
Unfavorable venous outflow correlates with poor prognosis in acute ischemic stroke due to large vessel occlusion (AIS-LVO) patients assessed dynamically and quantitatively based on four-dimensional computed tomography angiography/perfusion (4D-CTA/CTP)
Introduction
Acute ischemic stroke due to large blood vessel occlusion (AIS-LVO) is characterized by high incidence, high mortality, and disability rate. Timely and effective treatment is the key to obtaining a good prognosis for such patients (1). Many studies have shown that endovascular interventional therapy can benefit the AIS-LVO population (2-6), and imaging markers such as collateral circulation and venous outflow (VO) can assist in screening patients who are most likely to have a good prognosis from this treatment (7-11).
Previous studies have mainly focused on exploring the association between arterial collateral circulation on computed tomography (CT) angiography (CTA) and prognosis (3,12). However, cerebral microvascular perfusion is influenced not only by arterial blood inflow but also by VO. It has been shown that cortical venous filling (CVF) can reflect the function of the cerebral microcirculation, and tissue level collaterals (TLC) can reflect the success of leptomeningeal collateral blood perfusion into the microcirculation, and the above two imaging markers can realize an indirect assessment of cerebral microvascular perfusion, supplementing information other than arterial collaterals (13,14).
Several studies have reported that delayed flow in the cortical veins of AIS-LVO patients is associated with poor collateral circulation and poor prognosis (11,15), and patients with favorable cortical vein condition are more likely to benefit from endovascular intervention (9,10,16,17). As the technology has been updated and iterated, the evaluation of the cerebral venous system by CT has evolved from the application of single-phase CTA (sCTA) to multiple-phase CTA (mCTA) and to the current dynamic CTA evaluation. Existing one-stop 4-dimensional CT angiography/perfusion (4D-CTA/CTP) technology allows a comprehensive and rapid assessment of the velocity and extent of the CVF, as well as the acquisition of perfusion information. Previously published methods for the dynamic assessment of CVF based on 4D-CTA have mainly focused on the assessment of the velocity and extent of the CVF or the quantification of vein opacification at different time points (11,16,18,19), To our knowledge, there are no relevant studies comparing the predictive value of these two dynamic assessment methods. Therefore, this study aimed to utilize 4D-CTA/CTP technology to evaluate the cortical venous system in a multidirectional and multidimensional way and to investigate the association between it and clinical long-term prognosis, as well as to find out a relatively easier, more accurate, and more efficient method, so as to provide more basis for the clinical development of treatment strategies.
Moreover, the hypoperfusion intensity ratio (HIR) calculated by the CTP post-processing software can more accurately measure whether the inflow of artery successfully penetrates into the brain tissue, which could quantify the TLC (14). It has been shown that favorable VO evaluated by sCTA correlates with favorable TLC, but some patients with favorable TLC still present unfavorable VO (17). Hence, we would also like to investigate the association between VO and TLC based on 4D-CTA/CTP. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-669/rc).
Methods
Patients
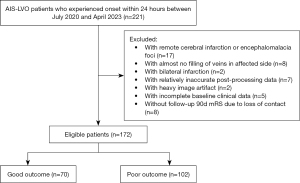
This was a retrospective, observational study aimed at AIS-LVO patients. We roughly determined the study size by combining previous studies and the ratio of outcome indicators to independent variables. We retrospectively collected and analyzed 221 consecutive patients with anterior circulation AIS-LVO who attended The First Affiliated Hospital of Chongqing Medical University between July 2020 and April 2023. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of The First Affiliated Hospital of Chongqing Medical University (No. 2021-274), which waived the requirement for informed consent. One hundred and seventy-two patients finally met the inclusion criteria, which were as follows: (I) underwent 4D-CTA/CTP at baseline within 24 h of symptoms onset; (II) AIS with unilateral occlusion of the internal carotid artery (ICA), and/or the middle cerebral artery (MCA), segment M1 or M2; (III) image quality met standards for proper analysis by post-processing software; (IV) detailed and complete demographic and clinical information was available, and follow-up visits were timely. The exclusion criteria were as follows: (I) baseline examination performed after more than 24 h of onset of symptoms; (II) CTP showed ischemia and infarction in the posterior circulation region or bilateral anterior circulation regions of the brain; (III) poor quality and unavailability of image data; (IV) lack of necessary demographics, clinical information or missed follow-up visits. The screening process for enrolled patients is shown in Figure 1.
Demographics, clinical data
Demographics, clinical data, and detailed baseline information of patients was recorded, including (I) age, gender, time from onset to 4D-CTA/CTP examination, the National Institutes of Health Stroke Scale (NIHSS) score at admission (admission NIHSS); (II) risk factors: any history of hypertension, coronary artery disease, diabetes, hyperlipidemia, atrial fibrillation, smoking; (III) treatment methods: conservative, thrombolytic, interventional or bridging. Patients’ long-term clinical prognosis was assessed using the 90-day modified Rankin Scale (90d mRS) (0–2 for good outcome and 3–6 for poor outcome).
Radiological data
The 4D-CTA/CTP was part of our routine scanning protocol. In the daily admission arrangement of our hospital, emergency patients with stroke symptoms (hemiplegia, aphasia, coma, etc.) will receive the 4D-CTA/CTP examination first.
The machine used for one-stop 4D-CTA/CTP scanning in all subjects was a CT scanner with a 320-row detector (Aquilion ONE, Canon Medical Systems Corporation, Otawara, Japan). During the scanning process, non-contrast images were first acquired using the dynamic volume single scanning mode, followed by CTA and CTP examination using the whole-brain dynamic volume intermittent scanning mode. Dynamic volume perfusion scanning was automatically initiated 7 seconds after contrast injection, thus ensuring synchronization of contrast perfusion and scanning; images were obtained at 2 seconds intervals during the arterial phase and at 5 seconds intervals during the venous phase, and the total time for obtaining non-contrast CT, CTA, and CTP correlation data was 60 seconds. The parameters of the CT scanner used in this study were as follows: 80 kv, 150–310 mA, coverage of 140–160 mm, layer thickness of 1.0 mm, spacing of 1.0 mm, and a total scanning dose of 5.0–6.0 mSv (k=0.0021). Adaptive iterative dose reduction reconstruction was used to improve the reconstruction speed. Regarding contrast agent injection: the amount and speed of contrast agent was automatically calculated according to the patient’s gender, height, weight, and contrast concentration, and the contrast agent was injected using the P3T high-pressure injector technique (Iopamidol, Bracco Sine, Milan, Italy).
