
Comparison of test-retest repeatability of DESPOT and 3D-QALAS for T1 and T2 mapping
Introduction
Magnetic resonance imaging (MRI) relaxation times are crucial parameters for tissue characterization. The longitudinal relaxation time, or T1, characterizes the exponential restoration of equilibrium spin-state populations. The transverse, or T2, relaxation time characterizes the exponential loss of phase coherence. T1 and T2 index different tissue properties and can be mapped across the brain using quantitative experiments that sample different stages of the relaxation processes. Quantitative assessment of T1 and T2 can enhance the diagnostic accuracy beyond conventional relaxation-weighted MRI in applications ranging from the detection and assessment of myocarditis (1-4), liver fibrosis and cirrhosis (5-9), cartilage degeneration to monitoring disease progression and response to therapy (10-12), neurologic disease (13-17), normal brain development (18,19), and aging (20-22). Quantitative MRI also allows for the objective comparison of pathological conditions across time and between individuals, making it an indispensable tool in both research and clinical settings.
The simplest, and gold standard, method for generating T1 maps involves acquiring images at multiple inversion times (TI), i.e., inversion recovery (IR) sequences, and modeling voxel-by-voxel with a T1 decay curve. However, IR sequences require very long repetition times (TR), resulting in scan times that are impractical for clinical use and may also lead to inaccuracies due to patient motion (23). The gold standard for generating T2 maps involves multi-echo spin-echo sequences. Several techniques for more rapid T1 mapping and combined T1-T2 mapping have been developed, including the modified look-locker IR (MOLLI) (24), shortened MOLLI (shMOLLI) (25), saturation recovery single-shot acquisition (SASHA) (26), true T1 mapping with SMART1Map (27), TrueFISP (28), variable flip angle imaging (29,30), and magnetization-prepared 2 rapid gradient echo (MP2RAGE) (31). Most of these techniques are either designed for T1 mapping only, require a long acquisition time or are 2D mapping methods, which limits their utility in clinical and research settings.
Driven Equilibrium Single Pulse Observation of T1 and T2 (DESPOT) (32,33) and three dimensional using an interleaved Look-Locker acquisition sequence with a T2 preparation pulse (3D-QALAS) (34) are two relatively new, fast 3D techniques that allow mapping of T1 and T2 at high spatial resolution. Here, we refer to DESPOT to collectively include DESPOT1 and DESPOT2 for T1 and T2 mapping, respectively. DESPOT1 acquires a series of spoiled gradient recalled-echo (SPGR) images with the same TR and different flip angles, while DESPOT2 acquires fully balanced steady-state free precession (bSSFP) images at different flip angles with constant TR (32,33). 3D-QALAS (QALAS hereafter), a newer technique, is based on 3D spoiled Turbo Field Echo sequences using IR interleaved T2 preparation. The QALAS acquisition consists of five turbo-fast low-angle shot (FLASH) readouts. A T2-preparation module precedes the first readout, followed by an inversion pulse, so that the following four readouts capture T1 dynamics (34). It combines T1, T2, and proton density (PD) mapping and evaluation of inversion efficiency (IE) in a single acquisition.
Measurements of T1 and T2 maps can be influenced by various factors, including the sequence type, acquisition sampling scheme, reconstruction technique, magnetization transfer, flow effects, T2 effects, and motion (23,35-37). Even for “gold standard” methods, measurements depend on the choice of inversion or echo times sampled. Establishing the repeatability and accuracy of T1 and T2 mapping is crucial for their adoption in clinical practice and longitudinal studies, particularly for newer methods, like DESPOT and QALAS, that map T1 and T2 from a complex set of mixed-contrast images. While other methods such as magnetic resonance fingerprinting (MRF) (38,39), IR-bSSFP or IR-TrueFISP (28), and triple echo steady-state (TESS) (40) are also promising hybrid mapping techniques, the current study focused on DESPOT and QALAS to directly compare two techniques that are currently being applied in multi-site studies. The purpose of this study is to evaluate and compare the test-retest repeatability of T1 and T2 mapping using DESPOT and QALAS techniques. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1870/rc).
Methods
MRI acquisition
Data were acquired from 10 healthy volunteers (5 male, 5 female, ages 23 to 49 years) after obtaining written informed consent in Johns Hopkins University. The sample size was determined based on availability of resources. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Johns Hopkins University (No. IRB00296552). All scans were conducted using a 3.0 Tesla Philips Ingenia Elition MRI scanner, equipped with a 32-channel receive head coil. For anatomical co-registration, we first collected a T1-weighted structural MPRAGE scan using the following parameters: TR/TE 2,000 ms/2 ms, flip angle 8°, 150 1-mm slices, voxel size 1 mm3 isotropic, total time 2 min 46 s. Then, we acquired DESPOT images at 1.3 mm3 isotropic resolution [field of view (FOV) 224×224×166 mm3] using an SPGR sequence with flip angles of 4°, 12°, and 18° and TR/TE of 6.3 ms/3.09 ms, and a bSSFP sequence with flip angles of 15°, 30°, and 60° TR/TE of 6.3 ms/3.09 ms. bSSFP images were acquired with phase cycling patters of 0° and 180° to allow correction for main magnetic field (B0) inhomogeneities (41). A B1 map using actual flip-angle imaging (AFI) (42) was also acquired. The total acquisition time for DESPOT including the field map was 11.87 min. Sensitivity encoding (SENSE) was applied for DESPOT with acceleration factor of 2. Similarly, QALAS was acquired with TR/TE of 5.7 ms/2.3 ms, 1.3 mm3 isotropic resolution, FOV 228×228×166 mm3, flip angle 4°. The five turbo-FLASH readouts were spaced 900 ms apart, and a 100-ms T2-preparation module was used. QALAS sequence parameters were adapted from the collaborative HEALthy Brain and Child Development (HBCD) (43) study (which is actively collecting longitudinal neuroimaging data from approximately 7,200 children), to maintain consistency with established protocols; DESPOT parameters were also based on on-going studies. For this study, we adjusted the FOV to better suit the adult population, and spatial resolution was matched for both sequences. The QALAS protocol also acquires B1 maps, using the 48.4 s long AFI (42) sequence. The total acquisition time for QALAS including the field map was 5.03 min. Compressed sensing (CS) SENSE was enabled for QALAS with acceleration factor of 2.5. The test-retest protocol involved repositioning the subjects between imaging sessions. The typical time between the end of the test scans and the start of the retest scans was approximately 2 min, ensuring that any changes in positioning did not significantly influence the repeatability of the measurements.
