
Aberrant static and dynamic functional network connectivity in patients with noise-induced hearing loss
Introduction
Noise-induced hearing loss (NIHL) (1) is caused by long-term unprotected or insufficiently protected exposure to high noise levels that exceed the limits of the national standards for noise in an environment. Exposure to excessive noise can promote the deterioration and death of auditory hair cells within the cochlea in the inner ear. With the advancement of modern industries, NIHL has become the most frequently recognized occupational disease worldwide (2). Presently, concerns about NIHL mostly focus on the improvement of hearing loss, tinnitus, and other auditory system-related symptoms; however, some clinical behavioral studies suggest that patients with NIHL can experience disorders such as anxiety, depression, decreased memory capacity, and reduced cognitive performance in addition to auditory system-related damage (3,4). Some studies (2,5) have suggested that early intervention is more crucial than long-term protection in the case of NIHL disease progression. It is critical to prevent primary noise exposure and provide secondary prevention before irreversible damage occurs to maintain the quality of life of workers. Therefore, a better understanding of the auditory systems and physical and mental health issues related to NIHL can help to evaluate the risks of NIHL in workers more comprehensively. This can help to improve the quality of life of patients with NIHL and reduce the national occupational health burden.
Brain alterations related to “cortical plasticity” in patients with sensorineural hearing loss (SNHL) have been reported in numerous hearing deprivation studies. Wang et al. (6) utilized a combination of brain functional imaging and diffusion tensor imaging (DTI) to reveal that congenital bilateral SNHL children exhibit reduced fractional anisotropy (FA) values in the white matter fiber skeleton microstructure, particularly in regions associated with auditory and language processing networks and increased resting-state functional connectivity (FC), indicating that auditory deprivation leads to less myelination and/or fewer fiber projections to the auditory cortex, resulting in functional remodeling of the auditory cortex. Liu et al. (7) and Zhang et al. (8) found that during the process of long-term hearing loss, patients may have “cross-functional remodeling” in the brain regions related to hearing, language, vision, and other networks. Luan et al. (9) found that long-term SNHL in adults involved significant connectivity impairment of multiple resting-state networks (RSNs) and revealed a neural substrate for possible cognitive and emotional disorders in SNHL. NIHL is a special class of SNHL, and there have been limited studies conducted on this particular class of hearing loss. Our previous study (1) showed that when gray matter (GM) volume was significantly different, the auditory cortex underwent functional reorganization in patients with NIHL, and the static functional network connectivity (sFNC) based on an independent component analysis (ICA) study (10) indicated that different degrees of NIHL involve different FNC alterations in RSNs. All of these findings may reveal neural mechanisms linked to emotion-related features and functional abnormalities following long-term NIHL. Recent research (11) has highlighted that the human brain is not a static entity but a highly adaptable and interactive system. By employing dynamic functional network connectivity (dFNC) analysis through resting-state functional magnetic resonance imaging (RS-fMRI), it becomes possible to identify varying connectivity states of the brain across time. This approach captures recurring large-scale connectivity patterns and their transitions, offering insights into the brain’s temporal dynamics (12). Compared with sFNC analysis, the prime advantage of dFNC is that it can capture temporal changes in spontaneous neural activity and may provide greater insight into the properties of brain RSNs (13). Li et al. (14) compared sFNC with dFNC in sudden SNHL (SSNHL) patients and found that the dFNC characteristics of SSNHL patients were different from those of sFNC, especially within the visual cortices. Subsequently, Li et al. (15) combined dFNC with structural study to examine the different processes of SSNHL. The study indicated that early-stage SSNHL patients have better therapeutic responses and hearing recovery.
The summary, assessment, and analysis of brain activity on a temporal scale could provide a more realistic picture of the ever-changing state of brain function, helping us to better understand the pathogenesis of some diseases. To the best of our knowledge, no study has investigated sFNC and dFNC features in patients with NIHL. Thus, we conducted the current study to provide neuroimaging evidence of the evolution of sFNC to dFNC brain alterations in patients with NIHL. The application of these methods may unravel the alterations among transmodal plasticity brain areas not involved in the hearing process, help to better understand NIHL, and identify new functional neuroimaging biomarkers to guide its treatment. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1511/rc).
Methods
Participants
A total of 79 NIHL patients and 69 age- and education-matched healthy controls (HCs) were recruited. Patients with NIHL were diagnosed by occupational doctors between 2014 and 2020 following China’s national occupational hygiene standards (GBZ 49-2014) (16). All participants were tested using the Mini-Mental State Examination (MMSE) and Hamilton Anxiety Rating Scale (HAMA). All patients were tested using the Tinnitus Handicap Inventory (THI), and some clinical data {age, education level, hearing threshold [monaural hearing threshold-weighted value (MTWV)], occupation type, and noise exposure time} were collected.
The criteria for scale tests according to the previous study were as follows (10): the MMSE assessed the cognitive status of all participants (criteria: normal, 27–30, cognitive dysfunction: <27). The HAMA scale contains 14 items, such as anxiety, tension, fear, and insomnia; the higher the score, the more serious the anxiety degree is. The THI score was obtained by adding the scores of all the 25 items; the higher the score, the higher the tinnitus disability level, and the worse the subjective feeling of the patients.
The criteria for diagnosing NIHL as follows (10): individuals with an average hearing threshold above 40 dB for binaural high frequency (3,000, 4,000, 6,000 Hz) should undergo diagnosis and classification based on the weighted value of good whisper frequency (500, 1,000, 2,000 Hz) and high frequency 4,000 Hz hearing threshold. MTWV ≥26 dB is used for diagnosing NIHL.
The inclusion and exclusion criteria were the same as those applied in our previous study (10): adult male (occupational restriction gender; 35–60 years); right-handed; Han Chinese; primary school to university level of education; normal mental state with no diagnoses of neuropsychiatric diseases; no systemic diseases or other factors that may affect brain structure or function; MMSE ≥27; no sedatives or central nervous system inhibitors were taken. Illiterate or uncooperative individuals or those with contraindications to magnetic resonance imaging (MRI) scanning were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Yantaishan Hospital (No. 2023014), and informed consent was provided by all individual participants.
