
Contrast-enhanced ultrasound combined with shear wave elastography in the diagnosis of C-TIRADS category 4 thyroid nodules
Introduction
Following the popularization of ultrasound examination, the diagnosis of thyroid nodules in the population is increasing. Thyroid cancer is currently the 13th most commonly diagnosed cancer and the 6th most commonly diagnosed cancer in women (1). Only 5–15% of thyroid nodules are malignant (2). Fine needle aspiration (FNA) and molecular testing have improved the diagnostic accuracy of thyroid nodules (2-4). Clinical practice guidelines on the management of thyroid cancer have been modified over the last 15 years to reduce overdiagnosis and any resultant overtreatment (1). The 2020 Chinese Thyroid Imaging Reporting and Data System (C-TIRADS) provides an effective approach for the differential diagnosis of thyroid nodules (5-11). The C-TIRADS uses counting methods, and has been shown to be more convenient and practical than weighting methods of the Thyroid Imaging Reporting and Data System (TI-RADS) that were developed by the American College of Radiology for clinical application. Due to overlapping in the imaging features of benign and malignant thyroid nodules in conventional ultrasound, the malignant risk of C-TIRADS category 4 nodules ranges from 2% to 90% (5,7). Thus, a combination of technical examinations is necessary to diagnose C-TIRADS category 4 nodules.
Contrast-enhanced ultrasound (CEUS) shows the distribution of microvessels in thyroid nodules, which is conducive to the diagnosis of nodules (12-14). Shear wave elastography (SWE) is also widely used in the diagnosis of thyroid nodules (15). For the differentiation of benign and malignant thyroid nodules, conventional ultrasound is first used to classify the nodules according to the C-TIRADS. Follow-up examinations may be recommended for C-TIRADS category 2 and 3 nodules; a combination of SWE, CEUS, or FNA may be recommended for C-TIRADS category 4 nodules; and FNA or surgery may be recommended for C-TIRADS category 5 nodules.
This study retrospectively analyzed the clinical data, sonographic features, and CEUS and SWE images of 219 patients with 219 C-TIRADS category 4 thyroid nodules confirmed by surgical pathology to investigate the diagnostic value of CEUS combined with SWE in the diagnosis of C-TIRADS category 4 nodules. We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-666/rc).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Jiangsu Corps Hospital, Chinese People’s Armed Police Forces (No. 20210201), and the requirement of individual consent for this retrospective analysis was waived. From July 2020 to July 2022, a total of 227 patients with thyroid nodules were identified for inclusion in the study. To be eligible for inclusion in the study, the patients had to meet the following inclusion criteria: (I) have a maximal thyroid nodule size ≥5 mm; (II) have C-TIRADS category 4A, 4B, or 4C nodules; (III) have undergone CEUS and SWE examinations; and (IV) have had the nodule diagnosis confirmed by surgical pathology. Patients were excluded from the study if they met any of the following exclusion criteria: (I) were aged <10 years old or >80 years old (n=3); and/or (II) did not have complete CEUS and SWE imaging data (n=5). For multiple nodules, the nodule most suspicious for malignancy was selected first, and the largest nodule was then evaluated. Ultimately, 219 patients with 219 nodules were enrolled in the study.
Conventional ultrasound and SWE
A LOGIQ E9 ultrasonic diagnostic instrument with a 6–15-MHz probe (GE Healthcare, Milwaukee, WI, USA) was used for conventional ultrasonic examinations by different doctors, who each had more than 10 years of experience in thyroid ultrasound diagnosis. The patient was placed in the supine position, fully exposing the anterior cervical region, and the size, echo, shape, boundary, microcalcification, capsule, blood supply, and cervical lymph nodes were observed. All the thyroid nodules included in the study were classified according to the C-TIRADS. The malignant ultrasonic features of thyroid nodule classification in the C-TIRADS include markedly hypoechoic, solid composition, irregular or ill-defined margins or extrathyroidal extensions, microcalcifications, and vertical orientation. One point was added for each feature. A comet-tail artifact is a benign ultrasonic feature, and one point was deducted if it was observed. Finally, the nodules were classified according to the total score. Any disagreements were resolved by consultation and discussion until a consensus was reached.