Determination of the responsible vessel and evaluation of cerebral cortical venous conditions were performed on the post-processing workstation (Vitrea, fX, 1.0, Canon Medical Systems Corporation, Japan). Nineteen CT perfusion packages from the scans were transferred to the workstation for image preprocessing, and the enhanced images were paired with the non-contrast scanned images for subtraction, resulting in 18 subtracted images, which were reconstructed as three-dimensional maximum intensity projection (3D-MIP) CTA images, and then we could observe the arterial inflow to VO dynamically according to arterial and venous curves, which yielded the 4D-CTA images for our evaluation, and meanwhile we determined and recorded the location of the responsible vessel occlusion. The two methods of dynamic assessment of VO conditions in this study are as follows.
The velocity combined with the extent
Referring to the method of Lin et al. (16), we dynamically observed the cortical vein filling of the affected side and the healthy side in all phases, and recorded the time points of the start of bilateral venous contrast inflow (CVF1), the time point for venous optimal opacification (CVF2), and the difference between the above two time points (CVF21) to assess the velocity of CVF, respectively. The bilateral time differences were calculated and counted as rCVF1, rCVF2, and rCVF21, respectively, and the corresponding medians were also calculated; if the difference was less than or equal to the median of the differences, it was defined as fast filling (Fast), and if the difference was greater than the median, it was defined as slow filling (Slow). Meanwhile, the number of cortical veins opacified bilaterally was compared during the peak venous phase to assess the extent of CVF. If the number of affected cortical veins opacified was ≥50% compared with the healthy side, it was defined as richly opacified (Good), and if the number was <50%, it was defined as sparsely opacified (Poor). Combining the velocity and extent indicators yielded three combined indicators: (I) the velocity for the veins start to fill combined with the extent of peak venous phase (V1&E), including Fast1 + Good, Fast1 or Good, and Slow1 + Poor; (II) The velocity for venous optimal opacification combined with the extent of peak venous phase (V2&E), including Fast2 + Good, Fast2 or Good, and Slow2 + Poor; (III) the velocity for the veins start to fill to the optimal opacification combined with the extent of peak venous phase (V21&E), including Fast21 + Good, Fast21 or Good, and Slow21 + Poor. During the process of data collection, we observed that a significant proportion of our subjects still had an incomplete VO of contrast agent from the affected side at the last phase, and previous relevant study have shown that the velocity of complete VO was not independently associated with prognosis (16), it is the reason for not including the complete VO time point in the assessment of the velocity.
Four-dimensional cortical venous collaterals score (4D-VCS)
The dynamic VO condition of the affected side was quantitatively evaluated according to the 4D-VCS (19). Four veins, including the superficial middle cerebral vein (SMCV), the vein of Trolard (VOT), the vein of Labbé (VOL), and the sphenoparietal sinus (SPS), which mainly drained the MCA supplying area and had relatively few anatomical variations, were selected and assessed by comparing with the veins of contralateral side in each phase. Each of the above four veins can be scored 0–4, 0 for no opacification in any phase; 1 for partial opacification till the late venous phase; 2 for partial opacification in the venous phase, and no further filling of contrast till the late venous phase; 3 for complete opacification in the late venous phase with no or partial filling of contrast before; 4 for complete opacification in the venous phase. Therefore, 4D-VCS can be scored from 0 to 16. During evaluation, partial opacification is defined as a vein that is filled with contrast but less dense than the contralateral vein, while full opacification is defined as a vein that is filled with contrast of nearly the same density as the contralateral vein. The venous phase is the peak phase of the venous curve, while the late venous phase is the phase after the venous phase.
In addition to the dynamical assessment of VO mentioned above, this study also concurrently assessed the VO of a single phase. Using the cortical vein opacification score (COVES) to evaluate the VO of the affected side at the peak venous phase according to the venous curve: We compared the filling of SMCV, VOL, and the SPS bilaterally, and a score of 2 for complete filling compared to the contralateral side, 1 for moderately incomplete filling, 0 for no filling at all. Final COVES can be scored from 0 to 6.
The arterial inflow sampling point of the arterial curve was localized at the beginning of the MCA or the basilar artery of the healthy side, and the VO sampling point was localized at the superior sagittal sinus or the great cerebral vein.
TLC was assessed in HIR, which is the ratio of time to maximum of the tissue residue function (Tmax) >10 s to Tmax >6 s, used to indicate the degree of severe hypoperfusion, and it can predict CTA collateral, infarction growth, and clinical outcomes (20-22). Evaluation of TLC was performed on a CTP post-processing software (Olea Sphere 3.0-SP28). The CTP data were imported into the software, and the HIR was calculated by the cSVD algorithm. In order to minimize bias, ischemic volumes that were clearly misdrawn outside the brain (e.g., ventricles, soft tissues at the base of the skull) were manually erased from the post-processed images. The result obtained from the calculation is a good state of TLC if HIR ≤0.4 (TLC+) and vice versa if HIR >0.4 (TLC−) (20).
The evaluation of the above imaging data was determined separately by two experienced radiologists, and in case of disagreement, the final unified opinion was summarized after consultation; the evaluators were blinded to the clinical data and the prognosis during the evaluation process.
Statistical analysis
All subjects were categorized into the good outcome group (mRS 0–2) and the poor outcome group (mRS 3–6) based on their long-term clinical prognosis. All statistical analyses covered in this study were performed by SPSS version 22.0 (IBM, Armonk, NY, USA) and Medcalc version 19.0.4. Measurement data was described as mean ± standard deviation (SD) or median [interquartile range (IQR)], and counting data was described as the number of cases (percentage). Since measurement data didn’t conform to normal distribution, we used the Mann-Whitney U test for the comparison of measurement variables, while the Chi-squared test was used for the counting variables. We chose the binary logistic regression analysis to explore the association between cerebral venous manifestations and clinical prognosis, and variables with P<0.1 in the univariable analysis were included in a multivariable logistic regression model to analyze the influences on poor prognosis; the diagnostic ability of models were also explored by receiver operating characteristic (ROC) curve analysis and compared by DeLong test. In addition to this, binary logistic regression analysis was also used to explore the association between cerebral venous manifestations and TLC. The level of statistical significance was the two-sided test and P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 172 subjects meeting the criteria were enrolled, including 90 (52%) males and 82 (48%) females, aged 41–94 years, with a mean age of approximately 70.866±11.789 years old. Tables 1,2 demonstrated the clinical and imaging characteristics of the subject patients, respectively. Subject patients were divided into two groups, 70 in the good outcome group and 102 in the poor outcome group. Among the clinical characteristics, the advanced age [73.137±11.020 years old; 76 (IQR, 66–81) years old], high admission NIHSS [12.34±6.308; 12 (IQR, 8–16)], and atrial fibrillation were more likely to be found in the poor outcome group (P<0.05), whereas there was no statistically significant difference between the gender, the remaining risk factors, the onset time of symptoms, and the kind of the treatment (P>0.05) between the groups. Among the imaging characteristics, the long rCVF1 [3.781±3.900 seconds; 2.6 (IQR, 2–4.9) seconds], long rCVF2 [5.169±4.36 seconds; 4 (IQR, 2.15–5.95) seconds], and poor extent of VO, favorable and unfavorable indicators of velocity combined with extent, low 4D-VCS [5.67±3.988; 5 (IQR, 3–8)], low COVES [3.088±1.904; 3 (IQR, 2–5)], and high HIR [0.444±0.215; 0.45 (IQR, 0.28–0.61)] were more likely to be found in the poor outcome group (P<0.05), whereas the moderate indicators of velocity combined with extent were not statistically different between the groups (P>0.05).
