscope (Mira LMS, Tescan, Brno, Czech).
Statistical analysis
For normally distributed data, we used one-way analysis of variance (ANOVA) to detect significant differences between groups, switching to Welch’s ANOVA when variances were unequal. Where ANOVA showed significant differences, Tukey’s honestly significant difference test was used for specific comparisons, and the Games-Howell test was applied in cases of unequal variances. Non-normally distributed data were analyzed using the Kruskal-Wallis test, followed by the Mann-Whitney U test with Bonferroni correction for significant findings. A P value of <0.05 was considered statistically significant. All statistical analyses were conducted using SPSS Statistics v21.0 (IBM, Armonk, NY, USA).
Results
Coil insertion
All embolization coils were successfully inserted into the flow model (Figure 3). No silicone tubes were occluded by the inserted coil. Repositioning was required for eight out of 12 hydrogel coils and six out of 12 fibered coils due to unsatisfactory packing. Detachment issues occurred with two 0.018-inch hydrogel coils when using the battery-operated detachment controller. Despite initial failures, both coils were eventually detached and successfully inserted after repeated attempts, which involved wiping the trailing end and reconnecting it to the controller. The median insertion times were approximately 5.3 [interquartile range (IQR), 3.8–7.7] minutes for hydrogel coils, 3.7 (IQR, 3.1–4.3) minutes for fibered coils, and 2.7 (IQR, 2.4–2.9) minutes for bare coils. There was a significant difference in the median insertion time among the three types of coils (P<0.001). Hydrogel coils had a significantly longer median insertion time compared to fibered coils (P=0.005), and fibered coils had a significantly longer median insertion time compared to bare coils (P<0.001).
Embolization length
The mean embolization length showed no significant difference for the 0.018-inch 4-mm diameter coils (P=0.396) (Table 2 and Figure 4A). However, significant differences were observed for the 0.018-inch 6-mm diameter coils (P=0.019) and the 0.035-inch coils (6-mm diameter: P<0.001; 8-mm diameter: P=0.008). Specifically, hydrogel coils had significantly longer mean embolization lengths compared to both fibered (0.018-inch 6-mm diameter: P=0.022; 0.035-inch 6-mm diameter: P<0.001; 0.035-inch 8-mm diameter: P<0.001) and bare coils (0.018-inch 6-mm diameter: P=0.045; 0.035-inch 6-mm diameter: P<0.001; 0.035-inch 8-mm diameter: P<0.001). In contrast, no significant differences were found between fibered and bare coils (0.018-inch 6-mm diameter: P=0.823; 0.035-inch 6-mm diameter: P=0.091; 0.035-inch 8-mm diameter: P=0.245).
Table 2
| Coil specification | Coil type | Embolization length (mm) | Packing density (%) |
|---|---|---|---|
| 0.018-inch | |||
| 4-mm diameter | Hydrogel | 12.1±2.4 | 15.6±2.9 |
| Fibered | 11.4±3.9 | 12.9±4.1 | |
| Bare | 9.0±1.0 | 22.9±2.7 | |
| 6-mm diameter | Hydrogel | 16.8±2.4 | 7.6±1.1 |
| Fibered | 10.3±2.8 | 8.1±2.1 | |
| Bare | 11.3±0.5 | 12.0±0.5 | |
| 0.035-inch | |||
| 6-mm diameter | Hydrogel | 28.8±1.4 | 13.9±0.7 |
| Fibered | 11.7±0.7 | 21.1±1.2 | |
| Bare | 14.3±1.4 | 31.9±3.0 | |
| 8-mm diameter | Hydrogel | 35.2±4.2 | 9.1±1.2 |
| Fibered | 11.3±1.1 | 12.4±1.3 | |
| Bare | 15.0±1.1 | 24.3±1.8 |
Data are presented as mean ± standard deviation.
