
Relationship between imaging, clinical, pathological features, and alpha-fetoprotein-producing gastric cancer: diagnostic performance of enhanced CT conventional features
Introduction
Alpha-fetoprotein (AFP) is produced in the liver and yolk sac (1). Elevated AFP levels in adults are associated with certain types of tumors, including hepatocellular carcinoma, germ cell tumors, and in rare cases, gastric cancer (GC) (2,3). Alpha-fetoprotein-producing gastric cancer (AFPGC) is a specific subtype of GC in which tumor cells produce elevated AFP levels. This subtype is uncommon, accounting for only a small percentage of all GC cases (4). Bourreille et al. first reported a case of AFPGC with liver metastasis in 1970 (5). Subsequent studies have revealed similar cases, and AFPGC has been consistently associated with tumor progression, liver metastasis, and an unfavorable prognosis (6,7).
This unique GC subtype challenges traditional paradigms and has a triad of distinctive features, including clinical manifestations, pathological characteristics, and radiological attributes. The hallmark of this subtype is an elevated serum AFP level, which often serves as a potential diagnostic indicator. Histologically, AFP-producing gastric adenocarcinomas can manifest as various subtypes (4).
Computed tomography (CT) is a non-invasive tool for the preoperative evaluation of GC. CT manifestations of GC primarily involve the thickening of the gastric wall, the narrowing of the gastric cavity, ulcer formation, serosal invasion, necrosis, cystic changes, and lymph node metastasis (8). These indicators play crucial roles in the early diagnosis and treatment of GC. Recent studies have highlighted the important contribution of CT in distinguishing between GC subtypes. For example, Li et al. showed that standardized arterial phase iodine concentration values can help to differentiate between poorly differentiated GCs (9). Zeydanli et al. showed that texture features derived from venous phase-enhanced CT images are not only effective in distinguishing between gastric adenocarcinoma, gastric lymphoma, and gastrointestinal stromal tumors, but are also effective in distinguishing between well-differentiated and poorly differentiated adenocarcinomas (10). Xu et al. identified differences in the clinicopathological features and CT manifestations between gastric papillary and tubular adenocarcinomas. By amalgamating demographic data, tumor markers, CT morphological features, and CT value-related parameters, Xu et al. found that a satisfactory diagnostic efficiency could be attained for the preoperative identification of papillary gastric adenocarcinoma (11).
However, current imaging reports on AFPGC present a potential challenge to radiologists and clinicians. Some research on the clinical and pathological aspects of AFPGC has been conducted; however, few studies have focused on its imaging characteristics and how they differ from those of typical GC. Current diagnostic protocols are insufficient for distinguishing AFPGC from other GC subtypes, which can lead to potential delays in diagnosis and treatment. This study ultimately aimed to provide a comprehensive understanding of the unique imaging features of AFPGC to assist clinicians to differentiate between AFPGC and other GC subtypes and improve the diagnostic accuracy of this disease. This could also facilitate the early diagnosis and development of tailored treatment strategies, and ultimately improve the outcomes of patients with this rare and challenging form of GC. We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1867/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Lanzhou University Second Hospital (approval No. [2023A-749]), and the requirement of individual consent for this retrospective analysis was waived.
Study design and population
This retrospective study focused on routine medical examinations at healthcare facilities. Currently, the most effective and convenient method for assessing AFPGC involves determining the serum AFP level. As per existing literature, after excluding hepatitis, cirrhosis, and primary liver cancer, a serum AFP level of ≥20 ng/mL within 2 weeks before surgery in GC patients is recognized as an independent indicator for diagnosis and prognosis evaluation (12-14). We retrospectively collected the pathological and imaging data of patients diagnosed with AFPGC at Lanzhou University Second Hospital between 2019 and 2021. The patients were selected using a consecutive sampling method. To be eligible for inclusion in the study, the patients had to meet the following inclusion criteria: (I) have a histopathological diagnosis of gastric adenocarcinoma through biopsy or postoperative pathology, and the patient’s serum AFP level was ≥20 ng/mL within 2 weeks before surgery; (II) have undergone abdominal contrast-enhanced CT 2 weeks before the operation; and (III) have not previously undergone chemotherapy before the CT examination or surgery. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had incomplete clinical or pathological data; (II) had lesions that were not identifiable on CT images; and/or (III) had cirrhosis or active hepatitis (Figure 1). As controls, we randomly selected 100 conventional gastric cancer (CGC) samples from the surgical specimen archives at Lanzhou University Second Hospital, covering the period from 2019 to 2021.
The clinical characteristic data of the patients, including age, sex, tumor location, surgery date, and serum levels of AFP, carcinoembryonic antigen (CEA), cancer antigen 125 (CA125), and cancer antigen 199 (CA199), were systematically collected from the hospital information system. Patient follow-up regarding metastatic status was meticulously performed using the medical records and radiological examinations spanning a follow-up period ranging from 12 to 36 months. Based on previously reported literature, the AFP cut-off was set at 100 ng/mL, and the patients were divided into high AFP (AFP-H) and low AFP (AFP-L) groups accordingly to further explore the relationship between the AFP levels and clinicopathological features, as well as the imaging characteristics (15).