T1 and T2 reconstruction and mapping
DESPOT
Following acquisition, SPGR, bSSFP and B1 map, were linearly co-registered to account for subtle head movement (44) and non-parenchyma signal was removed (41). The rapid combined T1 and T2 mapping approaches called DESPOT1 and DESPOT2-FM, respectively were used (32). T1 is estimated from the SPGR data acquired at different flip angles, modeled according to:
where is the SPGR signal intensity as a function of flip angle , is a factor proportional to the equilibrium longitudinal magnetization, and is expressed in Eq. [2]. Similarly, is estimated by fitting the bSSFP images acquired at three different flip angles to the following:
where SISSFP is the SSFP signal intensity associated with flip angle α. NIFTI-format T1 and T2 maps were generated in a python environment using the qmri-neuropipe tool (45).
QALAS
To estimate the QALAS T1 and T2 maps, a dictionary-based matching algorithm (46,47) that incorporates IE and B1 field corrections (48) was used. The dictionary encompassed IE values ranging from 0.75 to 1, in 10 equal steps, B1 field corrections ranging from 0.65 to 1.35, in 25 equal steps, T1 ranges (5:5:1,200 ms, 1,200:10:2,100 ms, 2,100:20:3,100 ms, 3,100:50:5,000 ms), and T2 ranges (1:2:150 ms, 150:5:360 ms, 360:10:1,100 ms, 1,100:100:2,500 ms). The dictionary was generated from simulations of the five sub-experiments using MATLAB R2023b (The MathWorks, Natick, MA, USA) on a high-capacity server cluster. The signals after the IR were adjusted based on their respective IE values. The B1+ inhomogeneity maps generated were applied to the flip angles within the turbo-FLASH readouts to account for spatial variations. The dictionary-matching used to generate T1 and T2 maps in NIFTI format was also performed using MATLAB R2019b on the same server clusters.
Postprocessing
All DESPOT and QALAS test and retest T1 and T2 maps were first co-registered and resliced to the high-resolution T1-weighted image [magnetization-prepared rapid gradient-echo (MP-RAGE)]. Gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) masks were derived from SPM 12 segmentation (49) of the T1-weighted MP-RAGE (with a probability threshold of 0.8) and subsequently applied to the T1 and T2 maps. To ensure consistency across sessions, the reslicing process was performed using the SPM12 tool’s ‘Estimate and Reslice’ function, with the T1-weighted MPRAGE image as the reference. This alignment was applied to both test-retest session acquired images of DESPOT and QALAS, allowing for accurate voxel-to-voxel comparisons. All post-processing, including image co-registration, re-slicing, segmentation, and brain region masking was performed using MATLAB R2023a. Figure 1 shows the post-processing procedures.
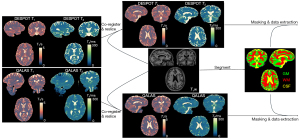
Statistical analysis
After segmentation and masking, histograms were plotted for T1 and T2 measurements across the GM, WM and CSF masks. The average T1 and T2 within each tissue mask was plotted for the test and retest acquisitions of each subject. These values are then used to generate within-subject and between-subject coefficients of variation (CoVs) for test-retest acquisitions, according to:
where the bar represents a mean across subjects, and x and y are the test and retest values, respectively. The between-subject CoV is determined from the ratio of standard deviations (SDs) of test-retest subject mean values and average of these test-retest subject mean values. The test-retest repeatability of DESPOT and QALAS was separately visualized with Bland-Altman plots of the tissue-mask values (x and y) values. Voxel-by-voxel repeatability of the maps was examined using intraclass correlation coefficients (ICCs) to assess the reliability and repeatability of T1 and T2 measurements across the test-retest sessions.
In addition to test and retest evaluation of each method, the agreement between DESPOT and QALAS measurements was visualized using Bland-Altman plots, plotting the difference between DESPOT and QALAS ‘test’ measurements against the average of the two ‘test’ measurements (i.e., ignoring retest for both methods). Voxel-to voxel ICCs were also evaluated between the two methods for ‘test’ measurements. This analysis provided insights into the bias and limits of agreement between the techniques.
Results
Regional T1 and T2 values
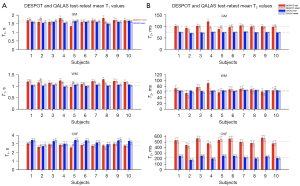
Histograms of T1 and T2 derived from DESPOT and QALAS for a representative subject, for each segmented tissue type, are shown in Figure 2. Test and retest generally show excellent agreement for each of the two methods. While there is slight disagreement between DESPOT and QALAS for T1 and T2 of GM and WM tissues, this is much more pronounced for CSF. The mean T1 and T2 values across GM, WM, and CSF masks for test-retest measurements using DESPOT and QALAS is shown for all 10 subjects in Figure 3.
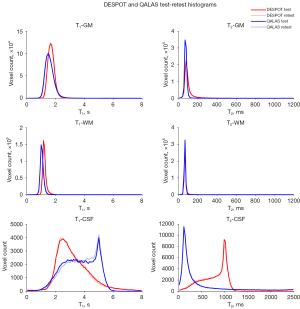
CoVs
Table 1 shows the within- and between-subject CoVs for mean T1 and T2 values across tissue masks, for DESPOT and QALAS. For DESPOT, the within-subject mean CoVs vary from a minimum of 2.1% for T2 in CSF to a maximum of 6.7% for T2 in GM. For QALAS, mean CoVs vary from a minimum of 0.6% for T2 in CSF to a maximum of 5.8% for T2 in WM. The between-subject CoVs for DESPOT range from 3.7% for T2 in WM, to 9.3% for T2 in CSF, and for QALAS range from a minimum of 2.5% for T2 in GM to a maximum of 12% for T2 in CSF. In almost all cases, CoVs were smaller for QALAS than DESPOT.