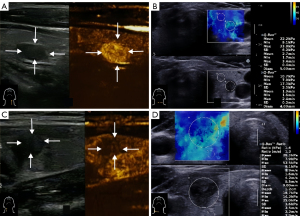
The SWE was operated by the senior doctor with 18 years of experience in thyroid ultrasound diagnosis. The doctor used the French Supersonic Aixplorer instrument with the 4–15-MHz probe (Supersonic Imagine, Aix-en-Provence, France), and the SWE indicators were measured. The transverse section and the longitudinal section of all the nodules were measured five times each, and the average values were taken. In terms of the SWE indicators, this study only included the maximum value of Young’s modulus (Emax; kPa) (Figure 1).
CEUS examination and analysis
An Esaote MyLab Twice ultrasonic diagnostic instrument with a LA523 probe (Esaote, Genoa, Italy) was used for CEUS, which was performed by a senior doctor with 18 years of experience in thyroid ultrasound diagnosis. The mechanical index was adjusted to 0.06. The probe was fixed on the optimal longitudinal section of the nodule, the imaging mode was switched, and 1.5 mL of SonoVue (Bracco Suisse SA, Paradiso, Switzerland), was injected into the patient’s elbow vein quickly, and the vein was then flushed with 5 mL of physiological saline. The nodule was dynamically observed, and the videos and images were saved. The enhancement level of the nodule was classified as high, equal, or low. The enhancement mode was divided into centripetal, eccentric and diffuse enhancement. The enhancement uniformity was classified as uniform or non-uniform. After enhancement, the boundary enhancement was classified as clear or unclear. The peripheral annular enhancement was classified as yes or no. The resolution mode was divided into fast, synchronous, and slow resolution. The CEUS features of the malignant nodules included low enhancement, centripetal enhancement, uneven enhancement, an unclear boundary after enhancement, and fast resolution (Figure 1).
When CEUS was combined with SWE for diagnosis, if CEUS showed to typical benign signs (i.e., annular enhancement, equal enhancement, or high enhancement, and slow regression or synchronous regression), the nodule was assessed as negative; for the rest of the nodules, if one of the methods was positive, the combined diagnosis was assessed as positive.
Statistical analysis
SPSS 20.0 (IBM, Chicago, IL, USA) was used for the statistical analysis. The normally distributed quantitative data are expressed as the mean ± standard deviation, and the t-test was used for inter-group comparisons. The non-normally distributed quantitative data are expressed as the interquartile range, and the non-parametric Mann-Whitney test was used for inter-group comparisons. The sex ratio and the diagnostic efficiency index were compared using the chi-square test. A P value <0.05 indicated a statistically significant difference.
Results
Clinical data and postoperative pathologies
There were 66 males and 153 females in this study. The age of the patients ranged from 19 to 75 years (average age: 45.0±13.2 years). The sizes of the nodules ranged from 5 to 28 mm. There were 39, 75, and 105 C-TIRADS category 4A, 4B, and 4C nodules, respectively. Of the 219 nodules, 168 (76.7%) were diagnosed as malignant and 51 (23.3%) as benign by postoperative pathology. The nodules included 26 nodular goiters, 16 follicular adenomas, 3 chronic lymphocytic thyroiditis, 2 subacute thyroiditis, 2 Hashimoto nodules, 2 adenomatous goiters, 164 thyroid papillary carcinomas, and 4 follicular thyroid carcinomas. There were no significant differences between the patients with benign and malignant nodules in terms of the gender ratio and size of the nodules (P>0.05). However, there was a statistically significant difference between the patients with benign and malignant nodules in terms of age (t =2.224, P=0.027; Table 1).
Table 1
| Clinical data | Benign (n=51) | Malignant (n=168) | Total (n=219) | P value |
|---|---|---|---|---|
| Size (mm), median (Q1, Q3) | 9 (7, 13) | 9 (7, 12) | 9 (7, 12) | 0.830 |
| Male, n (%) | 15 (29.4) | 51 (30.4) | 66 (30.1) | 0.897 |
| Age (years), mean ± SD | 48.9±11.5 | 43.9±13.5 | 45.0±13.2 | 0.027 |
SD, standard deviation.