CT imaging acquisition process
The patients underwent a fasting period of 10–12 hours before the examination and consumed 800–1,000 mL of purified water at divided doses 1 hour before the examination to distend the stomach. The CT examinations were performed using a GE Discovery CT750 HD scanner. The scanning range extended from the diaphragmatic apex to the anterosuperior iliac spine. Both plain and three-phase contrast-enhanced scans were performed in all cases. The scanning parameters were as follows: 120 kV, 320 mA, a slice thickness of 5 mm, a pitch of 1.0, a matrix of 512×512, and a high-pressure injection for contrast. A non-ionic contrast agent at a concentration of 370 mg/mL (Iohexol, 80–100 mL) was administered via the right cubital vein, followed by 40 mL of normal saline. Upon reaching a threshold of 100 Hounsfield units (HU) in the aorta, an arterial phase scan was automatically triggered. Images of the portal venous phase were acquired 30 seconds later, followed by a delayed phase at 60 seconds. The scan data were automatically reconstructed with a 1.25-mm slice thickness, transferred to the workstation, and subjected to post-processing techniques, including multiplanar reconstruction and maximum density projection, to visualize the tumor morphology and its relationship with the surrounding tissues.
Radiologic assessment
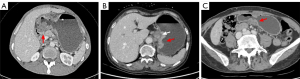
Two radiologists, reader 1 (Q.Z.) with 13 years of experience and reader 2 (C.X.) with 10 years of experience, performed the radiological evaluation without prior knowledge of the histological results. The evaluation adhered to established methods, and the following CT imaging features were assessed: (I) tumor growth location (cardia, gastric body, or gastric antrum); (II) axial maximum length of the tumor; (III) axial maximum thickness of the tumor; (IV) presence or absence of serosal infiltration (Figure 2A); (V) presence or absence of tumor ulceration (Figure 2B); (VI) presence or absence of tumor necrosis (which was considered present when the low-density CT value within the lesion was <20 HU or when there was no enhancement in the low-density area) (Figure 2B); (VII) enhancement uniformity (uniformity was defined as a difference between the strongest and weakest enhanced parts of the lesion of <10 HU, and non-uniformity as a difference of ≥10 HU) (Figure 2C); (VIII) the CT values of the solid area of the tumor, which were measured and recorded in the plain phase and at each phase after three-phase enhancement (the area of interest was outlined in the axial position of the tumor at its maximum and most precise level, avoiding the necrotic area); and (IX) the short axis length of the largest lymph node (lymph nodes <5 mm were recorded as 0 mm) (16). All cases were initially scored individually and then jointly reviewed by both readers 1 week after the individual assessments.
Immunohistochemistry
Consecutive tissue sections (4-µm thick) prepared from formalin-fixed and paraffin-embedded tissues were subjected to immunohistochemical staining. The immunohistochemical staining was performed on the Ventana automated staining system (BenchMark ULTRA). The following antigens were detected: AFP (EP209, 1:1,000; Maixin, Fujian, China), glypican 3 (clone MAXIM001, 1:200; Maixin), spalt like transcription factor 4 (SALL4; clone 6E3, 1:100; Maixin), and hepatocyte paraffin-1 (HepPar-1) (MX119, 1:100; Maixin).
Histologic and immunohistochemistry assessment
Postoperative histopathological specimens and immunohistochemical staining results were evaluated by two pathologists: reader 1 (P.Z.) with 9 years of work experience and reader 2 (F.W.) with 26 years of work experience. Both pathologists had received subspecialty training in gastrointestinal pathology. The evaluation included various aspects, including gross classification, histological classification, Lauren classification, differentiation grade, lymphatic or vascular invasion, depth of invasion (pT staging), and lymph node metastasis, for all patients. Histological classification covered intestinal-type adenocarcinoma, hepatoid adenocarcinoma, yolk sac tumor-like adenocarcinoma, and other subtypes. Tumor staging was performed according to the guidelines outlined in the eighth edition of the American Joint Committee on Cancer (17). In the assessment of AFP, glypican-3, HepPar-1, and SALL4, the staining of AFP and HepPar-1 in the cytoplasm and both the cytoplasm and cell membrane of glypican-3 were considered positive; however, for SALL4, only nuclear staining was considered positive.
Statistical analysis
The normally distributed quantitative variables are reported as the mean and standard deviation, and were compared using the independent sample t-test. The non-normally distributed quantitative variables are reported as the quartile range (Q1 and Q3), and were compared using the Mann-Whitney U test. The qualitative variables are presented as the raw number and percentage, and the chi-square test or Fisher’s exact test was employed for comparisons. All the tests were two-tailed, and a P value ≤0.05 was considered statistically significant. The statistical analyses were performed using SPSS software (version 26.0; IBM Corp.) and R Software (version 4.2.3; http://www.rproject.org).
Intraobserver consistency was assessed using intraclass correlation coefficients (ICCs) derived from a two-way random-effects mixed variance analysis model. ICCs were employed to evaluate the continuous radiological features, while the Cohen kappa value was used to assess the consistency of the categorical radiological features. The reported ICCs were calculated with corresponding 95% confidence intervals (CIs), with ICC values <0.5 indicating poor reliability, ICC values of 0.5–0.75 indicating moderate reliability, ICC values of 0.75–0.9 indicating good reliability, and ICC values >0.90 indicating excellent reliability.
Receiver operating characteristic (ROC) curves were used to appraise the diagnostic value of the CT features in differentiating between various diagnoses, and the sensitivity, specificity, and area under the curve (AUC) values of these features were determined. Further, multiple logistic regression and ROC curve analyses were conducted to assess the diagnostic value of combining multiple CT features to distinguish between AFPGC and CGC.