Table 1
| Variables | Good outcome (n=70) | Poor outcome (n=102) | All (n=172) | P value |
|---|---|---|---|---|
| Age (years) | 68 [59.25–76.75] | 76 [66–81] | 71 [65–80.25] | 0.004 |
| Female | 32 [46] | 50 [49] | 82 [48] | 0.670 |
| Admission NIHSS | 4 [2–9] | 12 [8–16] | 9 [4–15] | <0.001 |
| Risk factors | ||||
| Hypertension | 45 [64] | 74 [73] | 119 [69] | 0.249 |
| Diabetes mellitus | 25 [36] | 44 [43] | 69 [40] | 0.329 |
| Coronary heart disease | 9 [13] | 22 [22] | 31 [18] | 0.144 |
| Hyperlipemia | 21 [30] | 24 [24] | 45 [26] | 0.343 |
| Atrial fibrillation | 21 [30] | 53 [52] | 74 [43] | 0.004 |
| Smoking | 26 [37] | 36 [35] | 62 [36] | 0.804 |
| Time from symptom onset to admission (hour) | ||||
| t<4.5 | 33 [47] | 44 [43] | 77 [45] | 0.604 |
| 4.5≤ t ≤6 | 5 [7] | 10 [10] | 15 [9] | 0.543 |
| 6< t ≤ 24 | 27 [39] | 35 [34] | 62 [36] | 0.568 |
| Wake-up stroke (>24 h) | 5 [7] | 13 [13] | 18 [10] | 0.238 |
| Treatment methods | ||||
| Conservative treatment | 27 [39] | 45 [44] | 72 [42] | 0.469 |
| Intravenous thrombolysis | 19 [27] | 18 [18] | 37 [22] | 0.137 |
| Arterial thrombectomy | 15 [21] | 28 [27] | 43 [25] | 0.37 |
| Bridging treatment | 9 [13] | 11 [11] | 20 [12] | 0.677 |
Data were presented as median [interquartile range] or n [%]. P<0.05 as the threshold for statistical significance. Admission NIHSS, NIHSS score at admission; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; t, time.
Table 2
| Variables | Good outcome (n=70) | Poor outcome (n=102) | All (n=172) | P value |
|---|---|---|---|---|
| The velocity of venous outflow (in seconds) | ||||
| rCVF1 | 2 [0–4] | 2.6 [2–4.9] | 2 [1.9–4] | 0.004 |
| rCVF2 | 3 [2–4.45] | 4 [2.15–5.95] | 4 [2–5] | 0.003 |
| rCVF21 | 0 [0–2.6] | 1 [−0.95 to 3.15] | 0–65 [0–2.6] | 0.573 |
| The extent of venous outflow | ||||
| Good (≥50%) | 62 [89] | 67 [66] | 129 [75] | 0.001 |
| Poor (<50%) | 8 [11] | 35 [34] | 43 [25] | 0.001 |
| Favourable velocity and extent of venous outflow | ||||
| Fast1 + Good | 42 [60] | 40 [39] | 82 [48] | 0.007 |
| Fast2 + Good | 45 [64] | 40 [39] | 85 [49] | 0.001 |
| Fast21 + Good | 34 [49] | 33 [32] | 67 [39] | 0.032 |
| Moderate velocity and extent of venous outflow | ||||
| Fast1 or Good | 24 [34] | 37 [36] | 61 [35] | 0.789 |
| Fast2 or Good | 24 [34] | 42 [41] | 66 [38] | 0.361 |
| Fast21 or Good | 34 [49] | 58 [57] | 92 [53] | 0.284 |
| Unfavourable velocity and extent of venous outflow | ||||
| Slow1 + Poor | 4 [6] | 25 [25] | 29 [17] | 0.001 |
| Slow2 + Poor | 1 [1] | 20 [20] | 21 [12] | <0.001 |
| Slow21 + Poor | 2 [3] | 11 [11] | 13 [8] | 0.053 |
| 4D-VCS | 10 [6–13] | 5 [3–8] | 7 [3–11] | <0.001 |
| COVES | 5 [4–6] | 3 [2–5] | 4 [2–5] | <0.001 |
| HIR | 0.35 [0.13–0.47] | 0.45 [0.28–0.61] | 0.42 [0.24–0.55] | <0.001 |
Data were presented as median [interquartile range] or n [%]. If the bilateral time difference was less than or equal to the median, it was defined as fast filling (Fast), and if the difference was greater than the median, it was defined as slow filling (Slow). Nine combined indicators were obtained according to the combination of velocity (Fast or Slow), extent (Good or Poor), which were divided into three categories: favorable, moderate and unfavorable. P<0.05 as the threshold for statistical significance. 4D-VCS, four-dimensional venous collateral score; COVES, cortical vein opacification score; Fast1, fast filling of the start of bilateral venous contrast inflow; Fast2, fast filling of venous optimal opacification; Fast21, fast filling between the start of venous contrast inflow and optimal opacification; HIR, hypoperfusion intensity ratio; IQR, interquartile range; rCVF, relative cortical venous filling.
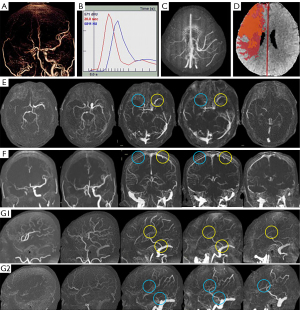
Figure 2 illustrated the vascular and perfusion images of a patient with moderate cerebral VO in the poor outcome group; another two patients with good and poor cerebral VO were illustrated in Figures S1,S2.
Multivariable regression analysis for prognosis
Table 3 demonstrated the results of the univariate logistic analysis, and we regarded indicators with P<0.1 as statistically significant univariate indicators for outcome. Among the clinical indicators, age, admission NIHSS, and atrial fibrillation were statistically significant (P<0.1), indicating that they were associated with prognosis, whereas gender, the remaining risk factors, the onset time of symptoms, and the treatment methods were not associated with prognosis (P>0.1). As for the imaging indicators, except moderate indicators of velocity and extent, other indicators were all associated with prognosis (P<0.1).
