
Dynamic cerebral perfusion changes in stretch syncope: a comprehensive case study and review
Introduction
Stretching syncope is a relatively rare clinical syndrome, with a higher prevalence in males compared to females (1,2). Characterized by a transient loss of consciousness, it typically occurs during neck hyperextension. This loss of consciousness is often preceded by prodromal symptoms such as visual disturbances, dizziness, headaches, and sensory abnormalities (3,4). Additionally, episodes may be accompanied by tachycardia, a drop in blood pressure, and occasionally by focal spasmodic jerking of limbs (5,6). Due to these symptoms, stretch syncope is frequently mistaken for epilepsy, but it is frequently resistant to antiepileptic drug therapy (7).
Case presentation
A 39-year-old male was admitted to the Department of Neurology due to recurrent transient loss of consciousness over the past 2 years, specifically triggered by stretching movements. These movements included neck and back hyperextension, upper extremity flexion, shoulder abduction and external rotation, and breath holding. Notably, there were no associated limb convulsions or incontinence. After each episode, the patient experienced approximately 2–3 minutes of spontaneous recovery. The episodes had recurred more than 30 times. No additional symptoms were observed before or after these episodes, and no specific precipitating stimuli were identified. The patient had a history of lumbar disc herniation, smoking, and alcohol consumption but no history of epilepsy, head trauma, or family history of similar symptoms.
During the interictal period, the patient underwent several diagnostic tests, including an upright tilt test, cranial magnetic resonance imaging (MRI), electrocardiogram (ECG), electroencephalogram (EEG), echocardiogram, and computed tomography angiography (CTA), as well as computed tomography perfusion (CTP) of the carotid and intracranial arteries. These tests did not reveal significant abnormalities.
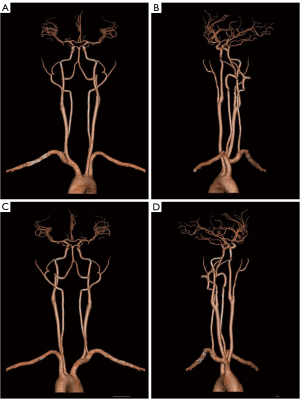
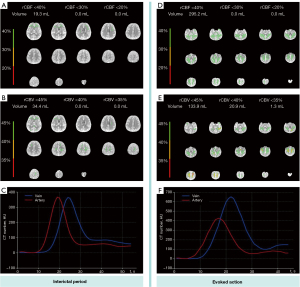
To investigate potential triggers, multiple episodes were elicited and monitored under similar conditions to ensure the consistency of findings. A transcranial Doppler (TCD) examination was performed during an evoked action, revealing a substantial decrease in the flow velocity of the middle cerebral artery (MCA), particularly during the diastolic phase at the end of stretching (Figure 1). TCD monitoring of bilateral MCA blood flow velocity showed that at rest, the right-MCA (R-MCA) had a systolic velocity of 67.2 cm/s and a diastolic velocity of 23.4 cm/s, whereas the left-MCA (L-MCA) side had a systolic velocity of 73.2 cm/s and a diastolic velocity of 34.3 cm/s. During the evoked action, the velocity dropped to the lowest point, with the R-MCA showing a systolic velocity of 44.1 cm/s and a diastolic velocity of 11.7 cm/s, and the L-MCA showing a systolic velocity of 48.2 cm/s and a diastolic velocity of 18.0 cm/s. Notably, when the blood flow velocity decreased, the patient reported feeling lightheaded, and the symptoms gradually improved after the evoked action ended, as the blood flow velocity increased. Meanwhile, concurrent cerebral vascular CTA showed no significant stenosis (Figure 2). Cerebral perfusion was further assessed using the ShuKun Technology Cerebral Perfusion Intelligent Assessment System (Figure 3). This evaluation indicated that 295.2 mL of brain tissue exhibited a reduction in regional cerebral blood flow (rCBF) to below 40% and a decrease in regional cerebral blood volume (rCBV) in certain areas. Importantly, during the resting state, the time-density curves showed similar heights and widths for both the arterial and venous peaks, whereas in the evoked state, the venous peaks markedly exceeded the arterial peaks in both height and breadth (Figure 3), suggesting significant contrast agent accumulation in the venous system.