CEUS characteristics of thyroid nodules
There were statistically significant differences in the CEUS characteristics between the benign and malignant nodules (P<0.05; Table 2). The malignant nodules mainly showed low enhancement (72.6%), centripetal enhancement (76.2%), enhancement heterogeneity (83.9%), an unclear enhancement boundary (81.5%), no peripheral enhancement ring (94.0%), and earlier wash-out (69.6%). While the benign nodules showed a peripheral enhancement ring (76.5%), and later or equal wash-out (82.4%). Considering the enhanced degree, pattern, homogeneity, boundary, peripheral enhancement ring, and wash-out time together, the most observed pattern in 64 (38.1%) malignant nodules was low enhancement, an unclear enhancement boundary, and later wash-out, while the second most observed pattern in 51 (30.6%) malignant nodules was low enhancement, no peripheral enhancement ring, and enhancement heterogeneity.
Table 2
| Characteristics | Benign, n (%) | Malignant, n (%) | P value |
|---|---|---|---|
| Enhancement degree | <0.001 | ||
| Hypo-enhancement | 10 (19.6) | 122 (72.6) | |
| Iso-enhancement | 25 (49.0) | 39 (23.2) | |
| Hyper-enhancement | 16 (31.4) | 7 (4.2) | |
| Enhancement pattern | 0.008 | ||
| Centripetal | 30 (58.8) | 128 (76.2) | |
| Centrifugal | 3 (5.9) | 14 (8.3) | |
| Diffuse | 18 (35.3) | 26 (15.5) | |
| Enhancement homogeneity | <0.001 | ||
| Homogeneity | 33 (64.7) | 27 (16.1) | |
| Heterogeneity | 18 (35.3) | 141 (83.9) | |
| Enhanced rear boundary | <0.001 | ||
| Clear | 41 (80.4) | 31 (18.5) | |
| Unclear | 10 (19.6) | 137 (81.5) | |
| Peripheral enhancement ring | <0.001 | ||
| Yes | 39 (76.5) | 10 (6.0) | |
| No | 12 (23.5) | 158 (94.0) | |
| Wash-out time | <0.001 | ||
| Earlier | 9 (17.6) | 117 (69.6) | |
| Later | 32 (62.7) | 28 (16.7) | |
| Equal | 10 (19.6) | 23 (13.7) | |
| Total | 51 (100.0) | 168 (100.0) | – |
Diagnostic efficiency of CEUS and SWE
The receiver operator characteristic (ROC) curves showed that the cut-off value of the Emax of SWE was 40.85 kPa, and the areas under the curve (AUCs) of the ROC curves for CEUS and SWE were 0.899 [95% confidence interval (CI): 0.847–0.951] and 0.838 (95% CI: 0.775–0.901), respectively (Figure 2). The sensitivity, specificity, accuracy, negative predictive value (NPV), positive predictive value (PPV), positive likelihood ratio (LR+), and negative likelihood ratio (LR−) of CEUS were 81.0% (136/168), 86.3% (44/51), 82.2% (180/219), 57.9% (44/76), 95.1% (136/143), 5.90, and 0.22, while those for SWE were 78.6% (132/168), 74.5% (38/51), 77.6% (170/219), 51.4% (38/74), 91.0% (132/145), 3.08, and 0.29, respectively (Table 3). For the C-TIRADS category 4A nodules, the AUCs of CEUS and SWE were 0.933 (95% CI: 0.853–0.983) and 0.840 (95% CI: 0.719–0.991), respectively. For the C-TIRADS category 4B nodules, the AUCs of CEUS and SWE were 0.886 (95% CI: 0.783–0.990) and 0.805 (95% CI: 0.699–0.912), respectively. For the C-TIRADS category 4C nodules, the AUCs of CEUS and SWE were 0.886 (95% CI: 0.810–0.963) and 0.826 (95% CI: 0.669–0.982), respectively.
