
Isolated homonymous quadrantanopia linked to temporal polymicrogyria: a case study utilizing magnetic resonance imaging and diffusion tensor imaging
Introduction
Homonymous quadrantanopia, a visual field defect characterized by vision loss in a quarter of the field in both eyes on the same side, often results from damage or disruption to the visual pathways within the brain, specifically the optic radiation pathways (1,2). Among these pathways, Meyer’s loop, which is situated in the temporal lobe and forms part of the optic radiation, is a frequent site of lesion formation in this condition. As a critical neural pathway transmitting visual information from the thalamus to the visual cortex, Meyer’s loop is crucial for visual processing, particularly in the upper quadrant of the visual field (3).
Polymicrogyria (PMG) is a rare cortical development disorder that involves the formation of an excessive number of small and irregularly formed cerebral gyri and sulci (4). Although the estimated prevalence of PMG is approximately 2.3 per 10,000 children in a population-based pediatric cohort, it remains one of the more commonly encountered malformations of cortical development, accounting for approximately 16% of such anomalies (5). Detection typically involves structural magnetic resonance imaging (MRI), which can reveal the severity of the condition and its unique ‘stippled’ or ‘bumpy’ appearance at the gray-white junction, a feature not observed in other cortical malformations (6). The etiology of PMG is multifactorial, potentially involving genetic influences (7), abnormal cortical migration, axonal myelination (8), and other developmental factors. Advanced imaging techniques like structural and diffusion MRI are crucial for investigating the relationship between PMG’s cortical abnormalities and white matter changes (9).
In the presented case, PMG specifically affects Meyer’s loop, leading to a distinct visual field defect that was initially challenging to detect using standard neuroimaging tests. Notably, the patient exhibited no other neurological symptoms. This case underscores the utility of combining high-resolution MRI and diffusion tensor imaging (DTI) in clinical practice for the diagnosis of conditions like PMG affecting Meyer’s loop.
Case presentation
A 24-year-old woman visited the neuro-ophthalmology clinic at Jeonbuk National University Hospital following the incidental detection of quadrantanopia during an eyeglass prescription fitting. Her visual acuity measured 0.4 in the left eye and 0.8 in the right eye. Humphrey visual field tests confirmed homonymous left upper quadrantanopia (Figure 1A). All other physical, ophthalmic, and neurological examinations, as well as laboratory tests, were unremarkable.
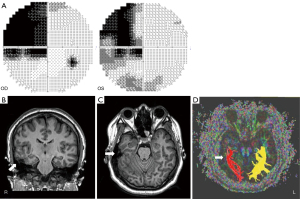
To identify potential underlying cause, a brain MRI was performed using a SIEMENS Verio scanner (Erlangen, Germany) for a more precise location of the patient’s pathology. DTI was acquired using a 2D echo planar imaging diffusion sequence. The imaging parameters included an echo time (TE) of 93 ms, repetition time (TR) of 6,100 ms, 30 diffusion sampling directions, a b-value of 1,000 s/mm2, an in-plane resolution of 0.898 mm, and a slice thickness of 3 mm.
MRI revealed PMG in the right temporal lobe (Figure 1B,1C). Volumetric analysis using volBrain (10), an automated volumetric pipeline, applied to T1-weighted and fluid attenuated inversion recovery (FLAIR) images indicated a general reduction in both grey and white matter volume in the patient’s right temporal hemisphere, relative to her left hemisphere and to normative data for her sex and age (Table 1).