Table 1
| CoV | T1 | T2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | WM | CSF | GM | WM | CSF | ||||||||||||
| D (%) | Q (%) | D (%) | Q (%) | D (%) | Q (%) | D (%) | Q (%) | D (%) | Q (%) | D (%) | Q (%) | ||||||
| Mean within-subject CoVs | 6.5 (0, 13.7) |
2.1 (0.4, 3.4) |
6.3 (0, 13.2) |
2.2 (0, 4.04) |
4.6 (0.4, 10.6) |
1.9 (0, 4.5) |
6.7 (0, 16.8) |
2.2 (0.03, 3.3) |
5.1 (0.08, 10.5) |
5.8 (0.08, 6.1) |
2.1 (0, 4.5) |
0.6 (0.02, 1.2) |
|||||
| Mean between-subject CoVs | 4.7 | 3.3 | 4.8 | 2.8 | 4.6 | 7.3 | 5.2 | 2.5 | 3.7 | 2.6 | 9.3 | 12.0 | |||||
The numbers in brackets show the minimum and maximum within-subject CoVs from all subjects. CoVs, coefficients of variation; CSF, cerebrospinal fluid; DESPOT, Driven Equilibrium Single Pulse Observation of T1 and T2; GM, gray matter; QALAS, Quantification using an interleaved Look-Locker acquisition Sequence with a T2 preparation pulse; WM, white matter.
Test-retest Bland-Altman plots
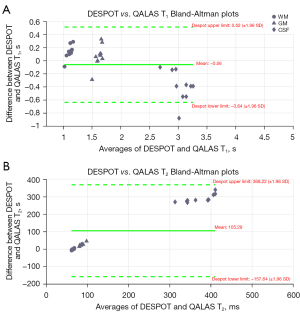
DESPOT and QALAS Bland-Altman plots for GM, WM and CSF T1 and T2 values of 10 subjects are shown in Figure 4. The DESPOT T1 plot shows a mean bias of −0.02 s and relatively wide 95% confidence intervals (CIs) (−0.31 to 0.27 s). The QALAS T1 plot shows a similar bias of −0.02 s with narrower 95% CIs (−0.14 to 0.11 s), indicating good consistency. For T2 measurements, the DESPOT plot shows a mean difference of 2.56 ms with a limit of agreement from −17.29 to 22.41 ms, while the QALAS plot exhibits a smaller mean difference of 1.08 ms and narrower limits of agreement (−1.88 to 4.04 ms).
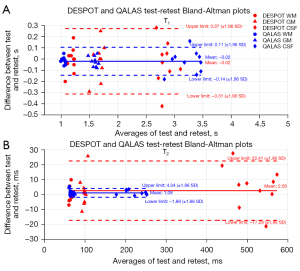
Test-retest voxel-to-voxel comparisons
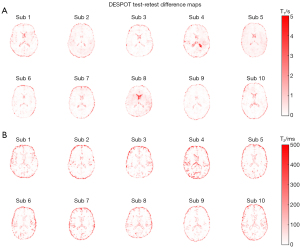
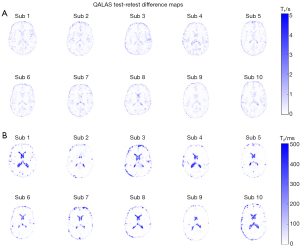
Single representative subject test-retest T1 and T2 scatter plots for DESPOT and QALAS are shown in Figure 5. The ICCs for all subjects are given in Tables 2,3. Higher mean voxel-to-voxel ICCs were observed in DESPOT for T2 (0.901±0.028) and in QALAS for T1 (0.841±0.039). Figures 6,7 illustrate the difference maps between the test and retest sessions of all subjects for DESPOT and QALAS, respectively. Qualitatively, relative lower differences are observed in T1 maps for QALAS and T2 maps for DESPOT. A notable difference is observed in the CSF regions of the QALAS T2 test and retest difference maps.
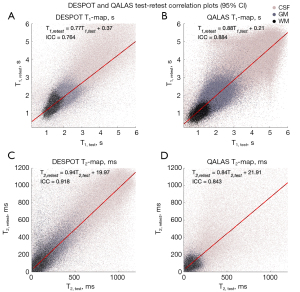
Table 2
| Subject | GM | WM | CSF | Whole brain | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D | Q | D | Q | D | Q | D | Q | ||||
| 1 | 0.635 | 0.559 | 0.623 | 0.426 | 0.633 | 0.677 | 0.754 | 0.846 | |||
| 2 | 0.649 | 0.39 | 0.714 | 0.265 | 0.655 | 0.689 | 0.646 | 0.747 | |||
| 3 | 0.781 | 0.539 | 0.855 | 0.617 | 0.747 | 0.663 | 0.815 | 0.828 | |||
| 4 | 0.538 | 0.476 | 0.498 | 0.457 | 0.577 | 0.642 | 0.707 | 0.882 | |||
| 5 | 0.745 | 0.631 | 0.827 | 0.604 | 0.698 | 0.706 | 0.706 | 0.859 | |||
| 6 | 0.689 | 0.58 | 0.824 | 0.601 | 0.684 | 0.632 | 0.767 | 0.842 | |||
| 7 | 0.635 | 0.588 | 0.77 | 0.572 | 0.696 | 0.766 | 0.756 | 0.862 | |||
| 8 | 0.412 | 0.542 | 0.283 | 0.484 | 0.58 | 0.721 | 0.642 | 0.825 | |||
| 9 | 0.665 | 0.576 | 0.72 | 0.513 | 0.673 | 0.667 | 0.718 | 0.837 | |||
| 10 | 0.711 | 0.693 | 0.74 | 0.627 | 0.756 | 0.804 | 0.764 | 0.884 | |||
| Mean ± SD | 0.646±0.106 | 0.557±0.082 | 0.685±0.177 | 0.517±0.114 | 0.670±0.061 | 0.697±0.054 | 0.728±0.055 | 0.841±0.039 | |||
CSF, cerebrospinal fluid; DESPOT, Driven Equilibrium Single Pulse Observation of T1 and T2; GM, gray matter; ICCs, intraclass correlation coefficients; QALAS, Quantification using an interleaved Look-Locker acquisition Sequence with a T2 preparation pulse; SD, standard deviation; WM, white matter.