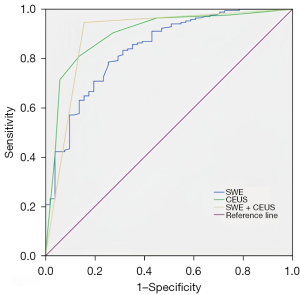
Table 3
| Methods | Pathology | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | NPV (%) (95% CI) |
PPV (%) (95% CI) |
Accuracy (%) (95% CI) | LR+ | LR− | |
|---|---|---|---|---|---|---|---|---|---|
| Benign | Malignant | ||||||||
| CEUS | 81.0 (75.0–86.9) | 86.3 (76.8–95.7) | 57.9 (46.8–69.0) | 95.1 (91.6–98.6) | 82.2 (77.1–87.3) | 5.90 | 0.22 | ||
| Negative | 44 | 32 | |||||||
| Positive | 7 | 136 | |||||||
| SWE | 78.6 (72.4–84.8) | 74.5 (62.5–86.5) | 51.4 (40.0–62.7) | 91.0 (86.4–95.7) | 77.6 (72.1–83.1) | 3.08 | 0.29 | ||
| Negative | 38 | 36 | |||||||
| Positive | 13 | 132 | |||||||
| CEUS + SWE | 94.6 (91.2–98.0) | 84.3 (74.3–94.3) | 82.7 (72.4–93.0) | 95.2 (92.0–98.4) | 92.2 (88.7–95.8) | 6.03 | 0.06 | ||
| Negative | 43 | 9 | |||||||
| Positive | 8 | 159 | |||||||
CEUS, contrast-enhanced ultrasound; CI, confidence interval; LR−, negative likelihood ratio; LR+, positive likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; SWE, shear wave elastography.
Diagnostic efficacy of CEUS + SWE
The AUC, sensitivity, specificity, NPV, PPV, accuracy, LR+, and LR− of CEUS and SWE combined were 0.895 (95% CI: 0.833–0.956), 94.6% (159/168), 84.3% (43/51), 82.7% (43/52), 95.2% (159/167), 92.2% (202/219), 6.03, and 0.06, respectively (Figure 2 and Table 3). CEUS + SWE compared with CEUS or SWE, there were no statistically significant differences in the specificity and PPV (all P>0.05), but there were a statistically significant differences in the sensitivity, NPV, and accuracy (all P<0.05) (Table 4).
Table 4
| Comparisons | P value | ||||
|---|---|---|---|---|---|
| Sensitivity | Specificity | NPV | PPV | Accuracy | |
| CEUS + SWE vs. CEUS | <0.001 | 0.603 | 0.003 | 0.966 | 0.002 |
| CEUS + SWE vs. SWE | <0.001 | 0.221 | <0.001 | 0.142 | <0.001 |
CEUS, contrast-enhanced ultrasound; NPV, negative predictive value; PPV, positive predictive value; SWE, shear wave elastography.
Discussion
C-TIRADS has been widely recognized for its ability to differentiate between thyroid nodules (5-11). The C-TIRADS has a number of features (5). First, the classification of thyroid nodules using the counting method of the C-TIRADS is simple, feasible, and easy to promote. Second, the classification of C-TIRADS is more in line with clinical practice than other classifications, and its nodule malignancy risk rate from class 2 to class 5 gradually increases from 0% to >90%, providing clear information for the clinical development of thyroid nodule diagnosis and treatment plans. Third, the C-TIRADS closely follows the new international concept of thyroid nodule diagnosis and treatment, and tries to avoid overtreatment of papillary thyroid microcarcinoma as much as possible, while ultrasound guided thermal ablation treatment under strict indications can be chosen. Fourth, the C-TIRADS classification must be combined with the patient’s clinical history. Fifth, the C-TIRADS is suitable for China’s national conditions. For hospitals in China that have not yet conducted FNA, the C-TIRADS classification may provide some reference opinions for surgeons as to whether (or not) to perform thyroid nodule surgery. For C-TIRADS category 4 nodules, ultrasound combining with other technologies to further improve the diagnostic ability is a current research hotpot (16,17).