Results
Clinical findings
Figure 1 provides a flowchart of the patient inclusion process. In total, 52 patients with AFPGC were ultimately included in the study, including two patients with early-stage GC and 50 patients with advanced stage GC. Among these 52 patients, 49 underwent surgical treatment, one underwent endoscopic submucosal dissection, and two received chemotherapy.
The clinical characteristics of the patients are summarized in Table 1. Among the 52 patients with AFPGC, the majority were male (n=43), and they had an average age of 60.35±9.82 years (range: 35–82 years). In terms of the tumor location, 25, 11, and 16 patients had tumors located in the gastric antrum, body, and cardia, respectively. There were no statistically significant differences between the two groups in terms of age, sex, or tumor location (P>0.05). The preoperative serum AFP levels varied from 10 to 2,000 ng/mL, averaging 241.89±439.77 ng/mL. The AFPGC group had significantly higher serum CEA levels than the control group (P=0.047). However, there were no significant differences between the two groups in terms of the serum CA125 and CA199 levels (P>0.05).
Table 1
| Characteristics | AFPGC (n=52) | CGC (n=100) | P value |
|---|---|---|---|
| Sex | 0.267 | ||
| Male | 43 (82.7) | 74 (74.0) | |
| Female | 9 (17.3) | 26 (26.0) | |
| Age (years) | 60.35±9.82 | 58.99±10.00 | 0.427 |
| Location of tumor | 0.837 | ||
| Upper | 16 (30.8) | 27 (27.0) | |
| Middle | 11 (21.2) | 20 (20.0) | |
| Lower | 25 (48.1) | 53 (53.0) | |
| AFP (ng/mL) | 241.89±439.77 | 2.09±0.96 | <0.001 |
| CEA (ng/mL) | 25.74±53.38 | 11.72±33.28 | 0.047 |
| CA199 (U/mL) | 17.31±24.31 | 39.88±133.49 | 0.231 |
| CA125 (U/mL) | 28.32±53.38 | 17.27±23.78 | 0.111 |
| Macroscopic type | 0.001 | ||
| Superficial | 2 (3.8) | 17 (17.0) | |
| Borrmann Type 1 | 9 (17.3) | 4 (4.0) | |
| Borrmann Type 2 | 11 (21.2) | 17(17.0) | |
| Borrmann Type 3 | 29 (55.8) | 56 (56.0) | |
| Borrmann Type 4 | 1 (1.9) | 6 (6.0) | |
| pT stage | 0.118 | ||
| pT1 | 2 (3.8) | 17 (17.0) | |
| pT2 | 10 (19.2) | 13 (13.0) | |
| pT3 | 30 (57.7) | 48 (48.0) | |
| pT4 | 10 (19.2) | 22 (22.0) | |
| pN stage | 0.001 | ||
| N0 | 11 (21.2) | 36 (36.0) | |
| N1 | 7 (13.4) | 12 (12.0) | |
| N2 | 11 (21.2) | 22 (22.0) | |
| N3 | 23 (44.2) | 30 (30.0) | |
| Lymphatic/venous invasion | 0.001 | ||
| Yes | 44 (84.7) | 58 (58.0) | |
| No | 8 (15.3) | 42 (42.0) | |
| Differentiation | 0.390 | ||
| Well/moderately differentiated | 21 (40.4) | 33 (33.0) | |
| Poorly differentiated | 31 (59.6) | 67 (67.0) |
Data are presented as mean ± standard deviation or number (%), and were compared using the independent sample t-test; the pT and pN stages are based on the 9th edition of the staging system of the American Joint Committee on Cancer. AFP, alpha-fetoprotein; AFPGC, alpha-fetoprotein-producing gastric cancer; CA199, carbohydrate antigen 199; CA125, carbohydrate antigen 125; CEA, carcinoembryonic antigen; CGC, conventional gastric cancer.
Pathological findings
The pathological characteristics of AFPGC and CGC are summarized in Table 1. In terms of the gross classification of AFPGC, 55.8% of cases had Borrmann Type III AFPGC. Two early-stage cancers presented as shallow ulcers, and nine presented as Type I, 11 as Type II, and one as Type IV cases. Statistically significant differences were observed between the two groups (P=0.001). In terms of lymph node staging, the AFPGC group had a significantly lower proportion of N0 cases than the control group, [11 (21.2%) vs. 36 (36.0%), P=0.001]. Conversely, the AFPGC group had a significantly higher proportion of N3 cases than the CGC group [23 (44.2%) vs. 30 (30.0%), P=0.001]. Further, 84.7% of the AFPGC patients had lymphatic vessel (venous vessel) invasion, which was statistically significantly different compared to that of the control group (P=0.001). However, there were no statistically significant differences between the two groups in terms of the degree of differentiation and T stage.
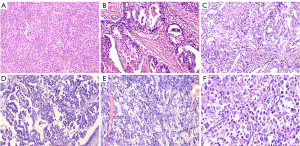
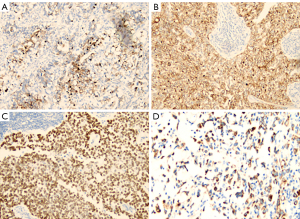
Among the 52 AFPGC patients, hepatoid adenocarcinoma was the most common histological pattern (n=31, 59.6%). Additionally, 18 patients (34.6%) had gastric adenocarcinoma with intestinal differentiation (one patient partially displayed a yolk sac tumor pattern), one patient had pure tubular and papillary adenocarcinoma, one patient had signet ring cell carcinoma, and one patient had a rhabdoid pattern (Figure 3). Immunohistochemical staining revealed that AFPGC expressed AFP, glypican-3, SALL4, and HepPar-1 (Figure 4). Glypican-3 (86.1%) had the highest positive rate, followed by SALL4 (80.5%), HepPar-1 (59.6%), and AFP (44.4%).