MRI acquisition and preprocessing
Routine craniocerebral and internal ear MRI examinations were performed using a General Electric (GE) Discovery MR 750 3.0-T scanner (GE Healthcare, Chicago, IL, USA) with an 8-channel head coil. Earplugs and headphones were used to alleviate the noise during scanning. All participants were required to lie flat, be quiet, close their eyes, not exercise, not sleep, not have systematic thinking activities, and try to ensure emotional stability during scanning (10). The structure of the brain was examined by sagittal scans using a three-dimensional T1-weighted imaging fast spoiled gradient echo (3D-T1WIFSPGR) sequence with the following parameters (10): repetition time (TR) =6.9 ms, echo time (TE) =3.4 ms, 1-mm section thickness, no gap, field of view (FOV) =25.6 cm × 25.6 cm, matrix =256×256, number of excitations (NEX) =1, and flip angle =12°. RS-fMRI blood oxygen level-dependent (BOLD) was performed at the same location with the following imaging parameters: TR =2,000 ms, TE =35 ms, 4-mm section thickness, no gap, FOV =24 cm × 24 cm, matrix =64×64, NEX =1, and flip angle =90°.
All RS-fMRI data were preprocessed using the Statistical Parametric Mapping software (SPM12) (http://www.fil.ion.ucl.ac.uk/spm) toolbox in MATLAB R2014b (MathWorks, Natick, MA, USA) (10). Data processing assistance for RS-fMRI: advanced edition (DPARSFA, version 4.2) (17). Preprocessing included the following steps: (I) removal of the first 10 time points. (II) Slice-timing adjustment (slice timing, slice number, and slice order). (III) Realignment for head motion correction (cases with a maximum head translation distance greater than 2.0 mm or axial rotation greater than 2.0° were excluded). (IV) Mean functional images were coregistered with T1-weighted structural images. Then, T1-weighted images were segmented into GM, white matter, and cerebrospinal fluid (CSF). Its space was normalized to the standardized Montreal Neurological Institute (MNI) space using diffeomorphic anatomical registration via the exponential Lie algebra (DARTEL) template. Subsequent normalized functional images were resampled to 3×3×3 mm3 voxels. (V) All data were smoothed using a 4 mm full-width at half maximum (FWHM) Gaussian kernel. (VI) Linear detrended removal and temporal bandpass filtering (0.01–0.1 Hz) were performed.
ICA
ICA was employed to parcellate the preprocessed RS-fMRI data using the Group ICA of fMRI Toolbook (GIFT) (http://ictab.sourceforge.net/) toolbox (age and education were already added as covariates at the time of the between-group analysis) (10). Optimal independent components (ICs) were automatically estimated using software based on previous studies (n=30) (10,18). The processing steps were as follows (10): (I) the number of components was estimated by the minimum description length criteria; (II) two-dimensional principal component analysis was used to reduce the dimension of RS-fMRI images; (III) dimension reduction and decomposition of RS-fMRI images were performed using ICA algorithm; and (IV) the composition of each participant was reconstructed and then the intensity values of each voxel were converted to z-scores. These were then compared with previously published ICA network templates (10,19-21), which were screened by spatial correlation and visual inspection for further analysis.
sFNC analyses
To explore FNC alterations among different functional networks, we conducted temporal correlations among the identified RSNs (10,22-24). The procedure was as follows (10): a single course of each identified RSN was obtained from the inverse reconstruction process of the ICA. The time correlation coefficients for each pair of 11 RSNs time courses were calculated and normalized using Fisher’s r-to-z transformation. The 23×23 (ICs) and 11×11 (RSNs) network connectivity matrices were used to compare the two groups (seven ICs containing noisy or irrelevant signals were removed). Within the two groups, the FNC differences for each pair of identified RSNs were compared using analysis of variance (ANOVA) with age and education as nuisance covariates [P<0.05 was considered statistically significant, corrected by the false discovery rate (FDR)].
dFNC analyses
To understand the nature of the dFNC, we used the temporal dFNC toolbox in the GIFT software. A sliding window approach was adopted to compute the dFNC between ICA time courses (25). The window length is a key parameter for obtaining dynamic, spontaneous brain activity (26), where a short window length may not adequately estimate dynamic changes, whereas an excessively long window length may fail to detect dynamic activity (27). Therefore, the window segment was tapered by convolving a rectangle (width 30, TR =60 s) with a Gaussian (σ=3 TRs) and advancing one TR at each step, which resulted in W=161 windows (14). The chosen window length of 60 s (2 s × 30 s) was deemed suitable for capturing the dynamics in the FNC (14). The covariance between components was estimated following a previously outlined procedure. The covariance matrices from each window were combined to create a 23×23×161 matrix, capturing the temporal changes in covariance (correlation) across networks (components). Using MATLAB, k-means clustering with squared Euclidean distance was applied to partition the 500 iterations and 150 replicates of the dFNC windows into five distinct clusters (25). The centroids of these clusters represent a compact set of prototype connectivity “states”, which can be interpreted as recurring average patterns that participants frequently revisit throughout the experiment. In each state, differences in dFNC between groups were investigated using two-sample t-tests (P<0.05, FDR-corrected). Dynamic indicators, including the fraction rate time (FT), mean dwell time (MDT), and number of transitions (NT), were computed to explore the temporal features of the different dFNC states.
Statistical tests
Differences in age, level of education, HAMA, MMSE, temporal correlation coefficient z values of sFNC and dFNC, and temporal features of different dFNC states between the groups were assessed using two-tailed independent sample t-tests (P<0.05).
The bivariate Pearson correlation was used to analyze the correlation between clinical symptoms (HAMA, THI, noise exposure time, and hearing loss level). The temporal correlation coefficient z values of the sFNC and dFNC abnormal networks and clinical symptoms, the temporal features of different dFNC states, and clinical symptoms were also analyzed by the bivariate Pearson correlation (P<0.05).
Results
General information
The study cohort comprised NIHL (n=69; mean age: 44.29±8.56 years; education level: 10.41±1.95 years) and HCs (n=57; mean age: 45.84±7.22 years; education level: 11.00±2.83 years) after a series of processing and elimination (10 NIHL and 12 HCs) (Figure 1A, Table 1). The ages, levels of education, and MMSE scores of the two groups were not significantly different (t=1.10, P>0.05; t=1.391, P>0.05; t=−0.522, P>0.05). The HAMA scores of the two groups were significantly different (t=−5.59, P<0.05) (Table 1). The occupations of the NIHL were predominantly drilled and welded (noise environment above 85 dB). The Pearson correlation coefficient was 0.263 (P=0.029) for noise exposure time and hearing threshold; 0.415 (P<0.001) for noise exposure time and THI scores (Figure 1B); and −0.058 (P=0.634) for exposure time and HAMA scores, but the Pearson correlation coefficient was −0.303 (P=0.011) for the HAMA scores and THI score (P<0.05).