Table 3
| Subject | GM | WM | CSF | Whole brain | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D | Q | D | Q | D | Q | D | Q | ||||
| 1 | 0.786 | 0.485 | 0.707 | 0.427 | 0.877 | 0.798 | 0.92 | 0.844 | |||
| 2 | 0.678 | 0.485 | 0.4323 | 0.216 | 0.841 | 0.8 | 0.85 | 0.72 | |||
| 3 | 0.807 | 0.458 | 0.582 | 0.362 | 0.815 | 0.797 | 0.929 | 0.839 | |||
| 4 | 0.605 | 0.445 | 0.414 | 0.352 | 0.774 | 0.7462 | 0.852 | 0.7808 | |||
| 5 | 0.808 | 0.496 | 0.408 | 0.562 | 0.832 | 0.808 | 0.903 | 0.846 | |||
| 6 | 0.806 | 0.458 | 0.599 | 0.491 | 0.829 | 0.802 | 0.916 | 0.842 | |||
| 7 | 0.783 | 0.511 | 0.68 | 0.578 | 0.817 | 0.824 | 0.911 | 0.845 | |||
| 8 | 0.75 | 0.448 | 0.625 | 0.474 | 0.823 | 0.806 | 0.899 | 0.819 | |||
| 9 | 0.876 | 0.486 | 0.626 | 0.485 | 0.844 | 0.806 | 0.911 | 0.84 | |||
| 10 | 0.854 | 0.536 | 0.648 | 0.55 | 0.812 | 0.837 | 0.918 | 0.843 | |||
| Mean ± SD | 0.775±0.081 | 0.481±0.029 | 0.572±0.112 | 0.450±0.113 | 0.826±0.026 | 0.802±0.023 | 0.901±0.028 | 0.822±0.041 | |||
CSF, cerebrospinal fluid; DESPOT, Driven Equilibrium Single Pulse Observation of T1 and T2; GM, gray matter; ICCs, intraclass correlation coefficients; QALAS, Quantification using an interleaved Look-Locker acquisition Sequence with a T2 preparation pulse; SD, standard deviation; WM, white matter.
Evaluation of agreement between DESPOT and QALAS
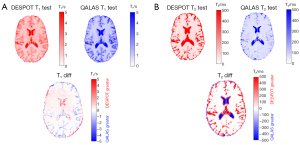
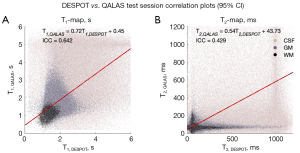
Single-subject single-session T1 and T2 maps from DESPOT and QALAS and the difference between them are shown in Figure 8. Major differences are observed in T2 maps, particularly in CSF region. For the same subject and session, the voxel-by-voxel T1 and T2 correlation plots between DESPOT and QALAS are shown in Figure 9, showing better agreement (ICC) in estimating T1 values than T2 values. The Bland-Altman plot for DESPOT against QALAS is depicted in Figure 10. The T1 plot shows a mean difference of −0.06 s with −0.64 to 0.52 s (95% CI) limits of agreement, while the T2 plot shows a mean difference of 105.29 ms with limits of −157.64 to 368.22 ms, indicating larger variability between the two methods in T2 measurements compared to T1, relative to quantity being measured.
Discussion
Mapping of T1 and T2 is an invaluable technique in the field of quantitative MRI. With a wealth of mapping techniques proposed in the literature, accurately characterizing and comparing the performance of these different methods is paramount to the success of the field. This study specifically investigates the repeatability of the DESPOT and QALAS techniques for T1 and T2 mapping. Both methods showed good test-retest repeatability across different brain regions, with some variations in their performance. For T1 mapping, QALAS exhibited better consistency, both within-subject and between-subject, than DESPOT. This is also evident in Figure 3, where the mean T1 and T2 values obtained from DESPOT across tissues exhibit greater variability compared to QALAS. The voxel-to-voxel ICCs for T1 were higher in QALAS, indicating more reliable performance in capturing T1 values. The Bland-Altman plots for QALAS T1 and T2 measurements also showed lower bias and variability than DESPOT. These findings suggest that QALAS may be more suitable for applications where accurate and consistent T1 mapping is critical, such as in longitudinal studies and clinical assessments of tissue characterization.
It is notable that QALAS achieved slightly better repeatability than DESPOT, in addition to significantly shorter acquisition time. To generate T1 and T2 maps at 1.3 mm3 resolution, the current DESPOT protocol acquired 9 separate images. SPGR images at three flip angles are used to determine T1, while 6 bSSFP images at varying flip angles and phase cycling patterns were used to estimate T2; acquisition of 0° and 180° phase cycling patterns was performed to address banding artifacts caused by main magnetic field inhomogeneities. While T1 and T2 mapping via DESPOT is possible with two varying flip angle SPGR images and two varying flip angle bSSFP images [with phase cycling patterns for B0-inhomogeneity correction (41)], offering opportunities to make the acquisition more efficient, the accuracy in the T1 and T2 model estimates is improved with the additional image information. In contrast, QALAS acquires only 5 images and determines T1 and T2 in a single dictionary-matching step. Therefore, QALAS may provide a more efficient acquisition process, using all 5 images to generate both T1 and T2 maps, optimizing the utility of acquired data. Furthermore, this single-step process is likely more efficient from the perspective of noise propagation.