Table 1
| Structure | Total, cm3 (%) [expected limits%]† |
Right, cm3 (%) [expected limits%] |
Left, cm3 (%) [expected limits%] |
Asymmetry‡ (%) [expected limits%] |
|---|---|---|---|---|
| Macrostructure segmentation | ||||
| Cerebrum | 1,035.65 (77.59) [76.30 to 81.44] | 497.07 (37.24)§ [38.15 to 40.75] | 538.58 (40.35) [38.10 to 40.73] | −8.02§ [−1.21 to 1.43] |
| Cerebrum WM | 407.20 (30.51) [30.08 to 33.85] | 196.44 (14.72)§ [15.04 to 16.92] | 210.76 (15.79) [15.02 to 16.94] | −7.03§ [−1.82 to 1.67] |
| Cerebrum GM | 628.45 (47.08) [45.54 to 48.66] | 300.62 (22.52)§ [22.80 to 24.36] | 327.82 (24.56)§ [22.71 to 24.32] | −8.66§ [−1.11 to 1.72] |
| Temporal lobe segmentation | ||||
| Temporal lobe | 103.37 (7.74)§ [7.89 to 9.26] | 39.82 (2.98)§ [3.94 to 4.69] | 63.55 (4.76)§ [3.90 to 4.62] | −45.90§ [−4.96 to 8.25] |
| Inferior temporal gyrus | 20.37 (1.53)§ [1.60 to 2.14] | 8.34 (0.63)§ [0.77 to 1.09] | 12.03 (0.90) [0.77 to 1.11] | −36.20§ [−20.30 to 15.36] |
| Middle temporal gyrus | 25.39 (1.90)§ [2.02 to 2.60] | 10.35 (0.78)§ [1.00 to 1.35] | 15.04 (1.12) [0.97 to 1.35] | −36.97§ [−12.94 to 20.47] |
| Superior temporal gyrus | 13.50 (1.011) [0.96 to 1.32] | 4.96 (0.37)§ [0.47 to 0.67] | 8.53 (0.64) [0.46 to 0.68] | −52.92§ [−22.17 to 20.62] |
| Transverse temporal gyrus | 3.56 (0.27) [0.17 to 0.30] | 1.56 (0.12) [0.08 to 0.15] | 2.00 (0.15) [0.09 to 0.16] | −24.77 [−48.57 to 24.51] |
| Temporal pole | 15.71 (1.18)§ [1.22 to 1.70] | 4.61 (0.35)§ [0.61 to 0.87] | 11.10 (0.83) [0.60 to 0.85] | −82.64§ [−10.14 to 14.30] |
†, values between brackets show expected limits (95% CI) of normalized volume in function of sex and age in the volBrain database for each measure for reference purpose. ‡, the Asymmetry index is calculated as the difference between right and left volumes divided by their mean (in percent). All the result images are located in the MNI space (neurological orientation). §, values outside the limits. CI, confidence interval; GM, grey matter; MNI, Montreal Neurological Institute; WM, white matter.
Tractography was performed using DTI Studio software (CMRM, John Hopkins Medical Institute, Baltimore, MD, USA). The initiation region of interest (ROI) was placed in the lateral geniculate nucleus (LGN) of the thalamus, with a target ROI in a ventral location corresponding to Meyer’s loop. The results showed a significant volume reduction in volume in the right Meyer’s loop compared to the left (Figure 1D). Furthermore, a noticeable decrease in fractional anisotropy (FA) was observed, measuring at 0.50 in the right Meyer’s loop, compared to 0.58 in the left.
This patient exhibited no other neurological symptoms aside from quadrantanopia and was managed with outpatient follow-up. Given the absence of additional neurological deficits, her follow-up care focused on monitoring her visual field. Nine months post-initial evaluation, her follow-up exam revealed persistent quadrantanopia without significant changes.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The clinical manifestations of PMG vary significantly in type and severity (6). For example, a case of unilateral PMG with brainstem atrophy, as documented by Roh et al., demonstrated less severe motor dysfunction than anticipated, given the extensive corticospinal tract volume reduction and ipsilateral brainstem atrophy. This milder-than-expected phenotype could be attributed to compensatory projections from the unaffected hemisphere (11). In severe PMG cases, symptoms can include mental retardation, epilepsy, and visual impairment (12). Our case is notable for its isolated involvement of Meyer’s loop, resulting in a subtle visual field defect that was not easily detected through conventional testing, yet without additional neurological impairments.
The underlying pathomechanisms of PMG, involving abnormal cortical lamination and white matter changes, remain an area of ongoing research. Understanding the relationship between polymicrogyric cortex malformation and its impact on white matter is crucial for elucidating the pathophysiological mechanisms behind neurological deficits, especially those affecting visual processing (4). Altered connectivity in visual pathways is a key factor in the development of homonymous quadrantanopia, as illustrated in our case.
Studies using various imaging techniques, including T1- and T2-weighted imaging ratios and histological analyses, support hypotheses related to altered axonal growth (8,9). DTI studies have demonstrated significantly diminished connectivity in affected regions, with reductions in both short U-fiber connectivity between neighboring primary gyri and long-range connectivity across hemispheres, possibly due to aberrant neuron migration and myelination issues (13). This case highlighted the effectiveness of combining structural and diffusion-based neuroimaging techniques not only for identifying PMG but also for exploring the integrity of the white matter alterations affecting visual pathway, particularly Meyer’s loop, which could contribute to homonymous quadrantanopia.
While structural MRI may not always reveal white matter abnormalities associated with PMG (6), DTI has proven invaluable in elucidating the underlying pathomechanisms and their correlation with symptomatology. Our findings align with prior research demonstrating reduced FA values in affected white matter regions, reinforcing the extensive impact of PMG on neural connectivity (14). Disrupted structural connectivity, as demonstrated by DTI, has highlighted the widespread impact of PMG on brain integrity, reflecting a complex interplay between cortical malformations and their downstream effects. These effects are particularly evident in structural and functional connectivity as well as information processing within the neural network (13,15,16). Moreover, magnetic resonance (MR) tractography has emerged as a valuable tool in diagnosing developmental anomalies, aiding both clinical management and a deeper understanding of PMG pathology. Our findings, which demonstrate reduced FA values in the white matter beneath Meyer’s loop affected by PMG, compared to both the unaffected contralateral regions and normative controls, are consistent with prior studies.