Table 1
| Characteristics | HCs (n=57) | NIHL (n=69) | t | P value |
|---|---|---|---|---|
| Age (years) | 45.84±7.22 | 44.29±8.56 | 1.10 | 0.272 |
| Education level (years) | 11.00±2.83 | 10.41±1.95 | 1.391 | 0.182 |
| MMSE | 28.74±1.19 | 28.84±1.01 | −0.522 | 0.603 |
| Noise exposure time (yeas) | – | 14.43±7.52 | – | – |
| Hearing threshold (superiority) (dB) | – | 32.45±4.33 | – | – |
| THI | – | 95.51±5.69 | – | – |
| HAMA | 3.81±1.11 | 9.30±7.35 | −5.59 | <0.001* |
Data are presented as mean ± standard deviation. *, comparison between HCs and NIHL using two independent sample t-test, P<0.05. HAMA, Hamilton Anxiety Rating Scale; HC, healthy control; MMSE, Mini-Mental State Examination; NIHL, noise-induced hearing loss; THI, Tinnitus Handicap Inventory.
RSNs
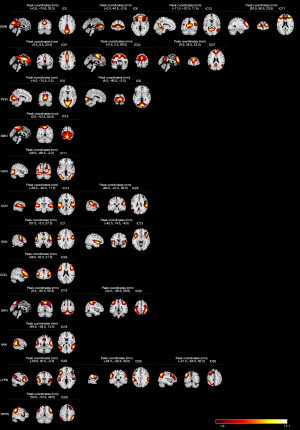
Totals of 11 RSNs and 23 ICs were identified, including default mode network (DMN) (IC3, 6, 12, 17, 21, 24, 27), primary visual network (PVIN) (IC5, 8), sensorimotor network (SMN) (IC16), higher visual network (HVIN) (IC11), auditory network (AUN) (IC14, 26), salience network (SAN) (IC7, 13), executive control network (ECN) (IC29), dorsal attention network (DAN) (IC18, 20), ventral attention network (VAN) (IC19), left frontoparietal network (LFPN) (IC22, 25, 30), and right frontoparietal network (RFPN) (IC28) (Figure 2).
Group differences in sFNC
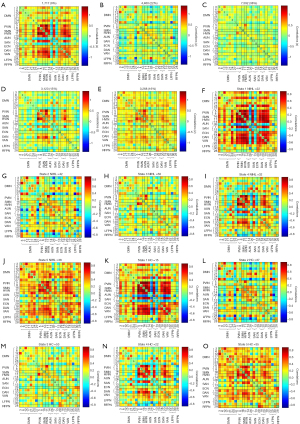
The between-network connectivity patterns of the two groups are illustrated by the averaged FNC matrices in Figure 3A,3B. The comparisons between groups (P<0.05, FDR uncorrected) (Table 2, Figure 3C,3D): NIHL compared with HCs, FNC in intra-LFPN (IC22, 30), inter-RSN [SAN (IC7) and DMN (IC12), HVIN (IC11) and DMN (IC12, 27), HVIN (IC11) and PVIN (IC8), HVIN (IC11) and VAN (IC19), PVIN (IC8) and VAN (IC19), DMN (IC24) and VAN (IC19), VAN (IC19) and LFPN (IC25), LFPN (IC22) and ECN (IC29), VAN (IC19) and ECN (IC29), and VAN (IC19) and VAN (IC20)] were significantly increased; inter-RSN [DMN (IC12) and RFPN (IC28)] was significantly decreased. Comparisons between groups (t=−3.454, P<0.05, FDR corrected) (Table 2, Figure 3E,3F): VAN (IC19) and ECN (IC29) were significantly increased in NIHL compared with HCs and FNC.

Table 2
| RSN, sFNC | MNI coordinates | NIHL vs. HCs | ||||
|---|---|---|---|---|---|---|
| X | Y | Z | t | P value | ||
| DAN (IC20), VAN (IC19) | 44.5, 59.5 | −39.5, −48.5 | 59.5, 14.5 | −2.206 | 0.029 | |
| DMN (IC12), HVIN (IC11) | −11.5, 48.5 | −57.5, −66.5 | 11.5, -2.5 | −2.569 | 0.011 | |
| DMN (IC27), HVIN (IC11) | 0.5, 48.5 | 56.5, −66.5 | 23.5, −2.5 | −2.220 | 0.028 | |
| DMN (IC12), RFPN (IC28) | −11.5, 50.5 | −57.5, −54.5 | 11.5, 48.5 | 1.998 | 0.048 | |
| DMN (IC12), SAN (IC7) | −11.5, 57.5 | −57.5, −3.5 | 11.5, 27.5 | −3.301 | 0.001 | |
| DMN (IC24), VAN (IC19) | −0.5, 59.5 | 2.5, −48.5 | 60.5, 14.5 | −2.134 | 0.035 | |
| ECN (IC29), VAN (IC19) | 48.5, 59.5 | 32.5, −48.5 | 21.5, 14.5 | −3.454 | 0.001/FDR <0.05* | |
| ECN (IC29), LFPN (IC22) | 48.5, −50.5 | 32.5, 20.5 | 21.5, −3.5 | −2.171 | 0.032 | |
| HVIN (IC11), PVIN (IC8) | 48.5, 8.5 | −66.5, −90.5 | −2.5, −2.5 | −2.136 | 0.035 | |
| HVIN (IC11), VAN (IC19) | 48.5, 59.5 | −66.5, −48.5 | −2.5, 14.5 | −2.060 | 0.042 | |
| LFPN (IC25), VAN (IC19) | −62.5, 59.5 | −32.5, −48.5 | 30.5, 14.5 | −2.237 | 0.027 | |
| LFPN (IC30), LFPN (IC22) | −41.5, −50.5 | −65.5, 20.5 | 50.5, −3.5 | −2.461 | 0.015 | |
| PVIN (IC8), VAN (IC19) | 8.5, 59.5 | −90.5, −48.5 | −2.5, 14.5 | −2.438 | 0.016 | |
*, comparison between HC and NIHL using two independent sample t-test, P<0.05, FDR corrected. DAN, dorsal attention network; DMN, default mode network; ECN, executive control network; FDR, false discovery rate; HC, healthy control; HVIN, higher visual network; IC, independent component; LFPN, left frontoparietal network; MNI, Montreal Neurological Institute; NIHL, noise induced hearing loss; PVIN, primary visual network; RFPN, right frontoparietal network; RSN, resting-state network; SAN, salience network; sFNC, static functional network connectivity; VAN, ventral attention network.