CEUS has been used in the clinic for many years. The main principle of CEUS is to generate scattering echo through microbubbles of contrast agents, strengthen blood flow signals in the lesions, dynamically observe microvascular changes in different phases of the target lesions, and improve diagnostic accuracy (12-14). It is a valuable new method for the diagnosis of thyroid nodules. It plays an important role in guiding the ablation procedure of nodules and accurately evaluating therapeutic efficacy (18). Huang et al. reported that CEUS was useful in differentiating between thyroid nodules, and had better accuracy for nodules with a size ≥0.66 cm (19). Yang et al. reported that a CEUS quantitative analysis could be helpful in the differential diagnosis of thyroid nodules in patients with Hashimoto’s thyroiditis (20). Similar to previous reports (16-18), this study showed that the CEUS features of malignant nodules mainly included low enhancement (72.6%), enhancement heterogeneity (83.9%), unclear enhancement boundary (81.5%), and earlier wash-out (69.6%), while those of benign thyroid nodules mainly included a peripheral enhancement ring (76.5%), and later or equal wash-out (82.4%). In a few nodules, two-dimensional ultrasound showed an extremely low echo, a blurred boundary, and a vertical position, while CEUS showed star-like enhancement, highly suggestive of necrotic lesions. For such nodules, excessive FNA, and excessive diagnosis and treatment can be avoided to some extent. Ruan et al. reported that according to the qualitative CEUS characteristics and the simplified regression coefficients of non-enhanced ultrasound, the CEUS TI-RADS of thyroid nodule malignant risk stratification had a high AUC of 0.93 (21). Petrasova et al. noted that ring enhancement is a powerful indicator of benign nodules, while heterogeneous enhancement is valuable for detecting malignant nodules (22). Jin et al. reported that the combination of the C-TIRADS and CEUS had higher diagnostic accuracy than any single method (23).
Ultrasonic elastography can be divided into two types according to whether or not an artificial external force is needed. SWE reflects tissue elasticity by transmitting an acoustic radiation pulse to generate shear waves without external force. Gao et al. reported that the C-TIRADS in combination with SWE had better diagnostic efficacy than the C-TIRADS or SWE alone (24). Brandenstein et al. noted that the use of elastography and CEUS provided new possibilities for distinguishing between benign and malignant thyroid nodules (25).
CEUS combined with the C-TIRADS is helpful in diagnosing thyroid nodules. Zhu et al. reported that combining CEUS with the C-TIRADS improved the sensitivity of the C-TIRADS, and had better diagnostic efficacy than C-TIRADS and CEUS alone (16). Cao et al. found that the differential diagnostic value of CEUS combined with the C-TIRADS for benign and malignant thyroid nodules was higher than that of the C-TIRADS alone (26). Cheng et al. adjusted the classification of C-TIRADS category 4 thyroid nodules through CEUS, and the results showed that the diagnostic performance after the adjustment was significantly improved, especially for the nodules recommended for FNA by C-TIRADS (17). Brandenstein et al. reported that the multiparametric ultrasound that consisted of B-mode, SWE, and CEUS, and included a time-intensity-curve analysis, provided new possibilities for the preoperative differential diagnosis of benign and malignant thyroid lesions (27). The results of our study showed that SWE combined with CEUS improved the sensitivity, NPV, and accuracy of diagnosing thyroid nodules.
This study had some limitations. First, as a retrospective study, selection bias was inevitable. Second, the analysis of the CEUS characteristics lacked any objective analysis of quantitative indicators. Third, the CEUS features of different sizes of nodules differed. Thus, the sample size for grouping research should be increased. Fourth, other SWE indicators should be added to the analysis in the future research. Finally, the comparisons did not account for multiplicity to control the type I error.