Radiographic findings
The measurements of the two radiologists demonstrated consistency (all ICCs >0.75), indicating good interobserver agreement. Table 2 summarizes the CT features of the 52 AFPGC patients and the 100 CGC patients. Ulcers were observed in 57.7% of the AFPGC patients on imaging, which differed significantly to the percentage observed in the CGC patients (P<0.001). AFPGC exhibited a noticeable trend toward internal necrosis, with 71.1% of the patients presenting with necrosis, and this difference was statistically significant (P=0.004). The arterial phase enhancement values differed significantly between the AFPGC and CGC groups (P<0.001). Patients in the AFPGC group often exhibited non-uniform enhancement (n=40, 76.9%), which was significantly different from that in the CGC group (P<0.001). In addition, AFPGC exhibited a significantly higher likelihood of liver metastasis in 18 cases (P<0.001). However, no other statistically significant differences were observed between the two groups in terms of the other imaging parameters, including tumor size, thickness, serosal infiltration, portal venous phase, or delayed phase CT values. Representative CT images of AFPGC are shown in Figure 5.
Table 2
| Characteristics | AFPGC (n=52) | CGC (n=100) | P value |
|---|---|---|---|
| L-max (cm) | 0.379 | ||
| ≥5 | 31 (59.6) | 50 (50.0) | |
| <5 | 21 (40.4) | 50 (50.0) | |
| D-max (cm) | 0.123 | ||
| ≥1.5 | 28 (53.8) | 39 (39.0) | |
| <1.5 | 24 (46.2) | 61 (61.0) | |
| Liver metastasis | <0.001 | ||
| Yes | 18 (34.6) | 8 (8.0) | |
| No | 34 (65.4) | 92 (92.0) | |
| Ulceration | <0.001 | ||
| Absent | 22 (42.3) | 76 (76.0) | |
| Present | 30 (57.7) | 24 (24.0) | |
| Necrotic | 0.004 | ||
| Absent | 15 (28.8) | 53 (53.0) | |
| Present | 37 (71.2) | 47 (47.0) | |
| Enhancement | <0.001 | ||
| Homogeneous | 12 (23.1) | 49 (49.0) | |
| Heterogeneous | 40 (76.9) | 51 (51.0) | |
| Serosal invasion | 0.203 | ||
| Absent | 24 (46.2) | 57 (57.0) | |
| Present | 28 (53.8) | 43 (43.0) | |
| CT value of AP (HU) | 83.14 (69.25, 96.75) | 67.14 (58.25, 74) | <0.001 |
| CT value of PVP (HU) | 82.53 (70.50, 89.50) | 79.12 (68.00, 89.75) | 0.338 |
| CT value of DP (HU) | 76.65 (66.50, 85.50) | 79.28 (64.00, 92.00) | 0.605 |
| LND (mm) | 14.49 (9.24, 19.29) | 12.26 (8.06, 14.58) | 0.105 |
Data are presented as number (%) or median (IQR). AFPGC, alpha-fetoprotein-producing gastric cancer; AP, arterial phase; CGC, conventional gastric cancer; CT, computed tomography; D-max, maximum depth of the tumor; DP, delayed period; IQR, interquartile range; L-max, maximum length of the tumor; LND, the short axis diameter of the largest lymph nodes discernible; PVP, portal venous phase.
To clarify the association between the serum AFP levels and CT variables, the AFPGC cohort was stratified into the AFP-H group (serum AFP ≥100 ng/mL) and the AFP-L group (serum AFP <100 ng/mL) based on an AFP cut-off value of 100 ng/mL. A detailed comparison of the CT characteristics between these two groups was performed (Table 3). The results revealed that the incidence of liver metastasis was significantly higher in the AFP-H group than the AFP-L group (P=0.006). Further, the maximum tumor diameter (P=0.003) and the largest lymph node diameter (P=0.01) differed significantly between these two groups. However, the other CT parameters did not differ significantly between these two groups.