Group differences in dFNC and temporal features
The dFNC among ICs was assessed using a sliding-window method. Subsequently, k-means clustering was applied to the dFNC data to identify stable functional state patterns. The 161×126 dFNC matrices were grouped into five distinct states, which consistently recurred during the RS-fMRI scans across all participants (Figure 4A-4E).
The most frequently connected was state-3 (38%) and the least frequently interconnected was state-1 (9%). Group-specific cluster centroids were identified by k-means clustering analysis of the NIHL (Figure 4F-4J) and HCs (Figure 4K-4O). Significant differences in the dFNC between the groups were observed in each state (P<0.05, FDR uncorrected) (Figure 5A-5J). Significant differences between groups in dFNC were observed only in state-5 and NIHL compared with HCs; FNC in the VAN (IC19) and ECN (IC29) were significantly increased (t=−4.202, P<0.05, FDR-corrected) (Figure 5K-5L). We subsequently investigated the differences in dFNC temporal features (FT, MDT, and NT) between patients with NIHL and HCs. Compared to HCs, there were no significant differences in the FT and MDT of all five states and NT in patients with NIHL (P>0.05) (Table 3, Figure 6).
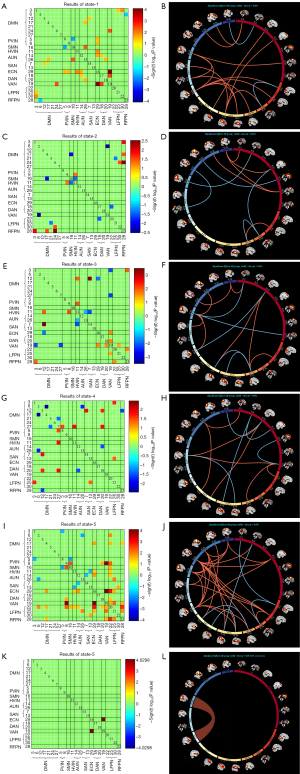
Table 3
| Temporal properties | HCs (n=57) | NIHL (n=69) | t | P value | Hearing loss level | Noise exposure time | THI | HAMA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | ||||||||
| FT (%) | |||||||||||||||
| State-1 | 5.76±12.37 | 10.80±20.90 | −1.603 | 0.111 | 0.027 | 0.827 | −0.098 | 0.424 | −0.255 | 0.035† | 0.243 | 0.006† | |||
| State-2 | 23.70±26.01 | 20.82±23.46 | 0.644 | 0.521 | 0.004 | 0.971 | −0.133 | 0.277 | 0.177 | 0.145 | −0.119 | 0.186 | |||
| State-3 | 38.85±25.15 | 36.68±30.45 | 0.431 | 0.667 | 0.145 | 0.235 | −0.008 | 0.951 | −0.090 | 0.460 | −0.044 | 0.621 | |||
| State-4 | 15.48±23.12 | 15.50±21.01 | −0.004 | 0.997 | 0.000 | >0.99 | 0.234 | 0.054 | 0.093 | 0.448 | 0.052 | 0.566 | |||
| State-5 | 16.22±17.43 | 16.20±20.95 | 0.004 | 0.997 | −0.242 | 0.045† | 0.022 | 0.855 | 0.094 | 0.443 | −0.065 | 0.471 | |||
| MDT (ms) | |||||||||||||||
| State-1 | 6.62±12.96 | 12.11±24.63 | −1.516 | 0.132 | −0.082 | 0.505 | −0.083 | 0.497 | −0.170 | 0.164 | 0.300 | 0.001† | |||
| State-2 | 18.94±19.14 | 17.07±17.84 | 0.565 | 0.573 | 0.013 | 0.914 | −0.059 | 0.631 | 0.247 | 0.041† | −0.128 | 0.152 | |||
| State-3 | 35.21±32.93 | 34.43±37.42 | 0.125 | 0.901 | 0.094 | 0.440 | −0.073 | 0.551 | −0.120 | 0.324 | −0.056 | 0.532 | |||
| State-4 | 12.86±21.26 | 12.93±16.57 | −0.020 | 0.984 | −0.044 | 0.722 | 0.173 | 0.155 | 0.052 | 0.669 | 0.043 | 0.634 | |||
| State-5 | 15.79±14.60 | 16.01±23.26 | −0.066 | 0.947 | −0.121 | 0.323 | 0.069 | 0.575 | 0.147 | 0.228 | −0.110 | 0.222 | |||
| NT | 4.89±2.15 | 4.54±2.40 | 0.882 | 0.379 | −0.097 | 0.429 | −0.035 | 0.777 | 0.048 | 0.698 | 0.019 | 0.834 | |||
Data are presented as mean ± standard deviation. Comparison between HC and NIHL using two independent sample t-test; P<0.05. †, the bivariate Pearson correlation was used to analyze the correlation, P<0.05. dFNC, dynamic functional network connectivity; FT, fraction rate time; HAMA, Hamilton Anxiety Rating Scale; HC, healthy control; MDT, mean dwell time; NIHL, noise-induced hearing loss; NT, number of transitions; THI, Tinnitus Handicap Inventory.
The comparisons of abnormal sFNC/dFNC and clinic correlation analysis
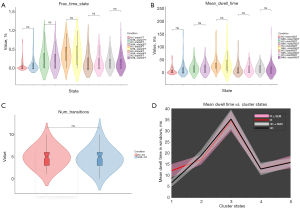
In addition to the FNC differences among the sFNC/dFNC in patients with NIHL compared with HCs, we performed a comparative analysis of the abnormal sFNC/dFNC correlation coefficients between sFNC and dFNC. The correlation coefficient of abnormal dFNC was significantly higher than that of sFNC in both NIHL patients and HCs (P<0.05) (Figure 7A).