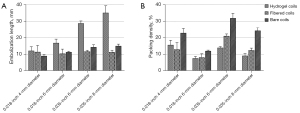
Packing density
The mean packing densities showed significant differences for the 0.018-inch coils (4-mm diameter: P=0.004; 6-mm diameter: P=0.007) and the 0.035-inch coils (6-mm diameter: P=0.002; 8-mm diameter: P<0.001) (Table 2 and Figure 4B). Specifically, bare coils had significantly higher mean packing densities compared to hydrogel coils (0.018-inch 4-mm diameter: P=0.013; 0.018-inch 6-mm diameter: P=0.009; 0.035-inch 6-mm diameter: P=0.013; 0.035-inch 8-mm diameter: P<0.001) and fibered coils (0.018-inch 4-mm diameter: P=0.005; 0.018-inch 6-mm diameter: P=0.015; 0.035-inch 6-mm diameter: P=0.028; 0.035-inch 8-mm diameter: P<0.001). In contrast, the mean packing densities of hydrogel coils were not significantly different from that of fibered coils (0.018-inch 4-mm diameter: P=0.627; 0.018-inch 6-mm diameter: P=0.859; 0.035-inch 8-mm diameter: P=0.070), except for the 0.035-inch 6-mm diameter coils, where hydrogel coils had a significantly lower packing density (P=0.006).
Hydrogel dislodgment
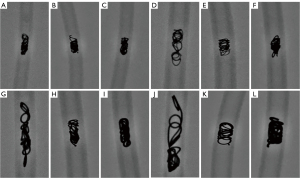
No hydrogel fragments were captured by the mesh filter within 30 minutes of coil insertion, but fragments were observed on days 3, 7, 14, and 28 (Figure 5). Over the course of 28 days, the filter captured a mean of 206.7±45.1 fragments from the 0.018-inch 4-mm diameter coils, 178.7±37.1 fragments from the 0.018-inch 6-mm diameter coils, 226.3±23.0 fragments from the 0.035-inch 6-mm diameter coils, and 211.0±27.1 fragments from the 0.035-inch 8-mm diameter coils (Figure 6A). For fragments measuring ≥1 mm in length, the filter captured a mean of 9.3±5.1 fragments from the 0.018-inch 4-mm diameter coils, 6.3±3.5 fragments from the 0.018-inch 6-mm diameter coils, 3.7±1.5 fragments from the 0.035-inch 6-mm diameter coils, and 6.3±4.5 fragments from the 0.035-inch 8-mm diameter coils (Figure 6B). There were no significant differences in the mean number of captured fragments or fragments measuring ≥1 mm in length across various coil specifications on days 3, 7, 14, and 28 (Tables 3,4). Hydrogel fragments were consistently found on the surface of all coils, especially at their ends. Notably, during the handling of a 0.035-inch 8-mm diameter coil, a loop of hydrogel dislodged from the end. The hydrogel fragments on the coil’s surface and those captured by the filter appeared similar under the scanning electron microscope.
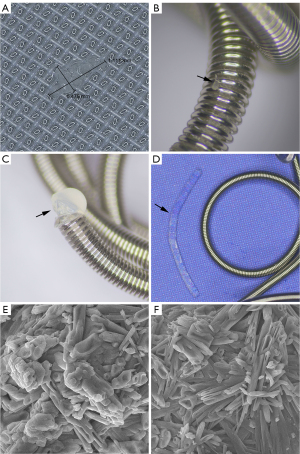

Table 3
| Time interval | Coil specification | P value | ||||
|---|---|---|---|---|---|---|
| 0.018-inch | 0.035-inch | |||||
| 4-mm diameter | 6-mm diameter | 6-mm diameter | 8-mm diameter | |||
| 3-day | 83.7±39.6 | 49.3±16.4 | 71.7±17.0 | 70.7±23.7 | 0.478 | |
| 7-day | 140.7±31.6 | 89.7±21.5 | 122.3±34.0 | 111.3±31.1 | 0.281 | |
| 14-day | 177.3±37.0 | 137.0±19.1 | 171.3±36.9 | 151.7±37.4 | 0.477 | |
| 28-day | 206.7±45.1 | 178.7±37.1 | 226.3±23.0 | 211.0±27.1 | 0.435 | |
Data are presented as mean ± standard deviation.
Table 4
| Time interval | Coil specification | P value | ||||
|---|---|---|---|---|---|---|
| 0.018-inch | 0.035-inch | |||||
| 4-mm diameter | 6-mm diameter | 6-mm diameter | 8-mm diameter | |||
| 3-day | 2.3±1.5 | 0.7±1.2 | 0.0±0.0 | 2.7±1.2 | 0.053 | |
| 7-day | 3.0±2.6 | 3.7±1.5 | 1.3±1.5 | 4.3±2.1 | 0.396 | |
| 14-day | 6.7±2.9 | 5.0±2.6 | 3.3±1.2 | 4.7±2.5 | 0.758 | |
| 28-day | 9.3±5.1 | 6.3±3.5 | 3.7±1.5 | 6.3±4.5 | 0.628 | |
Data are presented as mean ± standard deviation.