In conclusion, this unique case of temporal lobe PMG resulting in isolated homonymous quadrantanopia, without any additional neurological deficits, is exceedingly rare. It enhances our understanding of the complex interactions between polymicrogyric cortex malformation, white matter alterations, and their clinical consequences, as revealed through multimodal neuroimaging studies.
Acknowledgments
None.
Footnote
Funding: This work was supported by a
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1896/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jacobson DM. The localizing value of a quadrantanopia. Arch Neurol 1997;54:401-4. [Crossref] [PubMed]
- Miki A, Nakajima T, Fujita M, Takagi M, Abe H. Functional magnetic resonance imaging in homonymous hemianopsia. Am J Ophthalmol 1996;121:258-66. [Crossref] [PubMed]
- Meyer A. The connections of the occipital lobes and the present status of the cerebral visual affections. Transactions Association of the American Physicians 1907;22:7-23.
- Stutterd CA, Leventer RJ. Polymicrogyria: a common and heterogeneous malformation of cortical development. Am J Med Genet C Semin Med Genet 2014;166C:227-39. [Crossref] [PubMed]
- Kolbjer S, Martín Muñoz DA, Örtqvist AK, Pettersson M, Hammarsjö A, Anderlid BM, Dahlin M. Polymicrogyria: epidemiology, imaging, and clinical aspects in a population-based cohort. Brain Commun 2023;5:fcad213. [Crossref] [PubMed]
- Leventer RJ, Jansen A, Pilz DT, Stoodley N, Marini C, Dubeau F, Malone J, Mitchell LA, Mandelstam S, Scheffer IE, Berkovic SF, Andermann F, Andermann E, Guerrini R, Dobyns WB. Clinical and imaging heterogeneity of polymicrogyria: a study of 328 patients. Brain 2010;133:1415-27. [Crossref] [PubMed]
- Guerreiro MM, Andermann E, Guerrini R, Dobyns WB, Kuzniecky R, Silver K, et al. Familial perisylvian polymicrogyria: a new familial syndrome of cortical maldevelopment. Ann Neurol 2000;48:39-48. [Crossref] [PubMed]
- Miki Y, Tanji K, Mori F, Sakamoto N, Wakabayashi K. An autopsy case of refractory epilepsy due to unilateral polymicrogyria in a 65-year-old man: Histogenesis of four-layered polymicrogyric cortex. Neuropathology 2015;35:569-74. [Crossref] [PubMed]
- Takanashi J, Barkovich AJ. The changing MR imaging appearance of polymicrogyria: a consequence of myelination. AJNR Am J Neuroradiol 2003;24:788-93. [PubMed]
- Manjón JV, Coupé P. volBrain: An Online MRI Brain Volumetry System. Front Neuroinform 2016;10:30. [Crossref] [PubMed]
- Roh CH, Kim DS, Kim GW, Won YH, Ko MH, Seo JH, Park SH. Motor organization of unilateral polymicrogyria associated with ipsilateral brainstem atrophy - a case report. BMC Neurol 2022;22:303. [Crossref] [PubMed]
- Marqués-Fernández VE, Sánchez-Tocino H, Escudero-Caro MT, Cancho-Candela R, García-Zamora M. Visual Impairment Due to Lissencephaly. Neuroophthalmology 2016;40:229-33. [Crossref] [PubMed]
- Im K, Paldino MJ, Poduri A, Sporns O, Grant PE. Altered white matter connectivity and network organization in polymicrogyria revealed by individual gyral topology-based analysis. Neuroimage 2014;86:182-93. [Crossref] [PubMed]
- Paldino MJ, Hedges K, Gaab N, Galaburda AM, Grant PE. Failure to Identify the Left Arcuate Fasciculus at Diffusion Tractography Is a Specific Marker of Language Dysfunction in Pediatric Patients with Polymicrogyria. Behav Neurol 2015;2015:351391. [Crossref] [PubMed]
- Görkem SB, Doganay S, Gumus K, Bayram A, Kumandas S, Coskun A. The Role of Diffusion-Weighted Imaging in the Evaluation of the Whole Brain in Isolated Unilateral Polymicrogyria. J Child Neurol 2016;31:1575-8. [Crossref] [PubMed]
- Bonilha L, Halford J, Rorden C, Li LM, Patel A, Rumbolt Z, Morgan P. Microstructural white matter abnormalities in nodular heterotopia with overlying polymicrogyria. Seizure 2007;16:74-80. [Crossref] [PubMed]