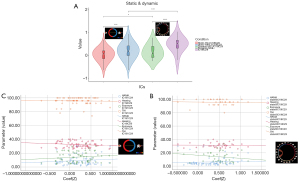
The abnormal correlation coefficient correlation analysis (Figure 7B,7C) revealed a significant positive correlation between the abnormal sFNC correlation coefficient and HAMA (r=0.188, P=0.035); however, there was no significant correlation between the abnormal sFNC/dFNC correlation coefficient and the hearing threshold, noise exposure time, THI scores, or abnormal dFNC and HAMA scores (P>0.05). Temporal features of the dFNC correlation analysis showed a significant positive correlation between FT (state-1) (r=0.243, P=0.006) and MDT (state-1) (r=0.300, P=0.001) with HAMA scores. There was a significant positive correlation between MDT (state-2) (r=0.247, P=0.041) and THI scores. There was a significant negative correlation between FT (state-1) (r=−0.255, P=0.035) and THI scores. There was a significant negative correlation between the FT (state-5) (r=−0.242, P=0.045) and the hearing threshold (Table 3).
Discussion
The sFNC or dFNC analysis based on ICA, sliding window approach, and k-means clustering was a data-drive method to analyze the changes in relative FNC among RSNs. During ICA analyses, we identified 23 ICs and 11 RSNs, including DMN (IC3, 6, 12, 17, 21, 24, 27), PVIN (IC5, 8), SMN (IC16), HVIN (IC11), AUN (IC14, 26), SAN (IC7, 13), ECN (IC29), DAN (IC18, 20), VAN (IC19), LFPN (IC22, 25, 30), and RFPN (IC28). Compared to HCs, the abnormal FNC differences in the VAN (IC19) and ECN (IC29) in NIHL patients were significantly increased in both sFNC and dFNC (state-5), and the correlation coefficient of abnormal dFNC was higher than that of sFNC in both NIHL and HCs. Furthermore, the sFNCs between the VAN (IC19) and ECN (IC29) may be linearly positively correlated with HAMA scores, whereas dFNC only showed a positive correlation. Interestingly, the differences in dFNC temporal features between NIHL and HCs showed no significant differences in the MDT and FT of all five states and the NT in NIHL patients, whereas the temporal features of dFNC correlation analysis showed various obvious correlations with clinical data. The clinical data correlation analysis also presented results consistent with previous research, such as the hearing threshold and THI scores were positively correlated with noise exposure time, confirming that long-term noise exposure can cause hearing damage (28). The HAMA score was negatively correlated with THI scores, which suggests that patients with NIHL adapt to noise, tinnitus, and hearing loss during long-term exposure to noisy environments, and their anxiety gradually decreases. Therefore, preventive measures are important for preserving the mental health of patients with NIHL. These findings support our expectations, deepen our understanding of the reconfiguration or integration of different functional networks in patients with NIHL, and contribute to our understanding of the pathological mechanisms underlying NIHL brain injury.
sFNC vs. dFNC
Multimodal imaging is an advantageous tool for providing clinicians with biomarkers to assist in diagnosis and prognosis. Previous research has shown that the human brain is intrinsically organized into functional networks and that the interrelationships between large-scale networks are thought to be more important than the activity of individual RSNs themselves, playing a key role in effective, dynamic communication across the brain (29). Until recently, most fMRI studies (30,31) have shown that multiple discrete, reoccurring connectivity states arise during rest and that brain neurons continuously integrate and transmit various biological information, which is time dynamic. Clearly, the sFNC ignores the dynamic nature of brain activity. Moreover, the previous research related to the diagnosis and classification of diseases has shown that dFNC has better classification performance and better reflects the brain functional status than sFNC (32). Zhu et al. (31) and Chen et al. (33) also found that the best support vector machine (SVM) classification result was obtained when both sFNC and dFNC were used, which implies that using both the sFNC and dFNC methods allows for a more comprehensive study of brain activity.
VAN and ECN
A prior sFNC study (10) showed that there were no significantly abnormal FNCs between patients with mild and relatively severe NIHL. This study did not group the degree of NIHL, which led to partially inconsistent results with the previous sFNC study. Our FDR uncorrected results showed that both sFNC and dFNC had abnormal FNCs in multiple intra-RSNs and inter-RSNs, including higher-order RSNs. Chen et al. (33) investigated the neural mechanism underlying white matter hyperintensity-related cognitive impairment based on sFNC and dFNC approaches (uncorrected) and found that a SVM model based on the uncorrected characteristic connectivity patterns can also achieve good prediction ability for patients. Hou et al. (34) characterized the spatial patterns of resting-state region of interest FC in obstructive sleep apnea and found that there was no significant alteration in FC by multiple comparison corrections; however, the SVM models showed a successful classification, and the selected FCs were associated with nearly all brain regions and were widely distributed in the whole brain, both within and between many resting-state functional networks. Based on these studies, we speculate that our FDR-uncorrected results mainly manifest as enhanced/reduced FNC of the sFNC or dFNC between RSNs to compensate for/adapt to auditory deprivation, and these aberrant FNC patterns have the potential to serve as neurological biomarkers of NIHL.
To avoid type I errors, further FDR correction showed that the abnormal FNCs in the VAN [IC19: bilateral temporoparietal junction (TPJ)] and ECN [IC29: bilateral dorsolateral prefrontal cortex (dlPFC)] in NIHL patients compared with HCs were significantly increased both in sFNC and dFNC (state-5), and the correlation coefficient of abnormal dFNC was higher than that of sFNC in both NIHL and HCs, confirming that dFNC can provide more detail, giving us additional confidence in the results. This is consistent with the results of previous studies (31,35). The VAN is responsible for bottom-up attention orientation, switching our attention between various tasks, and responding to bursts of signals (36). The VAN-related brain regions are mainly involved in the ventral frontal cortex and TPJ (37), which generally refers to an area of the cortex at the junction of the inferior parietal lobule (IPL) and lateral occipital cortex (38). Previous studies have suggested that the VAN is a right hemisphere network, but others have found that bilateral TPJ activation is observed when attention is redirected and in response to abnormal stimuli (39,40), and Donaldson et al. (38) concluded that the TPJ is related to auditory hallucinations and tinnitus. The ECN is involved in multiple high-level cognitive tasks and plays an important role in adaptive cognitive control, which includes multiple medial prefrontal cortical areas, the inferior frontal lobes, and the IPL; however, the core region is the dlPFC (41).