The clearest difference between DESPOT and QALAS lies in the T2 imaging of CSF. Histograms of areas with CSF probability over 0.8 show a large bias—with modal values of ~1 s for DESPOT and ~150 ms for QALAS, the longer values being closer to literature precedent (50). Reviewing the QALAS T2 images indicates that the larger CSF spaces, such as the lateral ventricles, reports long T2s in the core-CSF, and similarly shortened values at the edges, indicating a clear partial-voluming effect. The majority of voxels segmented as CSF are sulcal, rather than ventricular, and so the histograms primarily reflect sulcal CSF, which is often partial-volumed with GM to some extent. The ability of QALAS to determine T2 arises from a single T2-preparation module, which in our experiments was 100 ms in duration. This results in relatively good T2 contrast for T2 ~100 ms and relatively poor contrast for long-T2s. This increased contrast sensitivity at short-T2s may also be reflected in a non-linear response of the dictionary look-up to partial voluming. The relatively low resolution of imaging applied here (1.3 mm)3 accentuates the impact of partial voluming. To improve the precision of T2 quantification across the full range, it would be beneficial to incorporate additional T2-preparation pulses in the QALAS sequence to better suppress the longer T2 signals from CSF.
Relaxometry has a number of promising applications in the diagnosis and characterization of several diseases e.g., multiple sclerosis (51), stroke (52), and epilepsy (53,54). Historically, acquisition time was the main barrier to the clinical application of relaxometry mapping techniques, but the advent of rapid acquisition approaches, like those studied here, has circumvented this. The remaining barriers are the accessibility of new techniques, somewhat assuaged by the development of open-source sequence frameworks (55-57), and the establishment of measurement uncertainties, which is the focus of our own study.
The clinical utility of DESPOT for rapid T1 and T2 mapping, emphasizing its speed and feasibility for clinical applications, has been previously studied (32,33). DESPOT’s acquisition scheme makes it attractive for clinical use, especially in scenarios requiring rapid and high-resolution mapping of relaxation times. On the other hand, the capability of QALAS for simultaneous T1 and T2 mapping in the heart (34) and brain (58) has highlighted its efficiency in capturing multiple parameters in a single acquisition. This versatility is extended to GM, WM and CSF brain regions in our study, demonstrating QALAS’s reliability for T1 mapping with good ICC values and minimal bias observed in Bland-Altman plots. This method’s ability to integrate T1, T2, and PD mapping in one short scan makes it a powerful, clinically viable tool for comprehensive tissue characterization. However, QALAS showed lower consistency in T2 measurements between test and retest compared to DESPOT, as evident in the difference images (Figures 6,7) and Table 3, but performed better for T1. This may stem from the sequence’s sensitivity to noise and partial volume effects due to its fast acquisition strategy, leading to reduced SNR. Additional factors such as B1 inhomogeneity or scanner drift between sessions could further compromise T2 mapping accuracy.
Our findings add to the body of T1 and T2 mapping literature by directly comparing DESPOT and QALAS, demonstrating their respective strengths in repeatability and mapping accuracy. The mean T1 values for both DESPOT and QALAS exhibit similar distributions across GM, WM, and CSF. However, in DESPOT, GM shows a longer mean T2 compared to WM, whereas QALAS reveals only a slight T2 difference between the two tissues (see Figure 4). The DESPOT T1 values of this study were within acceptable agreement with prior 3T DESPOT T1 measurements (59) in GM (~1.6 s) and WM (~1 s). The DESPOT T2 values were on average higher than those found in (41) for GM (~75 ms) and WM (~50 ms). QALAS T1 values are significantly longer than in prior 3T QALAS work (60) (~1.5 vs. ~1.0 s in GM and ~1.0 vs. ~0.6 s in WM). QALAS T2 values are closer to (60) (~75 vs. 90 ms in GM and ~65 vs. 80 ms in WM). Both our T1 and T2 values agree with broader consensus (61), and prior work (46) using a similar analysis pipeline. The voxel-wise intra- and inter-site reproducibility of T1 and T2 measurements using DESPOT1 and DESPOT2 at 1.5 T, was evaluated and found to be robust (62). The repeatability of DESPOT has also been discussed in terms of percentage SD of T1 and T2 measurements from WM regions (i.e., 6.5% and 5.5%) (32) similar to these current findings. One prior QALAS repeatability study (60) showed a mean intrasubject CoV of 1.9%, similar or slightly better than these findings, although calculated across specific anatomical regions. When compared with other multiparametric mapping techniques, such as ME-MP2RAGE (31,63) and MRF (64,65), the presented methods demonstrated similar within-subject repeatability. Another multiparameter mapping protocol based on vendor product sequences [i.e., FLASH with magnetic transfer (MT), T1, and PD contrast weightings] reported an average intra-site CoV of 7% for T1 and 16% for T2 (66). Taken together, these findings imply both QALAS and DESPOT can be considered robust multiparameter mapping methods.
This is a study of modest scope, with several limitations. Firstly, the study was conducted in a small sample size of 10 healthy volunteers, which may not fully capture the variability in a larger, more diverse population. Secondly, the resolution of mapping was limited to 1.3 mm3 isotropic resolution, leading to meaningful partial voluming of cortical GM. Thirdly, only two relaxometry methods are compared. Future studies should include a larger cohort and investigate the reproducibility of these techniques in pathological conditions. Additionally, aberrant T1 and T2 values observed in some voxels (see Figure 2) could likely be due to partial volume artifacts. Improvements in the reconstruction algorithms could further enhance the repeatability and accuracy of both DESPOT and QALAS. We also acknowledge that, with quick subject repositioning between scans, this study does not fully assess inter-scan variability seen when imaging a subject at multiple time points on different scanners or software versions. While the findings establish test-retest repeatability under controlled conditions, further studies are needed to evaluate reproducibility in diverse clinical scenarios.