Table 3
| Predictors | Poor prognosis (mRS score of 3–6) | ||
|---|---|---|---|
| OR | 95% CI | P value | |
| Age (years) | 1.042 | 1.006–1.071 | 0.003 |
| Female | 1.142 | 0.621–2.101 | 0.670 |
| Admission NIHSS | 1.191 | 1.117–1.271 | <0.001 |
| Risk factors | |||
| Hypertension | 1.468 | 0.763–2.825 | 0.250 |
| Diabetes mellitus | 1.366 | 0.730–2.555 | 0.330 |
| Coronary heart disease | 1.864 | 0.801–4.335 | 0.148 |
| Hyperlipemia | 0.718 | 0.362–1.426 | 0.344 |
| Atrial fibrillation | 2.524 | 1.328–4.796 | 0.005 |
| Smoking | 0.923 | 0.490–1.737 | 0.804 |
| Treatment | |||
| Conservative treatment | 1.364 | 0.501–3.714 | 0.544 |
| Intravenous thrombolysis | 0.775 | 0.260–2.309 | 0.647 |
| Arterial thrombectomy | 1.527 | 0.518–4.503 | 0.443 |
| Bridging treatment | – | – | 0.429 |
| Favourable velocity and extent of venous outflow | |||
| Fast1 + Good | 0.430 | 0.231–0.801 | 0.008 |
| Fast2 + Good | 0.358 | 0.191–0.673 | 0.001 |
| Fast21 + Good | 0.506 | 0.271–0.947 | 0.033 |
| Moderate velocity and extent of venous outflow | |||
| Fast1 or Good | 1.091 | 0.577–2.064 | 0.789 |
| Fast2 or Good | 1.342 | 0.713–2.523 | 0.362 |
| Fast21 or Good | 1.396 | 0.758–2.571 | 0.285 |
| Unfavourable velocity and extent of venous outflow | |||
| Slow1 + Poor | 5.357 | 1.773–16.182 | 0.003 |
| Slow2 + Poor | 16.829 | 2.202–128.618 | 0.007 |
| Slow21 + Poor | 4.110 | 0.882–19.153 | 0.072 |
| 4D-VCS | 0.811 | 0.749–0.877 | <0.001 |
| COVES | 0.645 | 0.528–0.786 | <0.001 |
| TLC | 1.829 | 0.989–3.385 | 0.054 |
P<0.05 as the threshold for statistical significance. 4D-VCS, four-dimensional venous collateral score; Admission NIHSS, NIHSS score at admission; CI, confidence interval; COVES, cortical vein opacification score; Fast1, fast filling of the start of bilateral venous contrast inflow; Fast2, fast filling of venous optimal opacification; Fast21, fast filling between the start of venous contrast inflow and optimal opacification; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; TLC, tissue level collaterals.
Indicators with P<0.1 in the results of the univariate logistic analysis were included in the multivariate regression analysis, and we designed a total of 11 multivariable models, and the results of the multivariable regression analysis were summarized in Table 4. After adjusting for confounders, age and admission NIHSS were independent predictors of poor outcome in every model, and 4D-VCS [odds ratio (OR) =0.863; 95% CI: 0.791–0.941; P<0.05] in Model 2, Slow1 + Poor (OR =4.503; 95% CI: 1.313–15.441; P<0.05) in Model 3, Slow2 + Poor (OR =8.878; 95% CI: 1.063–74.150; P<0.05) in Model 4, COVES (OR =0.737; 95% CI: 0.591–0.919; P<0.05) in Model 6, 4D-VCS (OR =0.829; 95% CI: 0.752–0.915; P<0.05) in Model 8, Slow1 + Poor (OR =5.292; 95% CI: 1.461–19.170; P<0.05) in Model 9, Slow2 + Poor (OR =10.336; 95% CI: 1.196–89.312; P<0.05) in Model 10, and COVES (OR =0.690; 95% CI: 0.544–0.875; P<0.05) in Model 11 can independently predict poor outcome.
Table 4
| Model | OR (95% CI) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Admission NIHSS | 4D-VCS | Fast1 + Good | Fast1 or Good | Slow1 + Poor | Fast2 + Good | Fast2 or Good | Slow2 + Poor | Fast21 + Good | Fast21 or Good | Slow21 + Poor | COVES | TLC | |
| Model 1 (clinical information) | 1.041* (1.010–1.073) | 1.192* (1.115–1.275 | – | – | – | – | – | – | – | – | – | – | – | – |
| Model 2 (clinical information + 4D-VCS) | 1.037* (1.005–1.070) | 1.148* (1.071–1.231) | 0.863* (0.791–0.941) | – | – | – | – | – | – | – | – | – | – | – |
| Model 3 (clinical information + V1&E) | 1.039* (1.007–1.072) | 1.190* (1.111–1.274) | – | – | 0.938 (0.426–2.065) | 4.503* (1.313–15.441) | – | – | – | – | – | – | – | – |
| Model 4 (clinical information + V2&E) | 1.037* (1.005–1.070) | 1.179* (1.099–1.264) | – | – | – | – | – | 0.964 (0.444–2.091) | 8.878* (1.063–74.150) | – | – | – | – | – |
| Model 5 (clinical information + V21&E) | 1.039* (1.008–1.072) | 1.186* (1.108–1.270) | – | – | – | – | – | – | – | – | 1.142 (0.553–2.359) | 1.712 (0.300–9.790) | – | – |
| Model 6 (clinical information + COVES) | 1.038* (1.006–1.071) | 1.161* (1.084–1.243) | – | – | – | – | – | – | – | – | – | – | 0.737* (0.591–0.919) | – |
| Model 7 (clinical information + TLC) | 1.041* (1.010–1.073) | 1.200* (1.117–1.290) | – | – | – | – | – | – | – | – | – | – | – | 0.815 (0.385–1.729) |
| Model 8 (clinical information + 4D-VCS + TLC) | 1.038* (1.005–1.072) | 1.172* (1.088–1.262) | 0.829* (0.752–0.915) | – | – | – | – | – | – | – | – | – | – | 0.414 (0.171–1.004) |
| Model 9 (clinical information + V1&E + TLC) | 1.040* (1.008–1.073) | 1.208* (1.121–1.302) | – | – | 0.963 (0.436–2.124) | 5.292* (1.461–19.170) | – | – | – | – | – | – | – | 0.638 (0.286–1.423) |
| Model 10 (clinical information + V2&E + TLC) | 1.037* (1.005–1.070) | 1.194* (1.107–1.288) | – | – | – | – | – | 0.985 (0.453–2.144) | 10.336* (1.196–89.312) | – | – | – | – | 0.671 (0.305–1.476) |
| Model 11 (clinical information + COVES + TLC) | 1.038* (1.006–1.071) | 1.181* (1.097–1.270) | – | – | – | – | – | – | – | – | – | – | 0.690* (0.544–0.875) | 0.512 (0.221–1.182) |
*, P<0.05 as the threshold for statistical significance. 4D-VCS, four-dimensional venous collateral score; Admission NIHSS, NIHSS score at admission; CI, confidence interval; COVES, cortical vein opacification score; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; TLC, tissue level collaterals; V1&E, the velocity for the veins start to fill combined with the extent of peak venous phase; V2&E, the velocity for venous optimal opacification combined with the extent of peak venous phase; V21&E, the velocity for venous start to fill to optimal opacification combined with the extent of peak venous phase.