The dlPFC is the main cortical region that processes and integrates cognitive, sensory information, and emotion. Li (42) found that remodeling of the dlPFC was related to hearing and cognition in patients with presbycusis, suggesting that hearing loss leads to reduced auditory cortical stimulation and affects the integration of auditory and visual stimuli. Using a rat model, Luan et al. (43) found that the dlPFC may mediate cross-modal organization in long-term NIHL, and a subsequent study (44) found that the functional synchrony between the dlPFC and the auditory and visual cortices increased in patients with SNHL, which suggests that the dlPFC probably mediates cross-modal reorganization through top-down regulation in the case of hearing loss. The previous ICA study also showed that patients with mild NIHL had increased connectivity within the dlPFC and left IPL (part of the TPJ) compared with HCs, indicating that the functional network of the human brain exhibits plasticity under prolonged tolerance to noise, deafness, and tinnitus to adapt to the needs of survival and environmental changes (10). Andin and Holmer (45) have also shown that deafness induces large-scale network reorganization, with the middle/superior temporal cortex as a central node of plasticity. These results confirm that cross-modal reorganization may be related to behavioral adaptation to the environment in patients with hearing loss, which could be explained by the recruitment or compensatory reallocation of resources (46). Gromann et al. (47) found that frontotemporal connectivity was stronger between the TPJ and dlPFC during repetitive transcranial magnetic stimulation (rTMS) in patients with schizophrenia and auditory hallucinations; our results were consistent, which may explain the relevant tinnitus mechanism. Zou et al. (48) indicated that patients with chronic migraine had stronger FNC between the ECN and the attention network, disrupted control processes for pain, and cognitive impairments, such as depression.
Similar depression-mood-related abnormal connectivity in the sFNC and dFNC has been found in children with depressive disorders in childhood (49). Our results were consistent, which may explain why relevant abnormal moods, such as anxiety, correlated with long-term hearing loss in NIHL patients. The enhancement of the sFNC correlation coefficient between the VAN and ECN was significantly correlated with the HAMA score, and the scatter chart also showed a positive correlation trend in the dFNC (state-5) correlation coefficient, which was confirmed by clinical correlation analysis. This may be related to the patient’s hearing loss, leading to an uncontrolled focus on compensation. However, Li et al. (50), employing RS-fMRI, revealed significant and widespread reductions in connectivity patterns across three distinct levels—integrity, network, and edge—in patients with SSNHL, broadening our understanding of the brain’s network-level responses to sensory deprivation.
In our dFNC study, we identified five completely different sparse connectivity states across the entire group. However, there were no significant differences in FT and MDT in all five states and NT in patients with NIHL. This is not consistent with the results of previous research (51), which can be attributed to the small sample size, excessive states obtained in this study, or unstable or frequently changing dynamic network patterns. Interestingly, we found that global meta-state statistics occur in the same direction, even when failing to reach significance. This is something that should be investigated further. The correlation analysis showed that there were significant positive correlations between FT (state-1) and MDT (state-1) with HAMA scores, and MDT (state-2) with THI scores. Moreover, there were negative correlations between FT (state-1) and THI scores, and a negative correlation between FT (state-5) and hearing threshold. A previous study (52) found that sparse states in the long-term deprivation of auditory patients may be due to a combination of reduced sensory input, social isolation, and depression. Li et al. (14) also suggested that patients with SSNHL present a sparse dFNC state, which is significantly associated with social anxiety disorder. These findings highlight the utility of adding dFNC to more conventional methods than to traditional FC analysis. The dFNC analyses could yield additional subtle information regarding NIHL and offer a new perspective to enhance our understanding of NIHL, suggesting that functional deficits and compensatory plasticity occur after auditory deprivation in patients with NIHL. Cross-modal plasticity can occur as a result of reduced or abnormal sensory input (53); these results were all consistent with auditory deprivation leading to a cross-modal plasticity mechanism in the cortex. It is emphasized that these plasticity results will support the mechanism of NIHL-related brain injury and provide useful imaging markers for subsequent diagnosis and treatment.
Limitations
Several limitations of this study must be noted. The main limitation is that the NIHL patients included in this study were all male due to the type of occupation; therefore, the explanation of the results was limited. The other limitation is that the study did not assess these patients’ changes after drug treatment or in a noise-free environment for several months. Future studies should include patients of different genders and stages.
Conclusions
Our findings support the view that hearing impairment due to exposure to a noisy environment can lead to changes in a variety of brain functions and provide new evidence that abnormalities in both static and dynamic connectivity consist of shared and unique features in NIHL. dFNC provides more sensitive measures to characterize RSNs. The sFNC combined with dFNC can provide a comprehensive view of brain activity, which contributes to further elucidating the mechanism of abnormal brain functional activities in NIHL.