Conclusions
In summary, this study quantifies the test-retest repeatability of DESPOT and QALAS for T1 and T2 mapping. Given the shorter acquisition time and the slightly better results, QALAS appears to be more reliable, particularly for T1 measurements, than DESPOT.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1870/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1870/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Johns Hopkins University (No. IRB00296552). Written informed consent was obtained from the volunteers.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferreira VM, Piechnik SK, Robson MD, Neubauer S, Karamitsos TD. Myocardial tissue characterization by magnetic resonance imaging: novel applications of T1 and T2 mapping. J Thorac Imaging 2014;29:147-54. [Crossref] [PubMed]
- Bohnen S, Radunski UK, Lund GK, Ojeda F, Looft Y, Senel M, Radziwolek L, Avanesov M, Tahir E, Stehning C, Schnackenburg B, Adam G, Blankenberg S, Muellerleile K. Tissue characterization by T1 and T2 mapping cardiovascular magnetic resonance imaging to monitor myocardial inflammation in healing myocarditis. Eur Heart J Cardiovasc Imaging 2017;18:744-51. [Crossref] [PubMed]
- Kim PK, Hong YJ. Im DJ, Suh YJ, Park CH, Kim JY, Chang S, Lee HJ, Hur J, Kim YJ, Choi BW. Myocardial T1 and T2 Mapping: Techniques and Clinical Applications. Korean J Radiol 2017;18:113-31. [Crossref] [PubMed]
- O'Brien AT, Gil KE, Varghese J, Simonetti OP, Zareba KM. T2 mapping in myocardial disease: a comprehensive review. J Cardiovasc Magn Reson 2022;24:33. [Crossref] [PubMed]
- Mesropyan N, Kupczyk PA, Dold L, Praktiknjo M, Chang J, Isaak A, Endler C, Kravchenko D, Bischoff LM, Sprinkart AM, Pieper CC, Kuetting D, Jansen C, Attenberger UI, Luetkens JA. Assessment of liver cirrhosis severity with extracellular volume fraction MRI. Sci Rep 2022;12:9422. [Crossref] [PubMed]
- Hoffman DH, Ayoola A, Nickel D, Han F, Chandarana H, Shanbhogue KP. T1 mapping, T2 mapping and MR elastography of the liver for detection and staging of liver fibrosis. Abdom Radiol (NY) 2020;45:692-700. [Crossref] [PubMed]
- Haimerl M, Verloh N, Zeman F, Fellner C, Müller-Wille R, Schreyer AG, Stroszczynski C, Wiggermann P. Assessment of clinical signs of liver cirrhosis using T1 mapping on Gd-EOB-DTPA-enhanced 3T MRI. PLoS One 2013;8:e85658. [Crossref] [PubMed]
- Luetkens JA, Klein S, Träber F, Schmeel FC, Sprinkart AM, Kuetting DLR, Block W, Uschner FE, Schierwagen R, Hittatiya K, Kristiansen G, Gieseke J, Schild HH, Trebicka J, Kukuk GM. Quantification of Liver Fibrosis at T1 and T2 Mapping with Extracellular Volume Fraction MRI: Preclinical Results. Radiology 2018;288:748-54. [Crossref] [PubMed]
- Breit HC, Block KT, Winkel DJ, Gehweiler JE, Henkel MJ, Weikert T, Stieltjes B, Boll DT, Heye TJ. Evaluation of liver fibrosis and cirrhosis on the basis of quantitative T1 mapping: Are acute inflammation, age and liver volume confounding factors? Eur J Radiol 2021;141:109789. [Crossref] [PubMed]
- Cao G, Gao S, Xiong B. Application of quantitative T1, T2 and T2* mapping magnetic resonance imaging in cartilage degeneration of the shoulder joint. Sci Rep 2023;13:4558. [Crossref] [PubMed]
- Mittal S, Pradhan G, Singh S, Batra R. T1 and T2 mapping of articular cartilage and menisci in early osteoarthritis of the knee using 3-Tesla magnetic resonance imaging. Pol J Radiol 2019;84:e549-64. [Crossref] [PubMed]
- Zhao H, Li H, Liang S, Wang X, Yang F. T2 mapping for knee cartilage degeneration in young patients with mild symptoms. BMC Med Imaging 2022;22:72. [Crossref] [PubMed]
- Shah NJ, Neeb H, Zaitsev M, Steinhoff S, Kircheis G, Amunts K, Häussinger D, Zilles K. Quantitative T1 mapping of hepatic encephalopathy using magnetic resonance imaging. Hepatology 2003;38:1219-26. [Crossref] [PubMed]
- Vrenken H, Geurts JJ, Knol DL, van Dijk LN, Dattola V, Jasperse B, van Schijndel RA, Polman CH, Castelijns JA, Barkhof F, Pouwels PJ. Whole-brain T1 mapping in multiple sclerosis: global changes of normal-appearing gray and white matter. Radiology 2006;240:811-20. [Crossref] [PubMed]
- Lescher S, Jurcoane A, Veit A, Bähr O, Deichmann R, Hattingen E. Quantitative T1 and T2 mapping in recurrent glioblastomas under bevacizumab: earlier detection of tumor progression compared to conventional MRI. Neuroradiology 2015;57:11-20. [Crossref] [PubMed]
- Müller A, Jurcoane A, Kebir S, Ditter P, Schrader F, Herrlinger U, Tzaridis T, Mädler B, Schild HH, Glas M, Hattingen E. Quantitative T1-mapping detects cloudy-enhancing tumor compartments predicting outcome of patients with glioblastoma. Cancer Med 2017;6:89-99. [Crossref] [PubMed]
- Eminian S, Hajdu SD, Meuli RA, Maeder P, Hagmann P. Rapid high resolution T1 mapping as a marker of brain development: Normative ranges in key regions of interest. PLoS One 2018;13:e0198250. [Crossref] [PubMed]
- Grossman R, Hoffman C, Mardor Y, Biegon A. Quantitative MRI measurements of human fetal brain development in utero. Neuroimage 2006;33:463-70. [Crossref] [PubMed]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex 1996;6:551-60. [Crossref] [PubMed]