Figure S3 and Table 5 demonstrated the ROC curve information of each imaging indicator for predicting prognosis. Among them, 4D-VCS had the largest area under the curve (AUC) of 0.744 (95% CI: 0.668–0.820, P<0.001), which indicated that 4D-VCS had a relatively better prognostic diagnostic ability. The results of the DeLong test among the above indicators suggested that 4D-VCS was statistically different from V1&E, V2&E, V21&E, and HIR respectively (P<0.05), except COVES (P>0.05); in addition, COVES was also statistically different from V21&E (P<0.05); and there was no statistical difference among the remaining indicators (P>0.05). In addition, we obtained an optimal cutoff value of 8.5, a sensitivity of 0.657, and a specificity of 0.755 for the 4D-VCS using Jordon’s Index Maximization method.
Table 5
| Curve source | AUC | 95% CI | P value |
|---|---|---|---|
| 4D-VCS | 0.744 | 0.668–0.820 | <0.001 |
| COVES | 0.703 | 0.625–0.781 | <0.001 |
| V1&E | 0.636 | 0.553–0.718 | 0.003 |
| V2&E | 0.656 | 0.575–0.737 | 0.001 |
| V21&E | 0.599 | 0.514–0.685 | 0.027 |
| HIR | 0.658 | 0.578–0.739 | <0.001 |
P<0.05 as the threshold for statistical significance. 4D-VCS, four-dimensional venous collateral score; AUC, area under curve; CI, confidence interval; COVES, cortical vein opacification score; HIR, hypoperfusion intensity ratio; V1&E, the velocity for the veins start to fill combined with the extent of peak venous phase; V2&E, the velocity for venous optimal opacification combined with the extent of peak venous phase; V21&E, the velocity for venous start to fill to optimal opacification combined with the extent of peak venous phase.
Figure 3 and Table 6 demonstrated the ROC curve information of the 9 multifactorial models for predicting prognosis. Model 8, which included clinical information, 4D-VCS, and TLC, had the largest area under the curve (AUC =0.827, 95% CI: 0.765–0.889; P<0.05), and the results of DeLong’s test suggested that it was statistically different from Model 1, which only included clinical information (P<0.05).
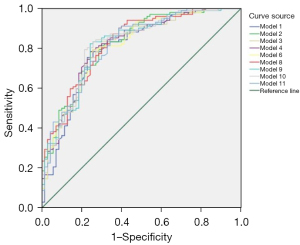
Table 6
| Model | AUC (95% CI) | P value (DeLong test) |
|---|---|---|
| Model 1 (clinical information) (age and Admission NHISS) | 0.798 (0.728–0.868) | – |
| Model 2 (clinical information + 4D-VCS) | 0.820 (0.757–0.884) | 0.241 |
| Model 3 (clinical information + V1&E) | 0.813 (0.747–0.878) | 0.2973 |
| Model 4 (clinical information + V2&E) | 0.817 (0.751–0.882) | 0.079 |
| Model 6 (clinical information + COVES) | 0.813 (0.748–0.879) | 0.3222 |
| Model 8 (clinical information + 4D-VCS + TLC) | 0.827* (0.765–0.889) | 0.0424 |
| Model 9 (clinical information + V1&E + TLC) | 0.819 (0.754–0.884) | 0.1462 |
| Model 10 (clinical information + V2&E + TLC) | 0.820 (0.755–0.885) | 0.0648 |
| Model 11 (clinical information + COVES + TLC) | 0.818 (0.753–0.882) | 0.2388 |
*, P<0.05 is the threshold for statistical significance. Admission NIHSS, NIHSS score at admission; 4D-VCS, four-dimensional venous collateral score; AUC, area under curve; CI, confidence interval; COVES, cortical vein opacification score; TLC, tissue level collaterals; V1&E, the velocity for the veins start to fill combined with the extent of peak venous phase; V2&E, the velocity for venous optimal opacification combined with the extent of peak venous phase.
Multivariable regression analysis for TLC
Regarding the TLC as the outcome index, the results of the univariate logistic analysis revealed that among the clinical indicators, only the admission NIHSS was associated with the TLC (P<0.1), whereas among the imaging indicators, the three combination indicators of velocity and extent, the 4D-VCS, and the COVES were all associated with TLC (P<0.1). Indicators with P<0.1 in the univariate logistic analysis were included in the multivariate logistic analysis, and we designed five multivariable models to investigate factors independently associated with TLC, as shown in Table S1. After adjusting for confounders, admission NIHSS was independently associated with TLC− in each model; additionally, 4D-VCS (OR =0.827; 95% CI: 0.759–0.902; P<0.05) in Model 1, and Slow1 + Poor (OR =2.717; 95% CI: 1.010–7.306; P<0.05) in Model 2 and COVES (OR =0.628; 95% CI: 0.505–0.782; P<0.05) were also independently associated with TLC−. However, none of the V2&E indicators in Model 3, none of the V21&E indicators in Model 4, and the VO in Model 5 were independently associated with TLC− (P<0.05).
Discussion
In this study, we performed dynamic assessment of cerebral VO in patients with AIS-LVO based on 4D-CTA/CTP technology and perfusion post-processing software, and explored the association between cerebral venous drainage and clinical long-term prognosis, and further compared the predictive ability of different methods of cerebral venous assessment as well as different predictive models on prognosis; at the same time, we also evaluated the TLC of all subjects through HIR, which further explored the relationship between cerebral VO and TLC.