Table 3
| Characteristics | AFP concentration | AFP staining | HepPar-1 staining | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AFP-L (n=38) | AFP-H (n=14) | P value | Negative (n=29) | Positive (n=23) | P value | Negative (n=21) | Positive (n=31) | P value | |||
| L-max (cm) | 0.003 | 0.870 | 0.009 | ||||||||
| ≥5 | 18 (47.4) | 13 (92.9) | 17 (58.6) | 14 (60.9) | 8 (38.1) | 23 (74.2) | |||||
| <5 | 20 (52.6) | 1 (7.1) | 12 (41.4) | 9 (39.1) | 13 (61.9) | 8 (25.8) | |||||
| D-max (cm) | 0.359 | 0.829 | 0.061 | ||||||||
| ≥1.5 | 19 (50.0) | 9 (64.3) | 16 (55.2) | 12 (52.2) | 8 (38.1) | 20 (64.5) | |||||
| <1.5 | 19 (50.0) | 5 (35.7) | 13 (44.8) | 11 (47.8) | 13 (61.9) | 11 (35.5) | |||||
| Liver metastasis | 0.006 | 0.182 | 0.127 | ||||||||
| Yes | 9 (23.7) | 9 (64.3) | 11 (37.9) | 13 (56.5) | 7 (33.3) | 17 (54.8) | |||||
| No | 29 (76.3) | 5 (35.7) | 18 (62.1) | 10 (43.5) | 14 (66.7) | 14 (45.2) | |||||
| Tumor ulceration | 0.961 | 0.473 | 0.281 | ||||||||
| Absent | 16 (42.1) | 6 (42.9) | 11 (37.9) | 11 (47.8) | 7 (33.3) | 15 (48.4) | |||||
| Present | 22 (57.9) | 8 (57.1) | 18 (62.1) | 12 (52.2) | 14 (66.7) | 16 (51.6) | |||||
| Necrotic | 0.507 | 0.696 | 0.002 | ||||||||
| Absent | 28 (73.7) | 9 (64.3) | 20 (69.0) | 17 (73.9) | 20 (95.2) | 17 (54.8) | |||||
| Present | 10 (26.3) | 5 (35.7) | 9 (31.0) | 6 (26.1) | 1 (4.8) | 14 (45.2) | |||||
| Enhancement | 0.361 | 0.386 | 0.439 | ||||||||
| Homogeneous | 10 (26.3) | 2 (14.3) | 8 (27.6) | 4 (17.4) | 6 (28.6) | 6 (19.4) | |||||
| Heterogeneous | 28 (73.7) | 12 (85.7) | 21 (72.4) | 19 (82.6) | 15 (71.4) | 25 (80.6) | |||||
| Serosal invasion | 0.359 | 0.143 | 0.061 | ||||||||
| Absent | 19 (50.0) | 5 (35.7) | 16 (55.2) | 8 (34.8) | 13 (61.9) | 11 (35.5) | |||||
| Present | 19 (50.0) | 9 (64.3) | 13 (44.8) | 15 (65.2) | 8 (38.1) | 20 (64.5) | |||||
| CT value of AP (HU) | 82.00 (69.25, 100.00) |
89.00 (67.00, 95.50) |
0.856 | 82.00 (66.75, 101.00) |
83.00 (69.00, 96.00) |
0.985 | 83.00 (70.00, 101.00) |
81.00 (66.75, 96.25) |
0.697 | ||
| CT value of PVP (HU) | 80.50 (70.25, 93.50) |
82.0 0 (72.00, 86.50) |
0.723 | 80.00 (70.75, 88.25) |
83.00 (70.00, 98.00) |
0.427 | 80.00 (67.00, 87.00) |
82.50 (77.00, 93.50) |
0.423 | ||
| CT value of DP (HU) | 77.50 (66.00, 86.25) |
77.00 (69.50, 84.00) |
0.724 | 78.00 (66.75, 85.00) |
77.00 (66.00, 87.00) |
0.780 | 77.00 (63.00, 88.00) |
77.50 (67.75, 84.75) |
0.905 | ||
| LND (mm) | 11.17 (7.82, 18.40) |
15.49 (12.61, 29.38) |
0.010 | 11.06 (7.47, 20.36) |
13.22 (11.09, 18.87) |
0.574 | 10.88 (7.71, 12.06) |
14.79 (9.99, 25.72) |
0.011 | ||
Data are presented as median (IQR) or number (%). AFP-L, serum AFP <100 ng/mL; AFP-H, serum AFP ≥100 ng/L. AFP, alpha-fetoprotein; AFPGC, alpha-fetoprotein-producing gastric cancer; AP, arterial phase; CT, computed tomography; D-max, maximum depth of the tumor; DP, delayed period; HU, Hounsfield unit; IQR, interquartile range; L-max, maximum length of the tumor; LND, the short axis diameter of the largest lymph nodes discernible; PVP, portal venous phase.
In addition, to explore the potential relationship between the hepatocyte biomarkers and CT imaging characteristics, AFP and HepPar-1 expression were analyzed in detail (Table 3). The findings revealed no statistically significant differences in the CT variables between the AFP-positive and AFP-negative groups. However, significant differences were observed between the HepPar-1-positive group and HepPar-1 negative group in terms of the tumor maximum diameter (P=0.009), largest lymph node diameter (P=0.011), and tumor necrosis rate (P=0.002).
Multivariate logistic regression and nomogram
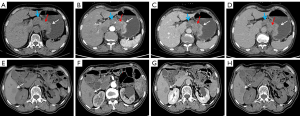
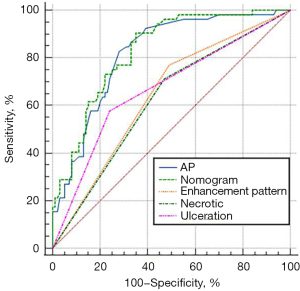
The multivariate logistic regression analysis revealed that ulceration, necrosis, enhancement pattern, and arterial phase CT value were independent risk factors for AFPGC (Table 4). Based on these findings, we developed an individualized nomogram using four independent risk factors to predict AFPGC (Figure 6). The logistic regression model based on these four features showed strong predictive performance for the sample. The AUC value, sensitivity, and specificity were 0.829, 0.904, and 0.650, respectively (Table 5 and Figure 7).