- Kumar R, Delshad S, Woo MA, Macey PM, Harper RM. Age-related regional brain T2-relaxation changes in healthy adults. J Magn Reson Imaging 2012;35:300-8. [Crossref] [PubMed]
- Callaghan MF, Freund P, Draganski B, Anderson E, Cappelletti M, Chowdhury R, Diedrichsen J, Fitzgerald TH, Smittenaar P, Helms G, Lutti A, Weiskopf N. Widespread age-related differences in the human brain microstructure revealed by quantitative magnetic resonance imaging. Neurobiol Aging 2014;35:1862-72. [Crossref] [PubMed]
- Carey D, Caprini F, Allen M, Lutti A, Weiskopf N, Rees G, Callaghan MF, Dick F. Quantitative MRI provides markers of intra-, inter-regional, and age-related differences in young adult cortical microstructure. Neuroimage 2018;182:429-40. [Crossref] [PubMed]
- Studler U, White LM, Andreisek G, Luu S, Cheng HL, Sussman MS. Impact of motion on T1 mapping acquired with inversion recovery fast spin echo and rapid spoiled gradient recalled-echo pulse sequences for delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in volunteers. J Magn Reson Imaging 2010;32:394-8. [Crossref] [PubMed]
- Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52:141-6. [Crossref] [PubMed]
- Piechnik SK, Ferreira VM, Dall'Armellina E, Cochlin LE, Greiser A, Neubauer S, Robson MD. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson 2010;12:69. [Crossref] [PubMed]
- Chow K, Flewitt JA, Green JD, Pagano JJ, Friedrich MG, Thompson RB. Saturation recovery single-shot acquisition (SASHA) for myocardial T(1) mapping. Magn Reson Med 2014;71:2082-95. [Crossref] [PubMed]
- Slavin GS, Stainsby JA. True T1 mapping with SMART1Map (saturation method using adaptive recovery times for cardiac T1 mapping): a comparison with MOLLI. Journal of Cardiovascular Magnetic Resonance 2013;15:3. [Crossref]
- Schmitt P, Griswold MA, Jakob PM, Kotas M, Gulani V, Flentje M, Haase A. Inversion recovery TrueFISP: quantification of T(1), T(2), and spin density. Magn Reson Med 2004;51:661-7. [Crossref] [PubMed]
- Fram EK, Herfkens RJ, Johnson GA, Glover GH, Karis JP, Shimakawa A, Perkins TG, Pelc NJ. Rapid calculation of T1 using variable flip angle gradient refocused imaging. Magn Reson Imaging 1987;5:201-8. [Crossref] [PubMed]
- Heule R, Ganter C, Bieri O. Variable flip angle T1 mapping in the human brain with reduced T2 sensitivity using fast radiofrequency-spoiled gradient echo imaging. Magn Reson Med 2016;75:1413-22. [Crossref] [PubMed]
- Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 2010;49:1271-81. [Crossref] [PubMed]
- Deoni SC, Rutt BK, Peters TM. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn Reson Med 2003;49:515-26. [Crossref] [PubMed]
- Deoni SC, Peters TM, Rutt BK. High-resolution T1 and T2 mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. Magn Reson Med 2005;53:237-41. [Crossref] [PubMed]
- Kvernby S, Warntjes MJ, Haraldsson H, Carlhäll CJ, Engvall J, Ebbers T. Simultaneous three-dimensional myocardial T1 and T2 mapping in one breath hold with 3D-QALAS. J Cardiovasc Magn Reson 2014;16:102. [Crossref] [PubMed]
- Roujol S, Weingärtner S, Foppa M, Chow K, Kawaji K, Ngo LH, Kellman P, Manning WJ, Thompson RB, Nezafat R. Accuracy, precision, and reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology 2014;272:683-9. [Crossref] [PubMed]
- Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz-Menger J, Schelbert EBSociety for Cardiovascular Magnetic Resonance Imaging. Cardiovascular Magnetic Resonance Working Group of the European Society of Cardiology. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013;15:92. [Crossref] [PubMed]
- Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson 2014;16:2. [Crossref] [PubMed]
- Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA. Magnetic resonance fingerprinting. Nature 2013;495:187-92. [Crossref] [PubMed]
- Dikaios N, Protonotarios NE, Fokas AS, Kastis GA. Quantification of T1, T2 relaxation times from Magnetic Resonance Fingerprinting radially undersampled data using analytical transformations. Magn Reson Imaging 2021;80:81-9. [Crossref] [PubMed]
- Heule R, Ganter C, Bieri O. Triple echo steady-state (TESS) relaxometry. Magn Reson Med 2014;71:230-7. [Crossref] [PubMed]
- Deoni SC. Transverse relaxation time (T2) mapping in the brain with off-resonance correction using phase-cycled steady-state free precession imaging. J Magn Reson Imaging 2009;30:411-7. [Crossref] [PubMed]
- Yarnykh VL. Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med 2007;57:192-200. [Crossref] [PubMed]
- Jordan CJ, Weiss SRB, Howlett KD, Freund MP. Introduction to the Special Issue on "Informing Longitudinal Studies on the Effects of Maternal Stress and Substance Use on Child Development: Planning for the HEALthy Brain and Child Development (HBCD) Study". Advers Resil Sci 2020;1:217-21. [Crossref] [PubMed]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17:825-41. [Crossref] [PubMed]
- Dean DC 3rd, Adluru N, Guerrero J. QMRI-neuropipe: A flexible software framework for the analysis of quantitative MRI data. 2023 ISMRM & ISMRT Annual Meeting & Exhibition. Toronto: Metro Toronto Convention Centre (MTCC); 2023.