Acknowledgments
The study was supported by the Yantaishan Hospital. The authors would like to thank the editor and the reviewers for their helpful, constructive comments, which were a great help in producing this improved version of the paper.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1511/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1511/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Yantaishan Hospital (No. 2023014), and informed consent was provided by all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huang R, Wang A, Ba X, Zhang G, Li C, Liu Q. Association Functional MRI Studies of Resting-State Amplitude of Low Frequency Fluctuation and Voxel-Based Morphometry in Patients With Occupational Noise-Induced Hearing Loss. J Occup Environ Med 2020;62:472-7. [Crossref] [PubMed]
- Tank ND, Rupani MP, Shah IA, Dhatrak SV. Prevalence and predictors of high-frequency hearing loss among mine workers in Gujarat, western India: a cross-sectional study on the need to implement a comprehensive hearing conservation program. Int Arch Occup Environ Health 2024;97:365-75. [Crossref] [PubMed]
- Mucci N, Traversini V, Lulli L, Vimercati L, Rapisarda V, Galea R, De Sio S, Arcangeli G. Neurobehavioral alterations in occupational noise exposure: a systematic review. Sustainability 2021;13:12224. [Crossref]
- Feng GW, Sun RC, Xu QY, Lan YJ. Literature analysis of hot topics on occupational noise-induced hearing loss. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2022;40:279-82. [PubMed]
- Sonstrom Malowski K, Gollihugh LH, Malyuk H, Le Prell CG. Auditory changes following firearm noise exposure, a review. J Acoust Soc Am 2022;151:1769. [Crossref] [PubMed]
- Wang S, Chen B, Yu Y, Yang H, Cui W, Li J, Fan GG. Alterations of structural and functional connectivity in profound sensorineural hearing loss infants within an early sensitive period: A combined DTI and fMRI study. Dev Cogn Neurosci 2019;38:100654. [Crossref] [PubMed]
- Liu B, Feng Y, Yang M, Chen JY, Li J, Huang ZC, Zhang LL. Functional Connectivity in Patients With Sensorineural Hearing Loss Using Resting-State MRI. Am J Audiol 2015;24:145-52. [Crossref] [PubMed]
- Zhang LL, Gong JP, Xu YW, Liu B. Brain plastic alterations in subjects with chronic right-sided sensorineural hearing loss: a resting-state MRI study. Zhonghua Yi Xue Za Zhi 2016;96:1850-2. [PubMed]
- Luan Y, Wang C, Jiao Y, Tang T, Zhang J, Teng GJ. Dysconnectivity of Multiple Resting-State Networks Associated With Higher-Order Functions in Sensorineural Hearing Loss. Front Neurosci 2019;13:55. [Crossref] [PubMed]
- Ranran H, Aijie W, Yafei Z, Xinru B, Yi L, Xianghua B, Yunxin L, Guochao L, Guowei Z. Alterations of resting-state functional network connectivity in patients with noise-induced hearing loss: A study based on independent component analysis. Eur J Neurosci 2024;59:2029-45. [Crossref] [PubMed]
- Fiorenzato E, Strafella AP, Kim J, Schifano R, Weis L, Antonini A, Biundo R. Dynamic functional connectivity changes associated with dementia in Parkinson's disease. Brain 2019;142:2860-72. [Crossref] [PubMed]
- Xue K, Liang S, Yang B, Zhu D, Xie Y, Qin W, Liu F, Zhang Y, Yu C. Local dynamic spontaneous brain activity changes in first-episode, treatment-naïve patients with major depressive disorder and their associated gene expression profiles. Psychol Med 2022;52:2052-61. [Crossref] [PubMed]
- Li Y, Ran Y, Chen Q. Abnormal static and dynamic functional network connectivity of the whole-brain in children with generalized tonic-clonic seizures. Front Neurosci 2023;17:1236696. [Crossref] [PubMed]
- Li YT, Chen JW, Yan LF, Hu B, Chen TQ, Chen ZH, Sun JT, Shang YX, Lu LJ, Cui GB, Wang W. Dynamic alterations of functional connectivity and amplitude of low-frequency fluctuations in patients with unilateral sudden sensorineural hearing loss. Neurosci Lett 2022;772:136470. [Crossref] [PubMed]
- Li YT, Bai K, Li GZ, Hu B, Chen JW, Shang YX, Yu Y, Chen ZH, Zhang C, Yan LF, Cui GB, Lu LJ, Wang W. Functional to structural plasticity in unilateral sudden sensorineural hearing loss: neuroimaging evidence. Neuroimage 2023;283:120437. [Crossref] [PubMed]
- National Health and Family Planning Commission of China. Diagnosis of occupational noise-induced deafness: GBZ 49-2014. Beijing: Standards Press of China; 2014.
- Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front Syst Neurosci 2010;4:13. [PubMed]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 2005;360:1001-13. [Crossref] [PubMed]
- Peraza LR, Nesbitt D, Lawson RA, Duncan GW, Yarnall AJ, Khoo TK, Kaiser M, Firbank MJ, O'Brien JT, Barker RA, Brooks DJ, Burn DJ, Taylor JP. Intra- and inter-network functional alterations in Parkinson's disease with mild cognitive impairment. Hum Brain Mapp 2017;38:1702-15. [Crossref] [PubMed]
- Huang H, Wang J, Seger C, Lu M, Deng F, Wu X, He Y, Niu C, Wang J, Huang R. Long-term intensive gymnastic training induced changes in intra- and inter-network functional connectivity: an independent component analysis. Brain Struct Funct 2018;223:131-44. [Crossref] [PubMed]
- Zhao Z, Wu J, Fan M, Yin D, Tang C, Gong J, Xu G, Gao X, Yu Q, Yang H, Sun L, Jia J. Altered intra- and inter-network functional coupling of resting-state networks associated with motor dysfunction in stroke. Hum Brain Mapp 2018;39:3388-97. [Crossref] [PubMed]
- Liu J, Zhu Q, Zhu L, Yang Y, Zhang Y, Liu X, Zhang L, Jia Y, Peng Q, Wang J, Sun P, Fan W, Wang J. Altered brain network in first-episode, drug-naive patients with major depressive disorder. J Affect Disord 2022;297:1-7. [Crossref] [PubMed]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011;106:1125-65. [Crossref] [PubMed]
- Zeng W, Fan W, Kong X, Liu X, Liu L, Cao Z, Zhang X, Yang X, Cheng C, Wu Y, Xu Y, Cao X, Xu Y. Altered Intra- and Inter-Network Connectivity in Drug-Naïve Patients With Early Parkinson's Disease. Front Aging Neurosci 2022;14:783634. [Crossref] [PubMed]
- Malhi GS, Das P, Outhred T, Bryant RA, Calhoun V. Resting-state neural network disturbances that underpin the emergence of emotional symptoms in adolescent girls: resting-state fMRI study. Br J Psychiatry 2019;215:545-51. [Crossref] [PubMed]