Conclusions
For C-TIRADS category 4 thyroid nodules, CEUS combined with SWE had high diagnostic efficacy, and the combination improved the diagnostic sensitivity, NPV, and accuracy, and could help guide the treatment and management of patients.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-666/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-666/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Jiangsu Corps Hospital, Chinese People’s Armed Police Forces (No. 20210201), and the requirement of individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kitahara CM, Schneider AB. Epidemiology of Thyroid Cancer. Cancer Epidemiol Biomarkers Prev 2022;31:1284-97. [Crossref] [PubMed]
- Patel J, Klopper J, Cottrill EE. Molecular diagnostics in the evaluation of thyroid nodules: Current use and prospective opportunities. Front Endocrinol (Lausanne) 2023;14:1101410. [Crossref] [PubMed]
- Fiorentino V. Dell' Aquila M, Musarra T, Martini M, Capodimonti S, Fadda G, Curatolo M, Traini E, Raffaelli M, Lombardi CP, Pontecorvi A, Larocca LM, Pantanowitz L, Rossi ED. The Role of Cytology in the Diagnosis of Subcentimeter Thyroid Lesions. Diagnostics (Basel) 2021;11:1043. [Crossref] [PubMed]
- Dell'Aquila M, Gravina C, Cocomazzi A, Capodimonti S, Musarra T, Sfregola S, Fiorentino V, Revelli L, Martini M, Fadda G, Pantanowitz L, Larocca LM, Rossi ED. A large series of hyalinizing trabecular tumors: Cytomorphology and ancillary techniques on fine needle aspiration. Cancer Cytopathol 2019;127:390-8. [Crossref] [PubMed]
- Zhou J, Yin L, Wei X, Zhang S, Song Y, Luo B, et al. 2020 Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: the C-TIRADS. Endocrine 2020;70:256-79. [Crossref] [PubMed]
- Hu Y, Xu S, Zhan W. Diagnostic performance of C-TIRADS in malignancy risk stratification of thyroid nodules: A systematic review and meta-analysis. Front Endocrinol (Lausanne) 2022;13:938961. [Crossref] [PubMed]
- Zhou J, Song Y, Zhan W, Wei X, Zhang S, Zhang R, et al. Thyroid imaging reporting and data system (TIRADS) for ultrasound features of nodules: multicentric retrospective study in China. Endocrine 2021;72:157-70. [Crossref] [PubMed]
- Chen Q, Lin M, Wu S. Validating and Comparing C-TIRADS, K-TIRADS and ACR-TIRADS in Stratifying the Malignancy Risk of Thyroid Nodules. Front Endocrinol (Lausanne) 2022;13:899575. [Crossref] [PubMed]
- Cai Y, Yang R, Yang S, Lu L, Ma R, Xiao Z, Lin N, Huang Y, Chen L. Comparison of the C-TIRADS, ACR-TIRADS, and ATA guidelines in malignancy risk stratification of thyroid nodules. Quant Imaging Med Surg 2023;13:4514-25. [Crossref] [PubMed]
- Yang J, Sun Y, Li X, Zhao Y, Han X, Chen G, Ding W, Li R, Wang J, Xiao F, Liu C, Xu S. Diagnostic performance of six ultrasound-based risk stratification systems in thyroid follicular neoplasm: A retrospective multi-center study. Front Oncol 2022;12:1013410. [Crossref] [PubMed]
- Lin Y, Lai S, Wang P, Li J, Chen Z, Wang L, Guan H, Kuang J. Performance of current ultrasound-based malignancy risk stratification systems for thyroid nodules in patients with follicular neoplasms. Eur Radiol 2022;32:3617-30. [Crossref] [PubMed]
- Trimboli P, Castellana M, Virili C, Havre RF, Bini F, Marinozzi F, D'Ambrosio F, Giorgino F, Giovanella L, Prosch H, Grani G, Radzina M, Cantisani V. Performance of contrast-enhanced ultrasound (CEUS) in assessing thyroid nodules: a systematic review and meta-analysis using histological standard of reference. Radiol Med 2020;125:406-15. [Crossref] [PubMed]