The role of VO in cerebral ischemic events
Under normal physiological conditions, blood flow into brain tissue via arteries is matched with VO after passing through the microcirculation (23); however, when the proximal artery is occluded, the brain tissue supplied by it is getting ischemic, the perfusion of the corresponding area is reduced, and the intracranial pressure increases; meanwhile, the veins collapses due to passive compression or constriction due to sympathetic excitation, or the microembolus builds up in the small vein causing obstruction, and if there is a lack of good collateral compensation at this time, the blood flow into the veins will be reduced, and the velocity of the blood flow will be slowed down relatively (24). Thus, VO plays a non-negligible and coherent role in cerebral ischemic events, and the condition of venous filling provides additional information about arterial inflow, actual perfusion of the microcirculation, and it is an indispensable component of the neurovascular network (25); notably, some studies have also found that the effect of VO on cerebral perfusion may be somewhat independent of arterial inflow (9,26), some patients demonstrated good VO but had poor arterial collateralization (27), it further emphasized the importance of venous assessment.
Several previous studies have established scoring methods for VO by sCTA, such as prognostic evaluation based on cortical vein score difference in stroke score (PRECISE), COVES, and others (9,28). In recent years, with the continuous innovation of imaging techniques, multiphase CTA and dynamic CTA have also been used in the assessment of cerebral VO and have demonstrated that the combined assessment of CVF extent and velocity has an additional prognostic value over established prognostic predictors (11,16,18,19,29). Above studies have demonstrated that cerebral VO is closely related to long-term clinical prognosis, and patients with favorable cerebral VO are more likely to benefit from endovascular interventions. However, there are various methods for assessing VO, and it is necessary to find a relatively simple, accurate, yet efficient way, which is one of the aims of our study.
The significance of the VO evaluation for AIS patients
The main findings of this study suggested that among the clinical indicators, age and admission NIHSS were independent predictors of long-term prognosis, and that both high NIHSS score and old age may predict poor long-term prognosis. Among the imaging indicators, low COVES, low 4D-VCS and poor opacified extent of the affected veins with slow inflow and peak velocity were independently associated with a poor clinical long-term prognosis, which is consistent with the results of previous studies (16,17,19). However, although two dynamic assessments of VO used in this study were both independently associated with clinical long-term prognosis, the predictive value of the 4D-VCS was superior. It quantified the filling velocity and extent of the major draining veins (VOT, VOL, SMCV, SPS) in the MCA supplying region, supplemented with time-resolved information, and a dynamic comparative assessment of the affected with healthy side is relatively more comprehensive and efficient. In comparison with the former, when using combined indicators of velocity and extent for assessment, we found that, for the velocity assessment, we used a fixed time gap to calculate the difference, which reduced the patient’s radiation dose but also led to some potential deficiencies: Some patients peaked at a period phase with a 2-second gap, and some peaked at a 5-second gap, with the before and after time differences varying by 2- or 5-second gaps. In addition, except for those who presented with significant large ischemia or infarction, the time difference of delayed venous drainage was mostly fixed in the remaining patients, which explained why the differences of velocity indicators were small. For the assessment of the extent of venous opacification, comparison of bilateral cerebral CVF in the venous peak phase was chosen for dichotomous evaluation, which is relatively easy but slightly more cursory. Therefore, although venous drainage was also considered comprehensively, the combined methods of velocity and extent assessment can be relatively more subjective and complex. Furthermore, in contrast to the findings by van den Wijngaard et al. and Lin et al. (11,16), we also found that not only the venous inflow velocity but also the venous peak velocity of combining indicators were independently associated with clinical long-term prognosis, which is an interesting and worthwhile point to explore. Blood flows into the microcirculatory system via the arteries to achieve microperfusion of brain tissue, and the outlet of the microcirculation is the small veins, and the regulation of blood outflow at the venous end is influenced by a number of factors, as described above: the passive pressure of cerebral parenchymal edema, the positive control of sympathetic nerves, the Windkessel effect, etc., and microemboli are more likely to accumulate in the small veins at the outlet of the microcirculation and cause the blockage (24). The start of venous opacification means that the posterior microcirculatory pathway is open and that microperfusion to at least some of the brain tissue is effective, so this is a very critical sign; it suggested that for ischemic brain tissue, the opening of the posterior microcirculatory pathway earlier or later may be reflective of the condition of microperfusion, and may had an impact on prognosis. It is noteworthy that Model 8, which contains clinical information, 4D-VCS and TLC, has a better predictive ability for long-term prognosis than Model 1, which contains only clinical information (P<0.05), while the remaining multivariable models are not statistically different from Model 1 (P>0.05). This can also suggest that 4D-VCS is a relatively good quantitative dynamic assessment method, and can also indicate the microperfusion status of brain tissue and dynamic cortical venous drainage status of corresponding areas in patients with ischemic stroke. In clinical work, 4D-VCS can be used as image markers similar to arterial collateral and estimated infarct core/penumbra volume to predict long-term clinical outcome and expand treatment time window. For example, according to the 4D-VCS cutoff value of our study, if the 4D-VCS ≥9 at baseline examination, it indicates that the VO status is good. Even if the time window of recanalization is exceeded, clinicians can still choose recanalization therapy after comprehensive evaluation with other indicators to achieve a good prognosis for such patients.
Other findings from the results
In addition, the results about prognosis provided us with some other information. The first point is that TLC was not independently associated with clinical long-term prognosis in the models, which differed from the results of some previous studies (17,20), which may be related to the different patient selection criteria and the post-processing software we used to calculate HIR. Secondary, there was no statistical difference in diagnostic efficacy among the multivariable models created by the combination of clinical and imaging indicators, which may be due to the greater variety of treatment methods in the included patients, and the greater clinical value of admission NIHSS, got the relatively minor impact of the inclusion of the imaging indicators.
As mentioned earlier, in addition to the arterial inflow aspect, VO may reflect additional information about the actual perfusion of the microcirculation. A relevant study has also shown that good VO is a strong predictor of brain tissue microperfusion condition (17). Nevertheless, previous studies have used VO assessed by sCTA to explore the relationship between VO and TLC, and this study utilized the 4D-CTA/CTP technique to dynamically assess VO, and then further explored the relationship. It was demonstrated that a high admission NIHSS, low 4D-VCS, low COVES, and poor venous extent of the affected side with slow velocity for the veins start to fill were all independently associated with poor TLC, again validating previous studies.
What needs to be added to the discussion is that patients who were older or had a history of atrial fibrillation were more likely to have a poor long-term prognosis in baseline characteristics other than admission NIHSS. The older the patient, the lower the metabolic capacity of the organism, and consequently, the higher the likelihood of concomitant underlying disease, and poor vascular condition. Previous studies have demonstrated that age is associated with the condition of pial meningeal collateral circulation, and all of the previously mentioned factors affect prognosis (8,30-32). Patients with a history of atrial fibrillation are more likely to develop thrombus that flow from the internal carotid arteries into the proximal intracranial arteries, resulting in cardioembolic stroke (33,34), which are prone to early or prolonged stroke recurrence and have the highest rate of in-hospital mortality compared with other types of stroke (35-37). The presence of both cardiac and cerebrovascular risks also increases the likelihood of a poor prognosis.