Table 4
| Risk factor | Z value | P value | OR | 95% CI |
|---|---|---|---|---|
| Necrotic | 2.987 | 0.0028 | 2.782 | 1.361 to 5.723 |
| Ulceration | 4.138 | <0.0001 | 2.651 | 1.145 to 6.137 |
| Enhancement pattern | 9.932 | <0.0001 | 2.302 | 0.463 to 11.437 |
| AP CT value | 9.137 | <0.0001 | 4.117 | 2.236 to 7.580 |
AFPGC, alpha-fetoprotein-producing gastric cancer; AP, arterial phase; CGC, conventional gastric cancer; CI, confidence interval; CT, computed tomography; OR, odds ratio.
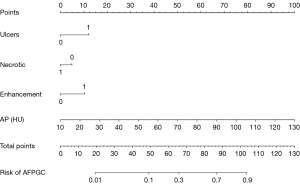
Table 5
| Risk factor | Necrotic | Ulceration | Enhancement pattern | AP CT value | Nomogram |
|---|---|---|---|---|---|
| AUC (95% CI) | 0.621 (0.539–0.698) | 0.668 (0.588–0.743) | 0.640 (0.558–0.716) | 0.818 (0.748–0.876) | 0.829 (0.760–0.885) |
| Accuracy (%) | 59.21 | 69.74 | 59.87 | 75.66 | 74.34 |
| Sensitivity (%) | 71.15 | 57.69 | 76.92 | 82.69 | 90.38 |
| Specificity (%) | 53.00 | 76.00 | 51.00 | 72.00 | 65.00 |
AFPGC, alpha-fetoprotein-producing gastric cancer; AP, arterial phase; AUC, area under the curve; CGC, conventional gastric cancer; CI, confidence interval; CT, computed tomography.
Discussion
The concept of AFPGC originated from a report in Japan in 1970, in which a GC patient had an elevated serum AFP level, and an autopsy revealed liver metastasis (4). Subsequently, AFP-positive cases of GC have been reported and termed hepatoid adenocarcinomas (6). AFP can be detected through the immunohistochemical staining of tumor cells or by measuring the serum AFP concentration. Due to its low incidence rate, no large-scale multicenter randomized controlled studies on AFPGC have been conducted to date, and there is a lack of clear serological diagnostic criteria. Some research results have suggested that a serum AFP level >20 ng/mL should be considered AFP-positive (12-14,18).
We systematically summarized the clinical, pathological, and radiological features of AFPGC. This type of GC exhibits high rates of vascular invasion, lymph node involvement, and liver metastasis (4,7,19). Our results are consistent with those of previous studies (4,7). The lymph node metastasis rate (78.7%) and probability of stage N3 (44.2%) were significantly higher in the AFPGC group than the CGC group, and most patients with AFPGC showed lymphatic and vascular invasion (84.7%). In addition to elevated serum AFP levels, the expression levels of CEA were significantly higher in the AFPGC group than the CGC group. Thus, further research at more centers needs to be conducted to determine whether CEA can be used as a diagnostic indicator for AFPGC.
Hepatoid adenocarcinoma is the most common histological type of AFPGC and is predominantly composed of large polygonal eosinophilic hepatocyte-like neoplastic cells or well-differentiated papillary- or tubular-type adenocarcinoma with a clear cytoplasm (i.e., adenocarcinoma with enteroblastic differentiation and yolk sac tumor-like carcinoma) (20-22). In our study, hepatoid adenocarcinoma was the most common histological form of AFPGC (61.5%), followed by enteroblastic differentiation (34.6%). There was one case with a partial yolk sac tumor-like area, which is consistent with findings in the literature (23). Additionally, there was one case with a histology resembling ordinary tubular or papillary adenocarcinoma, one case of signet ring cell carcinoma, and one case with a rhabdoid-like appearance, which has not been reported previously. Therefore, we discovered that the histological presentation of this type of GC is more complex than what has been previously reported, and the existence of other histological types has yet to be explored. The production of AFP is usually considered the result of the dedifferentiation of tumor cells back into primitive cells that can produce AFP. Therefore, a reverse differentiation may occur in the histology of AFPGC (4,24). The histogenesis of AFPGC is still unclear. It partly resembles the fetal primitive gut and yolk sac tumor, as well as the expression of SALL4 on immunohistochemical staining, which may be related to early embryonic development and indicate the higher invasive potential of the tumor (25).
CT tomography plays a crucial role in the diagnosis, staging, and treatment of AFPGC (26). This is the first imaging study of AFPGC, and we found that AFPGC typically demonstrated distinct features on CT scans that differentiated it from CGC. On CT, unlike CGC, AFPGC often presents with gastric wall thickening and ulcer formation (58%). Most of the GC patients were already in the advanced stages at the time of their initial presentation (96.2%). Internal necrosis was observed in 71% of the lesions, and the enhancement pattern was primarily heterogeneous, resulting in a heterogeneous appearance of the tumor. Research results have suggested that tumor enhancement in the arterial phase of GC is related to tumor angiogenesis and lymphatic invasion, and is associated with liver metastasis after curative GC resection (27,28). We found that AFPGC had significantly higher CT values in the arterial phase than CGC, which is consistent with its higher rates of liver metastasis and advanced lymph node staging.
Liver metastasis is the most common site of AFPGC metastasis. Previous studies have reported liver metastasis rates ranging from 31% to 63.4% (4,15,29). In our study, the liver metastasis rate of the AFPGC group was 34.6%, which was significantly higher than the 8% observed in the CGC group. During the follow-up period, we found that in the diagnosis of AFPGC: (I) some patients were misdiagnosed with primary liver cancer due to apparent liver lesions, leading to the omission of lesions in the stomach; and (II) early metastatic liver lesions were often overlooked in patients diagnosed with GC. Radiologists and clinicians should pay attention to these two aspects in treating patients.