- Cho J, Gagoski B, Kim TH, Wang F, Manhard MK, Dean D 3rd, Kecskemeti S, Caprihan A, Lo WC, Splitthoff DN, Liu W, Polak D, Cauley S, Setsompop K, Grant PE, Bilgic B. Time-efficient, high-resolution 3T whole-brain relaxometry using 3D-QALAS with wave-CAIPI readouts. Magn Reson Med 2024;91:630-9. [Crossref] [PubMed]
- Ben-Eliezer N, Sodickson DK, Block KT. Rapid and accurate T2 mapping from multi-spin-echo data using Bloch-simulation-based reconstruction. Magn Reson Med 2015;73:809-17. [Crossref] [PubMed]
- Ma D, Coppo S, Chen Y, McGivney DF, Jiang Y, Pahwa S, Gulani V, Griswold MA. Slice profile and B(1) corrections in 2D magnetic resonance fingerprinting. Magn Reson Med 2017;78:1781-9. [Crossref] [PubMed]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26:839-51. [Crossref] [PubMed]
- Spijkerman JM, Petersen ET, Hendrikse J, Luijten P, Zwanenburg JJM T. (2) mapping of cerebrospinal fluid: 3 T versus 7 T. MAGMA 2018;31:415-24. [Crossref] [PubMed]
- Granziera C, Wuerfel J, Barkhof F, Calabrese M, De Stefano N, Enzinger C, Evangelou N, Filippi M, Geurts JJG, Reich DS, Rocca MA, Ropele S, Rovira À, Sati P, Toosy AT, Vrenken H, Gandini Wheeler-Kingshott CAM, Kappos LMAGNIMS Study Group. Quantitative magnetic resonance imaging towards clinical application in multiple sclerosis. Brain 2021;144:1296-311. [Crossref] [PubMed]
- Bernarding J, Braun J, Hohmann J, Mansmann U, Hoehn-Berlage M, Stapf C, Wolf KJ, Tolxdorff T. Histogram-based characterization of healthy and ischemic brain tissues using multiparametric MR imaging including apparent diffusion coefficient maps and relaxometry. Magn Reson Med 2000;43:52-61. [Crossref] [PubMed]
- Chen H, Yu G, Wang J, Li F, Li G. Application of T2 relaxometry in lateralization and localization of mesial temporal lobe epilepsy and corresponding comparison with MR volumetry. Acta Radiol 2016;57:1107-13. [Crossref] [PubMed]
- Pell GS, Briellmann RS, Waites AB, Abbott DF, Jackson GD. Voxel-based relaxometry: a new approach for analysis of T2 relaxometry changes in epilepsy. Neuroimage 2004;21:707-13. [Crossref] [PubMed]
- Karakuzu A, Biswas L, Cohen-Adad J, Stikov N. Vendor-neutral sequences and fully transparent workflows improve inter-vendor reproducibility of quantitative MRI. Magn Reson Med 2022;88:1212-28. [Crossref] [PubMed]
- Layton KJ, Kroboth S, Jia F, Littin S, Yu H, Leupold J, Nielsen JF, Stöcker T, Zaitsev M. Pulseq: A rapid and hardware-independent pulse sequence prototyping framework. Magn Reson Med 2017;77:1544-52. [Crossref] [PubMed]
- Cordes C, Konstandin S, Porter D, Günther M. Portable and platform-independent MR pulse sequence programs. Magn Reson Med 2020;83:1277-90. [Crossref] [PubMed]
- Yamashita K, Yoneyama M, Kikuchi K, Wada T, Murazaki H, Watanuki H, Mikayama R, Ishigami K, Togao O. Reproducibility of quantitative ADC, T1, and T2 measurement on the cerebral cortex: Utility of whole brain echo-planar DWI with compressed SENSE (EPICS-DWI): A pilot study. Eur J Radiol Open 2023;11:100516. [Crossref] [PubMed]
- Deoni SC. High-resolution T1 mapping of the brain at 3T with driven equilibrium single pulse observation of T1 with high-speed incorporation of RF field inhomogeneities (DESPOT1-HIFI). J Magn Reson Imaging 2007;26:1106-11. [Crossref] [PubMed]
- Fujita S, Hagiwara A, Hori M, Warntjes M, Kamagata K, Fukunaga I, Andica C, Maekawa T, Irie R, Takemura MY, Kumamaru KK, Wada A, Suzuki M, Ozaki Y, Abe O, Aoki S. Three-dimensional high-resolution simultaneous quantitative mapping of the whole brain with 3D-QALAS: An accuracy and repeatability study. Magn Reson Imaging 2019;63:235-43. [Crossref] [PubMed]
- Stikov N, Boudreau M, Levesque IR, Tardif CL, Barral JK, Pike GB. On the accuracy of T1 mapping: searching for common ground. Magn Reson Med 2015;73:514-22. [Crossref] [PubMed]
- Deoni SCL, Williams SCR, Jezzard P, Suckling J, Murphy DGM, Jones DK. Standardized structural magnetic resonance imaging in multicentre studies using quantitative T1 and T2 imaging at 1.5 T. Neuroimage 2008;40:662-71. [Crossref] [PubMed]
- Metere R, Kober T, Möller HE, Schäfer A. Simultaneous Quantitative MRI Mapping of T1, T2* and Magnetic Susceptibility with Multi-Echo MP2RAGE. PLoS One 2017;12:e0169265. [Crossref] [PubMed]
- Buonincontri G, Biagi L, Retico A, Cecchi P, Cosottini M, Gallagher FA, Gómez PA, Graves MJ, McLean MA, Riemer F, Schulte RF, Tosetti M, Zaccagna F, Kaggie JD. Multi-site repeatability and reproducibility of MR fingerprinting of the healthy brain at 1.5 and 3.0 T. Neuroimage 2019;195:362-72. [Crossref] [PubMed]
- Körzdörfer G, Kirsch R, Liu K, Pfeuffer J, Hensel B, Jiang Y, Ma D, Gratz M, Bär P, Bogner W, Springer E, Lima Cardoso P, Umutlu L, Trattnig S, Griswold M, Gulani V, Nittka M. Reproducibility and Repeatability of MR Fingerprinting Relaxometry in the Human Brain. Radiology 2019;292:429-37. [Crossref] [PubMed]
- Leutritz T, Seif M, Helms G, Samson RS, Curt A, Freund P, Weiskopf N. Multiparameter mapping of relaxation (R1, R2*), proton density and magnetization transfer saturation at 3 T: A multicenter dual-vendor reproducibility and repeatability study. Hum Brain Mapp 2020;41:4232-47. [Crossref] [PubMed]