Limitation and prospect
Nevertheless, this study still had some limitations. First, as a retrospective single-center study with a concentrated group of patients could be chosen from, it may be subject to more uncontrollable factors and a higher possibility of selection bias. Secondly, the sample size is relatively small, so expanding the sample size might present the findings more objectively and accurately. Thirdly, the included patients were treated in a variety of ways; although the main venous indicators of the study still showed significant value, some of the other independent variables were more or less affected by this factor, and in the future, after further expansion of the sample size, we will group according to treatment to conduct a more detailed study. At last, recently, Drozdov et al. applied multiphase CTA to evaluate the performance of medullary venous drainage in AIS patients, and they found that compared with cortical or medullary venous asymmetry alone, the combined hemispherical asymmetry of cortical and medullary veins can predict the clinical prognosis of AIS patients better (38). This study only evaluated the cortical veins, so we expect that the deep venous system can be included in the evaluation system in future studies to explain the changes in the post-stroke blood flow pathway more completely. In addition, we also want to investigate the relationship between venous system changes in ischemic stroke and other post-stroke complications, such as malignant cerebral edema and hemorrhagic transformation in short term, which are also closely related to the long-term clinical prognosis (39,40).
Conclusions
In this study, three methods of imaging assessment of cortical veins have been used to investigate and compare the prognostic predictive value of cortical VO. The results suggested that among them, dynamic quantitative assessment of VO by 4D-VCS had relatively higher predictive value, and combining it with clinical indicators and TLC can predict the long-term prognosis of AIS-LVO patients relatively more stably, which reflected the advantages and value of 4D-CTA/CTP technology. In addition, this study also found that poor venous drainage status, whether assessed dynamically or in a single phase, was independently associated with poor TLC, which suggested that the cortical venous drainage status reflected the perfusion status of ischemic brain tissue and had the complementary value for cerebral perfusion information.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-669/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-669/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of The First Affiliated Hospital of Chongqing Medical University (No. 2021-274), granting a waiver of informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang YJ, Li ZX, Gu HQ, Zhai Y, Jiang Y, Zhao XQ, Wang YL, Yang X, Wang CJ, Meng X, Li H, Liu LP, Jing J, Wu J, Xu AD, Dong Q, Wang D, Zhao JZChina Stroke Statistics 2019 Writing Committee. China Stroke Statistics 2019: A Report From the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc Neurol 2020;5:211-39. [Crossref] [PubMed]
- Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11-20. [Crossref] [PubMed]
- Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009-18. [Crossref] [PubMed]
- Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019-30. [Crossref] [PubMed]
- Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296-306. [Crossref] [PubMed]
- Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285-95. [Crossref] [PubMed]
- Fanou EM, Knight J, Aviv RI, Hojjat SP, Symons SP, Zhang L, Wintermark M. Effect of Collaterals on Clinical Presentation, Baseline Imaging, Complications, and Outcome in Acute Stroke. AJNR Am J Neuroradiol 2015;36:2285-91. [Crossref] [PubMed]
- Lima FO, Furie KL, Silva GS, Lev MH, Camargo EC, Singhal AB, Harris GJ, Halpern EF, Koroshetz WJ, Smith WS, Yoo AJ, Nogueira RG. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke 2010;41:2316-22. [Crossref] [PubMed]
- Parthasarathy R, Kate M, Rempel JL, Liebeskind DS, Jeerakathil T, Butcher KS, Shuaib A. Prognostic evaluation based on cortical vein score difference in stroke. Stroke 2013;44:2748-54. [Crossref] [PubMed]
- Jansen IGH, van Vuuren AB, van Zwam WH, van den Wijngaard IR, Berkhemer OA, Lingsma HF, Slump CH, van Oostenbrugge RJ, Treurniet KM, Dippel DWJ, van Walderveen MAA, van der Lugt A, Roos YBWEM, Marquering HA, Majoie CBLM, van den Berg R. MR CLEAN Trial Investigators. Absence of Cortical Vein Opacification Is Associated with Lack of Intra-arterial Therapy Benefit in Stroke. Radiology 2018;286:643-50. [Crossref] [PubMed]
- van den Wijngaard IR, Wermer MJ, Boiten J, Algra A, Holswilder G, Meijer FJ, Dippel DW, Velthuis BK, Majoie CB, van Walderveen MA. Cortical Venous Filling on Dynamic Computed Tomographic Angiography: A Novel Predictor of Clinical Outcome in Patients With Acute Middle Cerebral Artery Stroke. Stroke 2016;47:762-7. [Crossref] [PubMed]
- Liebeskind DS. Collateral lessons from recent acute ischemic stroke trials. Neurol Res 2014;36:397-402. [Crossref] [PubMed]
- Faizy TD, Kabiri R, Christensen S, Mlynash M, Kuraitis G, Mader MM, Albers GW, Lansberg MG, Fiehler J, Wintermark M, Marks MP, Heit JJ. Association of Venous Outflow Profiles and Successful Vessel Reperfusion After Thrombectomy. Neurology 2021;96:e2903-11. [Crossref] [PubMed]
- de Havenon A, Mlynash M, Kim-Tenser MA, Lansberg MG, Leslie-Mazwi T, Christensen S, McTaggart RA, Alexander M, Albers G, Broderick J, Marks MP, Heit JJ. DEFUSE 3 Investigators. Results From DEFUSE 3: Good Collaterals Are Associated With Reduced Ischemic Core Growth but Not Neurologic Outcome. Stroke 2019;50:632-8. [Crossref] [PubMed]
- Volders D, Shewchuk JR, Marangoni M, Ni Mhurchu E, Heran M. Beyond the collaterals: Additional value of multiphase CTA in acute ischemic stroke evaluation. Neuroradiol J 2019;32:309-14. [Crossref] [PubMed]