We found that the AFPGC patients with high AFP levels (≥100 ng/mL) had a significantly higher rate of liver metastasis than those with low AFP levels (<100 ng/mL). These findings are consistent with previous reports, suggesting that elevated AFP levels may indicate stronger tumor spread, especially to the liver, possibly due to the hepatocellular-like characteristics of AFPGC tumor cells (30). For AFP-H patients, enhanced liver imaging should be performed preoperatively to detect and assess liver metastasis early. The larger tumor size and lymph node diameter observed in the AFP-H group suggest that elevated AFP levels may be linked to rapid tumor growth and lymphatic spread. Thus, AFP levels could serve as a diagnostic and risk stratification marker, and guide the clinical management of patients.
We also compared the CT parameters between the HepPar-1-negative group and HepPar1-positive group. The HepPar1-positive group had larger tumor and lymph node diameters and more necrosis than the HepPar1-negative group, which suggests that HepPar1 is associated with tumor aggressiveness and may serve as a prognostic marker. However, no significant differences were found between the AFP-negative and AFP-positive groups, which reflects the limitations of AFP immunohistochemical staining. Tissue AFP expression may be affected by antibody sensitivity or tumor heterogeneity, making serum AFP detection more reliable. Future studies should combine serum AFP levels with other markers for a more comprehensive assessment.
Due to the occurrence of misdiagnosis and missed diagnosis before surgery, we explored several meaningful combinations of imaging parameters to achieve a preoperative diagnosis of AFPGC. The combination model incorporating necrosis, ulceration, enhancement pattern, and arterial phase CT values showed excellent discriminative ability. This finding indicates that preoperative imaging can provide a basis for diagnosing AFPGC when AFP testing and biopsy diagnoses are unavailable. This will also allow surgeons to tailor perioperative treatments for patients and adopt more aggressive treatment plans when necessary.
This study had several limitations. First, as it was a retrospective study, further prospective studies need to be conducted to gain a more comprehensive understanding of these results. Second, the enhancement of GC lesions may be influenced by the volume and rate of contrast agent use. Finally, this study obtained data from a single center; thus, future prospective studies at multiple centers should be conducted.
Conclusions
In summary, we examined the clinical and histopathological characteristics of patients with AFPGC, and systematically explored the CT imaging features of AFPGC for the first time. AFPGC is a complex diagnostic and clinical challenge characterized by distinct CT imaging features, elevated serum AFP and CEA levels, and specific pathological features. The integration of these features is crucial for the precise diagnosis, staging, and management of this rare subtype of GC. AFP levels and HepPar-1 expression are important indicators of GC aggressiveness and metastatic potential. These findings suggest that more aggressive treatment strategies should be adopted for patients with high AFP levels or HepPar-1 positivity.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1867/rc
Funding: This work was supported by funding from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1867/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Lanzhou University Second Hospital (approval No. [2023A-749]), and the requirement of individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bergstrand CG, Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest 1956;8:174. [Crossref] [PubMed]
- Pedrazzoli P, Rosti G, Soresini E, Ciani S, Secondino S. Serum tumour markers in germ cell tumours: From diagnosis to cure. Crit Rev Oncol Hematol 2021;159:103224. [Crossref] [PubMed]
- Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, Schelman WR, Chintharlapalli S, Abada PB, Sherman M, Zhu AX. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int 2019;39:2214-29. [Crossref] [PubMed]
- Murakami T, Yao T, Mitomi H, Morimoto T, Ueyama H, Matsumoto K, Saito T, Osada T, Nagahara A, Watanabe S. Clinicopathologic and immunohistochemical characteristics of gastric adenocarcinoma with enteroblastic differentiation: a study of 29 cases. Gastric Cancer 2016;19:498-507. [Crossref] [PubMed]
- Bourreille J, Metayer P, Sauger F, Matray F, Fondimare A. Existence of alpha feto protein during gastric-origin secondary cancer of the liver. Presse Med (1893) 1970;78:1277-8. [PubMed]
- Ooi A, Okada Y, Minamoto T, Imabori T, Shima K. A case of alpha-fetoprotein-producing gastric carcinoma with histologic features of embryonal carcinoma. Gan No Rinsho 1985;31:334-40. [PubMed]
- Hirajima S, Komatsu S, Ichikawa D, Kubota T, Okamoto K, Shiozaki A, Fujiwara H, Konishi H, Ikoma H, Otsuji E. Liver metastasis is the only independent prognostic factor in AFP-producing gastric cancer. World J Gastroenterol 2013;19:6055-61. [Crossref] [PubMed]