Discussion
The majority of peripheral embolization coils available in the market are fibered coils. The fibers on these coils enhance thrombogenicity, thereby reducing the number of coils needed for vessel occlusion (12,13). However, these fibers occupy space within the delivery catheter, necessitating coils with smaller primary wind diameters and, consequently, smaller volumes. As a result, fibered coils rely more heavily on thrombus formation for vessel occlusion compared to bare coils, which may make them more susceptible to recanalization due to fibrinolysis of the thrombus. Our study demonstrated that bare coils achieved significantly higher packing densities than fibered coils, even though their embolization lengths were not significantly different. Similarly, a recent study has shown that the packing density of fibered coils was significantly lower than that of bare coils in a flow model (14). However, achieving vessel occlusion with bare coils requires a higher number of coils compared to fibered coils (12,13). This not only increases costs but also heightens the risk of complications, such as coil penetration into and compression of adjacent organs due to the mass effect of the coils (15-17).
Compared to fibered coils, hydrogel coils offer substantial advantages due to the characteristics of the polymer used in these coils. First, the polymer in hydrogel coils expands up to four times its original size upon contact with blood, directly aiding in the mechanical occlusion of the vessel, unlike fibers, which mainly promote thrombus formation. Second, because this expansion occurs only upon contact with blood, the polymer occupies less space within the delivery catheter, allowing for the use of coils with larger primary wind diameters. Third, the polymer in hydrogel coils is non-degradable, unlike thrombus, which is susceptible to fibrinolysis. Consequently, hydrogel coils depend less on thrombus formation for vessel occlusion. This has been demonstrated in animal studies, where first-generation peripheral hydrogel coils were shown to significantly reduce thrombus formation, resulting in a lower rate of recanalization compared to fibered coils (18,19). However, it remains unclear if the second-generation peripheral hydrogel coils provide the same benefits, since the polymer is now encased within the primary wind to reduce stiffness, rather than being coated on the surface as in the first-generation coils. Additionally, our study showed that despite this modification, these coils demonstrated significantly higher embolization lengths without significant improvements in packing densities compared to fibered coils. This result could be attributable to the stiffness of the second-generation coils, but it could also be due to their complex three-dimensional shape, which makes them longer than the helical-shaped coils.
Embolization coils are typically designed with minimal or no pitch in their primary wind, thereby minimizing the likelihood of blood bypassing the primary wind (20). However, the standard packing density calculation method does not account for the hollow nature of the primary wind (20). While excluding this factor may result in inaccuracies, treating the primary wind as entirely hollow would be even more misleading, as it does not reflect the coils’ functional design. Second-generation hydrogel coils and fibered coils further complicate packing density calculations; the polymer encased within the primary wind and the fibers on the exterior surface increase the coils’ volume, but no methods have been developed to accurately account for these contributions. Consequently, packing density values for hydrogel and fibered coils are usually calculated based only on the metal component, which likely leads to an underestimation of their actual packing density (21-23). A major concern with hydrogel coils is the potential dislodgment of the polymer, which could lead to embolization of the non-target vessels downstream (11). Although such complications have not been reported in the literature, it is imperative to assess the risk of hydrogel dislodgment, particularly given the increasing interest in using these coils for artery sacrifice (e.g., gastroduodenal, renal, and coronary arteries) (24-26). Despite the polymer being encased within the primary wind, our study showed that dislodgment can occur with second-generation peripheral hydrogel coils. Additionally, a considerable number of dislodged fragments were observed, although those measuring ≥1 mm were uncommon. However, it is unknown whether dislodged fragments could migrate to downstream vessels, as thrombosis around the coil may prevent migration and downstream flow can be reduced or stopped. Contrary to our expectations, there were no significant differences in the number of captured fragments or in those measuring ≥1 mm between 0.035-inch and 0.018-inch coils, or between larger and smaller diameter coils. One possible explanation for this result could be the larger interstices that form in the primary wind of the 0.018-inch and smaller diameter coils as they buckle, potentially facilitating hydrogel dislodgment.