- Lin J, Cheng Z, Shi Y, Cai X, Huang L. Evaluating the Velocity and Extent of Cortical Venous Filling in Patients With Severe Middle Cerebral Artery Stenosis or Occlusion. Front Neurol 2021;12:610658. [Crossref] [PubMed]
- Faizy TD, Kabiri R, Christensen S, Mlynash M, Kuraitis GM, Broocks G, Flottmann F, Marks MP, Lansberg MG, Albers GW, Fiehler J, Wintermark M, Heit JJ. Favorable Venous Outflow Profiles Correlate With Favorable Tissue-Level Collaterals and Clinical Outcome. Stroke 2021;52:1761-7. [Crossref] [PubMed]
- Singh N, Bala F, Kim BJ, Najm M, Ahn SH, Fainardi E, Rubiera M, Khaw AV, Zini A, Goyal M, Menon BK, Almekhlafi M. Time-resolved assessment of cortical venous drainage on multiphase CT angiography in patients with acute ischemic stroke. Neuroradiology 2022;64:897-903. [Crossref] [PubMed]
- Cao R, Jiang Y, Li L, Yang X, Wang H, Chen M, Chen J. Venous collaterals in acute ischemic stroke patients after endovascular treatments: a novel scoring system using 4D computed tomography angiography. Quant Imaging Med Surg 2022;12:5030-43. [Crossref] [PubMed]
- Olivot JM, Mlynash M, Inoue M, Marks MP, Wheeler HM, Kemp S, Straka M, Zaharchuk G, Bammer R, Lansberg MG, Albers GW. DEFUSE 2 Investigators. Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 Cohort. Stroke 2014;45:1018-23. [Crossref] [PubMed]
- Lyndon D, van den Broek M, Niu B, Yip S, Rohr A, Settecase F. Hypoperfusion Intensity Ratio Correlates with CTA Collateral Status in Large-Vessel Occlusion Acute Ischemic Stroke. AJNR Am J Neuroradiol 2021;42:1380-6. [Crossref] [PubMed]
- van Horn N, Broocks G, Kabiri R, Kraemer MC, Christensen S, Mlynash M, Meyer L, Lansberg MG, Albers GW, Sporns P, Guenego A, Fiehler J, Wintermark M, Heit JJ, Faizy TD. Cerebral Hypoperfusion Intensity Ratio Is Linked to Progressive Early Edema Formation. J Clin Med 2022;11:2373. [Crossref] [PubMed]
- Lassen NA. Control of cerebral circulation in health and disease. Circ Res 1974;34:749-60. [Crossref] [PubMed]
- Tong LS, Guo ZN, Ou YB, Yu YN, Zhang XC, Tang J, Zhang JH, Lou M. Cerebral venous collaterals: A new fort for fighting ischemic stroke? Prog Neurobiol 2018;163-164:172-93. [Crossref] [PubMed]
- Munuera J, Blasco G, Hernández-Pérez M. Daunis-I-Estadella P, Dávalos A, Liebeskind DS, Wintermark M, Demchuk A, Menon BK, Thomalla G, Nael K, Pedraza S, Puig J. Venous imaging-based biomarkers in acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2017;88:62-9. [Crossref] [PubMed]
- Parthasarathy R, Sohn SI, Jeerakathil T, Kate MP, Mishra SM, Nambiar VK, Ahmad A, Menon BK, Shuaib A. A Combined Arterial and Venous Grading Scale to Predict Outcome in Anterior Circulation Ischemic Stroke. J Neuroimaging 2015;25:969-77. [Crossref] [PubMed]
- Faizy TD, Mlynash M, Kabiri R, Christensen S, Kuraitis GM, Mader MM, Flottmann F, Broocks G, Lansberg MG, Albers GW, Marks MP, Fiehler J, Wintermark M, Heit JJ. The Cerebral Collateral Cascade: Comprehensive Blood Flow in Ischemic Stroke. Neurology 2022;98:e2296-306. [Crossref] [PubMed]
- Hoffman H, Ziechmann R, Swarnkar A, Masoud HE, Gould G. Cortical Vein Opacification for Risk Stratification in Anterior Circulation Endovascular Thrombectomy. J Stroke Cerebrovasc Dis 2019;28:1710-7. [Crossref] [PubMed]
- Wang J, Li J, Liu J, Wu J, Gu S, Yao Y, Luo T, Huang C, Huang F, Li Y. Significant Slowed Cortical Venous Blood Flow in Patients with Acute Ischemic Stroke with Large Vessel Occlusion Suggests Poor Collateral Circulation and Prognosis. Acad Radiol 2023;30:1896-903. [Crossref] [PubMed]
- Yeo LL, Paliwal P, Teoh HL, Seet RC, Chan BP, Ting E, Venketasubramanian N, Leow WK, Wakerley B, Kusama Y, Rathakrishnan R, Sharma VK. Assessment of intracranial collaterals on CT angiography in anterior circulation acute ischemic stroke. AJNR Am J Neuroradiol 2015;36:289-94. [Crossref] [PubMed]
- Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, Viñuela F, Liebeskind DSUCLA Collateral Investigators. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2008;79:625-9. [Crossref] [PubMed]
- Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, Lee KH, Liebeskind DS. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke 2011;42:693-9. [Crossref] [PubMed]
- Caplan LR. Brain embolism, revisited. Neurology 1993;43:1281-7. [Crossref] [PubMed]
- Bogousslavsky J, Cachin C, Regli F, Despland PA, Van Melle G, Kappenberger L. Cardiac sources of embolism and cerebral infarction--clinical consequences and vascular concomitants: the Lausanne Stroke Registry. Neurology 1991;41:855-9. [Crossref] [PubMed]
- Caplan LR, Hier DB, D'Cruz I. Cerebral embolism in the Michael Reese Stroke Registry. Stroke 1983;14:530-6. [Crossref] [PubMed]
- Hornig CR, Brainin M, Mast H. Cardioembolic stroke: results from three current stroke data banks. Neuroepidemiology 1994;13:318-23. [Crossref] [PubMed]
- Lodder J, Krijne-Kubat B, Broekman J. Cerebral hemorrhagic infarction at autopsy: cardiac embolic cause and the relationship to the cause of death. Stroke 1986;17:626-9. [Crossref] [PubMed]
- Drozdov AA, Arora M, Leon Guerrero CR, Sparks AD, Reza Taheri M. Appearance of medullary and cortical veins on multiphase CT-angiography in patients with acute ischemic stroke. Clin Neurol Neurosurg 2023;224:107523. [Crossref] [PubMed]
- Cao R, Ye G, Lu Y, Wang Y, Jiang Y, Sun C, Chen M, Chen J. The Predictive Value of Cerebral Veins on Hemorrhagic Transformation After Endovascular Treatment in Acute Ischemic Stroke Patients: Enhanced Insights From Venous Collateral Circulation Analysis Using Four-Dimensional CTA. Acad Radiol 2024;31:1024-35. [Crossref] [PubMed]
- Faizy TD, Kabiri R, Christensen S, Mlynash M, Kuraitis G, Meyer L, Marks MP, Broocks G, Flottmann F, Lansberg MG, Albers GW, Fiehler J, Wintermark M, Heit JJ. Venous Outflow Profiles Are Linked to Cerebral Edema Formation at Noncontrast Head CT after Treatment in Acute Ischemic Stroke Regardless of Collateral Vessel Status at CT Angiography. Radiology 2021;299:682-90. [Crossref] [PubMed]