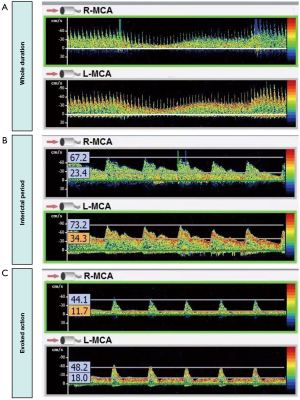
Additionally, there was a corresponding increase in heart rate from an average of 72 beats per minute to 100 beats per minute. This heart rate increase occurred synchronously with the observed changes in blood flow velocity, suggesting a compensatory response to the reduced cerebral perfusion. Despite multiple blood pressure measurements, no significant changes in mean arterial pressure were observed during the evoked actions compared to the interictal periods, confirming that the hemodynamic changes were not due to systemic hypotension. A 32-lead video EEG system was used for extensive dynamic monitoring, revealing no significant abnormalities during either the interictal or evoked phases, further excluding epilepsy as a cause.
Based on these findings, the patient was diagnosed with syncope induced by specific postures. It was recommended that the patient avoid movements known to trigger these episodes.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The case was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital (No. KT2023040). Written informed consent was provided by the patient for publication of this article and accompany images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Stretch syncope is a complex clinical syndrome with a multifaceted etiology, potentially involving vascular compression during stretching, reflex vascular inhibition, adrenergic dysfunction, genetics, and other factors. Despite its rarity, existing literature provides insights into its characteristics and management, as summarized in Table 1. Differentiating stretch syncope from epilepsy can be challenging, particularly in cases where spastic limb jerks are present at onset. Excessive testing and medication failures can negatively impact patients’ finances and adherence to treatment. However, tests such as TCD measurements and EEG monitoring generally yield consistent results during episode induction, suggesting common characteristics of this condition. Notably, TCD is highly sensitive and relatively easy to perform, although variations in other tests may contribute to the complexity of diagnosing stretch syncope.
Table 1
| Ref. | Number of cases | Age (years) | Sex | Imaging tools | Imaging findings | Key conclusions |
|---|---|---|---|---|---|---|
| (1) | 6 | 12–14 | 4 males, 2 females |
ECG, chest strain gauge, X-ray | Normal exams and cervical X-rays for most cases | Stretch syncope linked to familial syncope |
| (2) | 2 | 15, 18 | 1 male, 1 female |
TCD, DSA, MRI, cervical spine radiographs | Small posterior communicating arteries; extracranial compression; normal MRI | Decreased posterior circulation blood flow and vertebral artery compression during neck hyperextension are key in stretch syncope |
| (3) | 1 | 16 | 1 male | Video-EEG, TCD, DSA, MRI | TCD showed decreased blood flow in PCAs during attacks; normal DSA, EEG and MRI | Stretch syncope is a self-induced condition involving neck hyperextension |
| (4) | 3 | 20–26 | 3 males | Video-EEG, TCD | TCD showed drop in MCA flow; EEG slow waves; normal MRI and ECG | Stretch syncope is a vasodepressor faint, not caused by vertebral artery insufficiency |
| (5) | 1 | 11 | 1 female | ECG, EEG, MRI, DSA, TCD | TCD showed decreased blood flow during stretch; normal MRI and DSA | Stretch syncope was linked to exaggerated Valsalva maneuver responses and adrenergic dysfunction |
| (6) | 1 | 7 | 1 female | Video-EEG, ECG, TCD, MRI | EEG slow waves and tachycardia; normal MRI and TCD | Stretch syncope involves brainstem ischemia or vascular compression, mistaken for epilepsy |
| (7) | 1 | 21 | 1 male | EEG, MRI, MRA, PET, SPECT, video-EEG | Video-EEG showed polymorphic theta/delta activity during events. Normal MRI, MRA, PET and SPECT | Stretch syncope is difficult to differentiate from epilepsy |
DSA, digital subtraction angiography; ECG, electrocardiogram; EEG, electroencephalography; MCA, middle cerebral arteries; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; PCA, posterior cerebral arteries; PET, positron emission tomography; SPECT, single-photon emission computed tomography; TCD, transcranial Doppler.