- Cui Q, Sheng W, Chen Y, Pang Y, Lu F, Tang Q, Han S, Shen Q, Wang Y, Xie A, Huang J, Li D, Lei T, He Z, Chen H. Dynamic changes of amplitude of low-frequency fluctuations in patients with generalized anxiety disorder. Hum Brain Mapp 2020;41:1667-76. [Crossref] [PubMed]
- Zhang C, Dou B, Wang J, Xu K, Zhang H, Sami MU, Hu C, Rong Y, Xiao Q, Chen N, Li K. Dynamic Alterations of Spontaneous Neural Activity in Parkinson's Disease: A Resting-State fMRI Study. Front Neurol 2019;10:1052. [Crossref] [PubMed]
- Chen X, Liu M, Zuo L, Wu X, Chen M, Li X, An T, Chen L, Xu W, Peng S, Chen H, Liang X, Hao G. Environmental noise exposure and health outcomes: an umbrella review of systematic reviews and meta-analysis. Eur J Public Health 2023;33:725-31. [Crossref] [PubMed]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 2005;102:9673-8. [Crossref] [PubMed]
- Abrol A, Chaze C, Damaraju E, Calhoun VD. The chronnectome: Evaluating replicability of dynamic connectivity patterns in 7500 resting fMRI datasets. Annu Int Conf IEEE Eng Med Biol Soc 2016;2016:5571-4. [Crossref] [PubMed]
- Zhu J, Jiao Y, Chen R, Wang XH, Han Y. Aberrant dynamic and static functional connectivity of the striatum across specific low-frequency bands in patients with autism spectrum disorder. Psychiatry Res Neuroimaging 2023;336:111749. [Crossref] [PubMed]
- Yan B, Xu X, Liu M, Zheng K, Liu J, Li J, Wei L, Zhang B, Lu H, Li B. Quantitative Identification of Major Depression Based on Resting-State Dynamic Functional Connectivity: A Machine Learning Approach. Front Neurosci 2020;14:191. [Crossref] [PubMed]
- Chen H, Xu J, Lv W, Hu Z, Ke Z, Qin R, Xu Y. Altered static and dynamic functional network connectivity related to cognitive decline in individuals with white matter hyperintensities. Behav Brain Res 2023;451:114506. [Crossref] [PubMed]
- Hou A, Pang X, Zhang X, Peng Y, Li D, Wang H, Zhang Q, Liang M, Gao F. Widespread aberrant functional connectivity throughout the whole brain in obstructive sleep apnea. Front Neurosci 2022;16:920765. [Crossref] [PubMed]
- Niu H, Li W, Wang G, Hu Q, Hao R, Li T, Zhang F, Cheng T. Performances of whole-brain dynamic and static functional connectivity fingerprinting in machine learning-based classification of major depressive disorder. Front Psychiatry 2022;13:973921. [Crossref] [PubMed]
- Suo X, Ding H, Li X, Zhang Y, Liang M, Zhang Y, Yu C, Qin W. Anatomical and functional coupling between the dorsal and ventral attention networks. Neuroimage 2021;232:117868. [Crossref] [PubMed]
- Jiang LW, Qian RB, Fu XM, Zhang WM, Zhang D, Xia CS, Peng N, Lin B, Niu ZS, Wan YH. Altered functional and causal connectivity in attention and default mode network of postconcussional syndrome patients. Chinese Journal of Neuromedicine 2018;17:1008-13.
- Donaldson PH, Rinehart NJ, Enticott PG. Noninvasive stimulation of the temporoparietal junction: A systematic review. Neurosci Biobehav Rev 2015;55:547-72. [Crossref] [PubMed]
- Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist 2014;20:150-9. [Crossref] [PubMed]
- Markett S, Nothdurfter D, Focsa A, Reuter M, Jawinski P. Attention networks and the intrinsic network structure of the human brain. Hum Brain Mapp 2022;43:1431-48. [Crossref] [PubMed]
- Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, Dapretto M. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Dev Cogn Neurosci 2014;10:148-59. [Crossref] [PubMed]
- Li X. Investigation of the Neural Mechanisms of Presbycusis with Cognitive Impairment Using Sub-bands Analysis of ALFF and Functional Connectivity. Jinan: Shandong University; 2021.
- Luan Y, Salvi R, Liu L, Lu C, Jiao Y, Tang T, Liu H, Teng GJ. High-frequency Noise-induced Hearing Loss Disrupts Functional Connectivity in Non-auditory Areas with Cognitive Disturbances. Neurosci Bull 2021;37:720-4. [Crossref] [PubMed]
- Luan Y. Neural plasticity in hearing loss: a combined animal and clinical study using multimodal MRI techniques. Nanjing: Southeast University; 2021.
- Andin J, Holmer E. Reorganization of large-scale brain networks in deaf signing adults: The role of auditory cortex in functional reorganization following deafness. Neuropsychologia 2022;166:108139. [Crossref] [PubMed]
- Alzaher M, Vannson N, Deguine O, Marx M, Barone P, Strelnikov K. Brain plasticity and hearing disorders. Rev Neurol (Paris) 2021;177:1121-32. [Crossref] [PubMed]
- Gromann PM, Tracy DK, Giampietro V, Brammer MJ, Krabbendam L, Shergill SS. Examining frontotemporal connectivity and rTMS in healthy controls: implications for auditory hallucinations in schizophrenia. Neuropsychology 2012;26:127-32. [Crossref] [PubMed]
- Zou Y, Tang W, Qiao X, Li J. Aberrant modulations of static functional connectivity and dynamic functional network connectivity in chronic migraine. Quant Imaging Med Surg 2021;11:2253-64. [Crossref] [PubMed]
- Yu T, Zou Y, Nie H, Li Y, Chen J, Du Y, Peng H, Luo Q. The role of the thalamic subregions in major depressive disorder with childhood maltreatment: Evidences from dynamic and static functional connectivity. J Affect Disord 2024;347:237-48. [Crossref] [PubMed]
- Li J, Zou Y, Kong X, Leng Y, Yang F, Zhou G, Liu B, Fan W. Exploring functional connectivity alterations in sudden sensorineural hearing loss: A multilevel analysis. Brain Res 2024;1824:148677. [Crossref] [PubMed]
- Huang W, Fang X, Li S, Mao R, Ye C, Liu W, Deng Y, Lin G. Abnormal characteristic static and dynamic functional network connectivity in idiopathic normal pressure hydrocephalus. CNS Neurosci Ther 2024;30:e14178. [Crossref] [PubMed]
- Shukla A, Harper M, Pedersen E, Goman A, Suen JJ, Price C, Applebaum J, Hoyer M, Lin FR, Reed NS. Hearing Loss, Loneliness, and Social Isolation: A Systematic Review. Otolaryngol Head Neck Surg 2020;162:622-33. [Crossref] [PubMed]
- Glick H, Sharma A. Cross-modal plasticity in developmental and age-related hearing loss: Clinical implications. Hear Res 2017;343:191-201. [Crossref] [PubMed]