- Sorrenti S, Dolcetti V, Fresilli D, Del Gaudio G, Pacini P, Huang P, Camponovo C, Leoncini A, D'Andrea V, Pironi D, Frattaroli F, Trimboli P, Radzina M, Cantisani V. The Role of CEUS in the Evaluation of Thyroid Cancer: From Diagnosis to Local Staging. J Clin Med 2021;10:4559. [Crossref] [PubMed]
- Zhang Y, Zhang X, Li J, Cai Q, Qiao Z, Luo YK. Contrast-enhanced ultrasound: a valuable modality for extracapsular extension assessment in papillary thyroid cancer. Eur Radiol 2021;31:4568-75. [Crossref] [PubMed]
- Swan KZ, Nielsen VE, Bonnema SJ. Evaluation of thyroid nodules by shear wave elastography: a review of current knowledge. J Endocrinol Invest 2021;44:2043-56. [Crossref] [PubMed]
- Zhu T, Chen J, Zhou Z, Ma X, Huang Y. Differentiation of Thyroid Nodules (C-TIRADS 4) by Combining Contrast-Enhanced Ultrasound Diagnosis Model With Chinese Thyroid Imaging Reporting and Data System. Front Oncol 2022;12:840819. [Crossref] [PubMed]
- Cheng H, Zhuo SS, Rong X, Qi TY, Sun HG, Xiao X, Zhang W, Cao HY, Zhu LH, Wang L. Value of Contrast-Enhanced Ultrasound in Adjusting the Classification of Chinese-TIRADS 4 Nodules. Int J Endocrinol 2022;2022:5623919. [Crossref] [PubMed]
- Radzina M, Ratniece M, Putrins DS, Saule L, Cantisani V. Performance of Contrast-Enhanced Ultrasound in Thyroid Nodules: Review of Current State and Future Perspectives. Cancers (Basel) 2021;13:5469. [Crossref] [PubMed]
- Huang K, Bai Z, Bian D, Yang P, Li X, Liu Y. Diagnostic Accuracy of Contrast-Enhanced Ultrasonography in Papillary Thyroid Microcarcinoma Stratified by Size. Ultrasound Med Biol 2020;46:269-74. [Crossref] [PubMed]
- Yang W, Zhou J, Yue C, He Y, Lei J, Chen Y, Ma B. Clinical value of contrast-enhanced ultrasound quantitative analysis for differentiating thyroid lesions in Hashimoto's thyroiditis patients. Quant Imaging Med Surg 2024;14:944-57. [Crossref] [PubMed]
- Ruan J, Xu X, Cai Y, Zeng H, Luo M, Zhang W, Liu R, Lin P, Xu Y, Ye Q, Ou B, Luo B. A Practical CEUS Thyroid Reporting System for Thyroid Nodules. Radiology 2022;305:149-59. [Crossref] [PubMed]
- Petrasova H, Slaisova R, Rohan T, Stary K, Kyclova J, Pavlik T, Kovalcikova P, Kazda T, Valek V. Contrast-Enhanced Ultrasonography for Differential Diagnosis of Benign and Malignant Thyroid Lesions: Single-Institutional Prospective Study of Qualitative and Quantitative CEUS Characteristics. Contrast Media Mol Imaging 2022;2022:8229445. [Crossref] [PubMed]
- Jin Z, Zhu Y, Lei Y, Yu X, Jiang N, Gao Y, Cao J. Clinical Application of C-TIRADS Category and Contrast-Enhanced Ultrasound in Differential Diagnosis of Solid Thyroid Nodules Measuring ≥1 cm. Med Sci Monit 2022;28:e936368. [Crossref] [PubMed]
- Gao XQ, Ma Y, Peng XS, Wang LL, Li HX, Zheng XL, Liu Y. Diagnostic performance of C-TIRADS combined with SWE for the diagnosis of thyroid nodules. Front Endocrinol (Lausanne) 2022;13:939303. [Crossref] [PubMed]
- Brandenstein M, Wiesinger I, Jung F, Stroszczynski C, Jung EM. High-performance sonographical multimodal imaging of non cystic thyroid lesions: Chances of the preoperative diagnostics in relation to histopathology. Clin Hemorheol Microcirc 2021;79:27-38. [Crossref] [PubMed]
- Cao H, Fan Q, Zhuo S, Qi T, Sun H, Rong X, Xiao X, Zhang W, Zhu L, Wang L. The Value of Chinese Thyroid Imaging Report and Data System Combined With Contrast-Enhanced Ultrasound Scoring in Differential Diagnosis of Benign and Malignant Thyroid Nodules. J Ultrasound Med 2022;41:1753-61. [Crossref] [PubMed]
- Brandenstein M, Wiesinger I, Künzel J, Hornung M, Stroszczynski C, Jung EM. Multiparametric Sonographic Imaging of Thyroid Lesions: Chances of B-Mode, Elastography and CEUS in Relation to Preoperative Histopathology. Cancers (Basel) 2022;14:4745. [Crossref] [PubMed]