In the interictal period, our patient exhibited normal rCBF and rCBV across most brain areas, with similar heights and widths of arterial and venous peaks in the time–density curves. However, during evoked actions, venous peaks significantly exceeded arterial peaks in both height and breadth, which typically indicates more pronounced contrast agent accumulation in the venous system compared to the arteries. Under normal conditions, contrast agents should first flow into the arteries, followed by venous return. When venous peaks markedly exceed arterial peaks, it suggests a delay or obstruction in venous flow, possibly indicating impaired venous return. The decrease in rCBF and rCBV also supports the hypothesis of venous return obstruction. Venous outflow obstruction leads to blood accumulation in the brain, thereby reducing blood flow and blood volume. In contrast, if arterial perfusion is restricted, it typically results in a decrease in flow or perfusion in the arterial system, causing a significant increase in arterial peaks, without notably affecting venous flow.
The increased venous flow delay may be linked to impaired cerebral venous return due to neck vein compression, a potential cause of the syncope observed in our patient. This explanation aligns with recent literature on idiopathic intracranial hypertension (IIH), which has proposed that venous outflow resistance and compromised intracranial compliance (ICC) contribute to syncope by impairing brain perfusion during postural changes (8). In IIH, the abnormal collapsibility of dural sinuses may cause a feedback loop between cerebrospinal fluid (CSF) pressure and venous pressure, reducing CSF reabsorption and increasing CSF volume and intracranial pressure (ICP). This results in a decrease in ICC, making the brain more susceptible to hypoperfusion during posture changes or Valsalva maneuver due to a venous side-dependent reduction of cerebral perfusion pressure, thus triggering syncope.
Conclusions
This case highlights the diagnostic challenges associated with stretch syncope and underscores the need for a thorough and comprehensive evaluation approach. The findings advocate for incorporating dynamic, situation-specific testing modalities, such as TCD and CTP, into the standard diagnostic protocol for patients presenting with syncope under specific physical conditions. Moreover, this case contributes to the growing body of evidence that stretch syncope, although rare, is a clinically significant condition. Increased awareness and understanding within the medical community are crucial for accurate diagnosis and effective management.
Acknowledgments
We would like to thank Dr. Bo Jin (Department of Neurology, Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou, China) for his valuable assistance in analyzing the patient’s test results.
Footnote
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2056/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The case was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital (Approval No. KT2023040). Written informed consent was provided by the patient for publication of this article and accompany images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pelekanos JT, Dooley JM, Camfield PR, Finley J. Stretch syncope in adolescence. Neurology 1990;40:705-7. [Crossref] [PubMed]
- Sturzenegger M, Newell DW, Douville CM, Byrd S, Schoonover KD, Nicholls SC. Transcranial Doppler and angiographic findings in adolescent stretch syncope. J Neurol Neurosurg Psychiatry 1995;58:367-70. [Crossref] [PubMed]
- Sarrigiannis PG, Randall M, Kandler RH, Grunewald RA, Harkness K, Reuber M. Stretch syncope: reflex vasodepressor faints easily mistaken for epilepsy. Epilepsy Behav 2011;20:450-3. [Crossref] [PubMed]
- Mazzuca M, Thomas P. Self-induced stretch syncope of adolescence: a video-EEG documentation. Epileptic Disord 2007;9:413-7. [Crossref] [PubMed]
- Yeom JS, Kim Y, Lim JY, Woo HO, Youn HS. Exaggerated Valsalva maneuver may explain stretch syncope in an adolescent. Pediatr Neurol 2011;45:338-40. [Crossref] [PubMed]
- Routier L, Bourel-Ponchel E, Heberle C, Mathiron A, Urbina-Hiel B, Faucon C, Berquin P, Wallois F. Stretch syncope or epileptic seizure? A pathologic hypothesis for self-induced stretch syncope. Neurophysiol Clin 2020;50:383-6. [Crossref] [PubMed]
- Villamar MF, Taylor JA, Hamner JW, Voinescu PE. Clinical Reasoning: A Young Man With Daily Episodes of Altered Awareness. Neurology 2022;98:e1197-203. [Crossref] [PubMed]
- De Simone R, Sansone M, Curcio F, Russo CV, Galizia G, Miele A, Stornaiuolo A, Piccolo A, Braca S, Abete P. Recurrent reflex syncope in idiopathic intracranial hypertension patient resolved after lumbar puncture: pathogenetic implications. BMC Neurol 2023;23:416. [Crossref] [PubMed]