Our study has several important limitations. First, the flow model lacks important physiological functions, such as blood coagulation and vascular compliance, limiting the applicability of our results. Second, since first-generation peripheral hydrogel coils were not included, we cannot determine whether second-generation coils offer improvements over their predecessors. Third, the non-inclusion of second-generation coils without the polymer prevents us from assessing the polymer’s impact. Fourth, the small sample size may affect the reliability of our results. Fifth, the packing density was calculated with the assumption that the primary wind of the coil is solid, overlooking the fact that it is actually hollow and could therefore permit blood flow through its interstices. Additionally, this calculation did not account for the presence of hydrogel and fibers. Sixth, our evaluation focused only on individual coils rather than on multiple coils used together; therefore, our findings may not represent the outcomes when multiple coils are utilized together. Finally, the thrombogenicity of coils was not evaluated and compared.
Conclusions
In conclusion, in a flow model, second-generation peripheral hydrogel coils did not demonstrate superior packing densities compared to fibered and bare coils, attributed to their longer embolization lengths. Additionally, these coils may be susceptible to hydrogel dislodgment, potentially leading to embolization of non-target vessels downstream. Further studies are necessary to determine the effectiveness of second-generation coils compared to fibered and bare coils in clinical settings, and to assess the clinical impact of hydrogel dislodgment.
Acknowledgments
None.
Footnote
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2251/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xiao N, Lewandowski RJ. Embolic Agents: Coils. Semin Intervent Radiol 2022;39:113-8. [Crossref] [PubMed]
- Chaer RA, Abularrage CJ, Coleman DM, Eslami MH, Kashyap VS, Rockman C, Murad MH. The Society for Vascular Surgery clinical practice guidelines on the management of visceral aneurysms. J Vasc Surg 2020;72:3S-39S. [Crossref] [PubMed]
- Nassiri N, Cirillo-Penn NC, Thomas J. Evaluation and management of congenital peripheral arteriovenous malformations. J Vasc Surg 2015;62:1667-76. [Crossref] [PubMed]
- Chen J, Stavropoulos SW. Management of Endoleaks. Semin Intervent Radiol 2015;32:259-64. [Crossref] [PubMed]
- Loffroy R, Favelier S, Pottecher P, Estivalet L, Genson PY, Gehin S, Cercueil JP, Krausé D. Transcatheter arterial embolization for acute nonvariceal upper gastrointestinal bleeding: Indications, techniques and outcomes. Diagn Interv Imaging 2015;96:731-44. [Crossref] [PubMed]
- Ferral H. Hydrogel-Coated Coils: Product Description and Clinical Applications. Semin Intervent Radiol 2015;32:343-8. [Crossref] [PubMed]
- Maleux G, Deroose C, Fieuws S, Van Cutsem E, Heye S, Bosmans H, Verslype C. Prospective comparison of hydrogel-coated microcoils versus fibered platinum microcoils in the prophylactic embolization of the gastroduodenal artery before yttrium-90 radioembolization. J Vasc Interv Radiol 2013;24:797-803; quiz 804. [Crossref] [PubMed]
- Perdikakis E, Fezoulidis I, Tzortzis V, Rountas C. Varicocele embolization: Anatomical variations of the left internal spermatic vein and endovascular treatment with different types of coils. Diagn Interv Imaging 2018;99:599-607. [Crossref] [PubMed]
- Hongo N, Kiyosue H, Ota S, Nitta N, Koganemaru M, Inoue M, Nakatsuka S, Osuga K, Anai H, Yasumoto T, Tanoue S, Maruno M, Kamei N, Kichikawa K, Abe T, Hasebe T, Asayama Y. Vessel Occlusion using Hydrogel-Coated versus Nonhydrogel Embolization Coils in Peripheral Arterial Applications: A Prospective, Multicenter, Randomized Trial. J Vasc Interv Radiol 2021;32:602-609.e1. [Crossref] [PubMed]
- Shimohira M, Kawai T, Hashizume T, Muto M, Kitase M, Shibamoto Y. Usefulness of Hydrogel-Coated Coils in Embolization of Pulmonary Arteriovenous Malformations. Cardiovasc Intervent Radiol 2018;41:848-55. [Crossref] [PubMed]
- Chopra AM, Cruz JP, Hu YC. Polymer Embolism from Bioactive and Hydrogel Coil Embolization Technology: Considerations for Product Development. AJNR Am J Neuroradiol 2019;40:E34-5. [Crossref] [PubMed]
- Trerotola SO, Pressler GA, Premanandan C. Nylon Fibered Versus Non-Fibered Embolization Coils: Comparison in a Swine Model. J Vasc Interv Radiol 2019;30:949-55. [Crossref] [PubMed]
- White SB, Wissing ER, Van Alstine WG, Trerotola SO. Comparison of Fibered versus Nonfibered Coils for Venous Embolization in an Ovine Model. J Vasc Interv Radiol 2023;34:888-95. [Crossref] [PubMed]
- Yoon JT, Kwon B, Choi JH, Hwang SM, Kim M, Hwang S, Song Y, Lee DH. In Vitro Head-to-Head Comparison of Flow Reduction between Fibered and Non-Fibered Pushable Coils. Neurointervention 2024;19:31-8. [Crossref] [PubMed]
- Shah NA, Akingboye A, Haldipur N, Mackinlay JY, Jacob G. Embolization coils migrating and being passed per rectum after embolization of a splenic artery pseudoaneurysm, "the migrating coil": a case report. Cardiovasc Intervent Radiol 2007;30:1259-62. [Crossref] [PubMed]
- Dinter DJ, Rexin M, Kaehler G, Neff W. Fatal coil migration into the stomach 10 years after endovascular celiac aneurysm repair. J Vasc Interv Radiol 2007;18:117-20. [Crossref] [PubMed]
- Kao WY, Chiou YY, Chen TS. Coil migration into the common bile duct after embolization of a hepatic artery pseudoaneurysm. Endoscopy 2011;43 Suppl 2 UCTN:E364-5.