- Li W, Zhang L, Tian C, Song H, Fang M, Hu C, Zang Y, Cao Y, Dai S, Wang F, Dong D, Wang R, Tian J. Prognostic value of computed tomography radiomics features in patients with gastric cancer following curative resection. Eur Radiol 2019;29:3079-89. [Crossref] [PubMed]
- Li R, Li J, Wang X, Liang P, Gao J. Detection of gastric cancer and its histological type based on iodine concentration in spectral CT. Cancer Imaging 2018;18:42. [Crossref] [PubMed]
- Zeydanli T, Kilic HK. Performance of quantitative CT texture analysis in differentiation of gastric tumors. Jpn J Radiol 2022;40:56-65. [Crossref] [PubMed]
- Xu M, Liu S, Qiao X, Li L, Ji C, Zhou Z. Clinicopathological features and CT findings of papillary gastric adenocarcinoma. Abdom Radiol (NY) 2022;47:3698-711. [Crossref] [PubMed]
- Chen Y, Qu H, Jian M, Sun G, He Q. High level of serum AFP is an independent negative prognostic factor in gastric cancer. Int J Biol Markers 2015;30:e387-93. [Crossref] [PubMed]
- Lin HJ, Hsieh YH, Fang WL, Huang KH, Li AF. Clinical manifestations in patients with alpha-fetoprotein-producing gastric cancer. Curr Oncol 2014;21:e394-9. [Crossref] [PubMed]
- Zhan Z, Chen B, Yu J, Zheng J, Zeng Y, Sun M, Peng L, Guo Z, Wang X. Elevated Serum Alpha-Fetoprotein Is a Significant Prognostic Factor for Patients with Gastric Cancer: Results Based on a Large-Scale Retrospective Study. Front Oncol 2022;12:901061. [Crossref] [PubMed]
- Vatansever S, Özer MK, Erdoğan EI. Prognostic significance of α-fetoprotein in gastric adenocarcinoma. Prz Gastroenterol 2022;17:35-40. [Crossref] [PubMed]
- Park CJ, Seo N, Hyung WJ, Koom WS, Kim HS, Kim MJ, Lim JS. Prognostic significance of preoperative CT findings in patients with advanced gastric cancer who underwent curative gastrectomy. PLoS One 2018;13:e0202207. [Crossref] [PubMed]
- Olawaiye AB, Baker TP, Washington MK, Mutch DG. The new (Version 9) American Joint Committee on Cancer tumor, node, metastasis staging for cervical cancer. CA Cancer J Clin 2021;71:287-98. [Crossref] [PubMed]
- Kinjo T, Taniguchi H, Kushima R, Sekine S, Oda I, Saka M, Gotoda T, Kinjo F, Fujita J, Shimoda T. Histologic and immunohistochemical analyses of α-fetoprotein--producing cancer of the stomach. Am J Surg Pathol 2012;36:56-65. [Crossref] [PubMed]
- Lew DH, Jung WT, Kim HJ, Min HJ, Ha CY, Kim HJ, Kim TH, Ko GH. Clinicopathological characteristics and prognosis of alpha-fetoprotein producing gastric cancer. Korean J Gastroenterol 2013;62:327-35. [Crossref] [PubMed]
- Lu J, Jin M, Zhou X, Chen X, Shao Y, Jiang X. Clinicopathological and molecular characteristics of the alpha-fetoprotein-producing gastric cancer: emphasis on two major subtypes. APMIS 2022;130:169-80. [Crossref] [PubMed]
- Li M, Mei YX, Wen JH, Jiao YR, Pan QR, Kong XX, Li J. Hepatoid adenocarcinoma-Clinicopathological features and molecular characteristics. Cancer Lett 2023;559:216104. [Crossref] [PubMed]
- Zhou ZY, Sun J, Guo Q, Zhao HB, Zhou ZH. Clinicopathological significance of primitive phenotypes in early gastric cancer with differentiated histology. Diagn Pathol 2021;16:66. [Crossref] [PubMed]
- Satake N, Chikakiyo M, Yagi T, Suzuki Y, Hirose T. Gastric cancer with choriocarcinoma and yolk sac tumor components: case report. Pathol Int 2011;61:156-60. [Crossref] [PubMed]
- Yamazawa S, Ushiku T, Shinozaki-Ushiku A, Hayashi A, Iwasaki A, Abe H, Tagashira A, Yamashita H, Seto Y, Aburatani H, Fukayama M. Gastric Cancer With Primitive Enterocyte Phenotype: An Aggressive Subgroup of Intestinal-type Adenocarcinoma. Am J Surg Pathol 2017;41:989-97. [Crossref] [PubMed]
- Ushiku T, Shinozaki A, Shibahara J, Iwasaki Y, Tateishi Y, Funata N, Fukayama M. SALL4 represents fetal gut differentiation of gastric cancer, and is diagnostically useful in distinguishing hepatoid gastric carcinoma from hepatocellular carcinoma. Am J Surg Pathol 2010;34:533-40. [Crossref] [PubMed]
- Xu Q, Sun Z, Li X, Ye C, Zhou C, Zhang L, Lu G. Advanced gastric cancer: CT radiomics prediction and early detection of downstaging with neoadjuvant chemotherapy. Eur Radiol 2021;31:8765-74. [Crossref] [PubMed]
- Tsurumaru D, Nishimuta Y, Muraki T, Asayama Y, Nishie A, Oki E, Honda H. Gastric cancer with synchronous and metachronous hepatic metastasis predicted by enhancement pattern on multiphasic contrast-enhanced CT. Eur J Radiol 2018;108:165-171. [Crossref] [PubMed]
- Chen XH, Ren K, Liang P, Chai YR, Chen KS, Gao JB. Spectral computed tomography in advanced gastric cancer: Can iodine concentration non-invasively assess angiogenesis? World J Gastroenterol 2017;23:1666-75. [Crossref] [PubMed]
- Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long Z, Zhu H, Wang Y. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol 2010;102:249-55. [Crossref] [PubMed]
- Wang YK, Shen L, Jiao X, Zhang XT. Predictive and prognostic value of serum AFP level and its dynamic changes in advanced gastric cancer patients with elevated serum AFP. World J Gastroenterol 2018;24:266-73. [Crossref] [PubMed]