- Fohlen A, Namur J, Ghegediban H, Laurent A, Wassef M, Pelage JP. Peripheral Embolization Using Hydrogel-Coated Coils Versus Fibered Coils: Short-Term Results in an Animal Model. Cardiovasc Intervent Radiol 2018;41:305-12. [Crossref] [PubMed]
- Fohlen A, Namur J, Ghegediban H, Laurent A, Wassef M, Pelage JP. Midterm Recanalization after Arterial Embolization Using Hydrogel-Coated Coils versus Fibered Coils in an Animal Model. J Vasc Interv Radiol 2019;30:940-8. [Crossref] [PubMed]
- White JB, Ken CG, Cloft HJ, Kallmes DF. Coils in a nutshell: a review of coil physical properties. AJNR Am J Neuroradiol 2008;29:1242-6. [Crossref] [PubMed]
- Yasumoto T, Osuga K, Yamamoto H, Ono Y, Masada M, Mikami K, Kanamori D, Nakamura M, Tanaka K, Nakazawa T, Higashihara H, Maeda N, Tomiyama N. Long-term outcomes of coil packing for visceral aneurysms: correlation between packing density and incidence of coil compaction or recanalization. J Vasc Interv Radiol 2013;24:1798-807. [Crossref] [PubMed]
- Taschner CA, Chapot R, Costalat V, Machi P, Courthéoux P, Barreau X, et al. GREAT-a randomized controlled trial comparing HydroSoft/HydroFrame and bare platinum coils for endovascular aneurysm treatment: procedural safety and core-lab-assessedangiographic results. Neuroradiology 2016;58:777-86. [Crossref] [PubMed]
- Bendok BR, Abi-Aad KR, Ward JD, Kniss JF, Kwasny MJ, Rahme RJ, et al. The Hydrogel Endovascular Aneurysm Treatment Trial (HEAT): A Randomized Controlled Trial of the Second-Generation Hydrogel Coil. Neurosurgery 2020;86:615-24. [Crossref] [PubMed]
- López-Benítez R, Hallscheidt P, Kratochwil C, Ernst C, Kara L, Rusch O, Vock P, Kettenbach J. Protective embolization of the gastroduodenal artery with a one-HydroCoil technique in radioembolization procedures. Cardiovasc Intervent Radiol 2013;36:105-10. [Crossref] [PubMed]
- Kimura Y, Osuga K, Ono Y, Nakazawa T, Higashihara H, Tomiyama N. Long-Term Outcomes of Selective Renal Artery Embolization for Renal Arteriovenous Fistulae with Dilated Venous Sac. J Vasc Interv Radiol 2018;29:952-7. [Crossref] [PubMed]
- Abdelfattah OM, Saad AM, Kassis N, Shekhar S, Isogai T, Gad MM, Ahuja KR, Hariri E, Kaur M, Farwati M, Khatri J, Krishnaswamy A, Kapadia SR. Utilization and outcomes of transcatheter coil embolization for various coronary artery lesions: Single-center 12-year experience. Catheter Cardiovasc Interv 2021;98:1317-31. [Crossref] [PubMed]



