
Independent predictors of ischemic stroke: a comprehensive scan of the internal carotid and vertebrobasilar systems
Introduction
Ischemic cardiovascular disease, which includes coronary heart disease and stroke, continues to be the leading cause of morbidity and mortality worldwide (1). Among these diseases, stroke has the highest single-disease disability rate. Based on the current status of domestic and international research, stroke is preventable and controllable (2,3). Therefore, the early detection of stroke risk factors, which will facilitate timely, active, and effective interventions, is essential to reduce the incidence of stroke and poor neurological prognoses (e.g., hemisensory syndrome and mild dysarthria) (4).
Cervical vascular anomalies and intracranial artery stenosis or occlusion are prevalent etiological factors in ischemic stroke (5). The Carotid Artery System and the Vertebrobasilar Artery System are connected, and together, participate in the blood supply of brain tissue. To date, most studies have focused on the effects of internal carotid artery (ICA) stenosis, carotid plaque, and intracranial stenosis on stroke. However, there is a paucity of studies comprehensively analyzing these two systems as an integrated entity. The accurate and comprehensive assessment of the Carotid Artery System and Vertebrobasilar Artery System is key to preventing stroke, and the two systems should not be separated.
Further, in cases of acute stroke, cerebral collateral circulation serves as the most prompt safeguard against ischemic brain injury by facilitating persistent perfusion to regions beyond the occluded intracranial artery via alternative vascular pathways. Adequate collateral circulation enhances the survival of brain tissue in the compromised vascular area, consequently restricting the extent of the infarct core. The crucial role of optimal collateral flow in facilitating favorable clinical outcomes is now broadly acknowledged in medical practice.
Integrated cervicocerebral ultrasound (ICCUS) is a combination of both cervical duplex ultrasonography and transcranial color-coded duplex sonography. It has significant advantages in the early screening and assessment of ischemic stroke. Notably, it can be used to comprehensively assess the vascular hemodynamics of the Carotid Artery System and the Vertebrobasilar Artery System, and in the qualitative and quantitative analysis of lesions (5,6). Compared to traditional imaging modalities, such as computed tomography (CT) angiography, digital subtraction angiography, and magnetic resonance angiography (MRA), it has a number advantages (e.g., it is non-invasive, rapid, convenient, and cost effective).
This study performed comprehensive scans of the Carotid Artery Systemand the Vertebrobasilar Artery System using ICCUS to explore the predictive factors for the occurrence of ischemic stroke, and to identify valuable imaging features to guide clinical decision making. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1763/rc).
Methods
Patients
Between July 2023 and April 2024, this observational (non-interventional) study prospectively enrolled patients who had undergone ICCUS scanning and who had recently suffered from an ischemic stroke (<30 days). ICCUS is an ultrasound technique that can be used to comprehensively scan the Carotid Artery System and the Vertebrobasilar Artery System. Ischemic stroke was defined as a focal neurological deficit lasting more than 24 hours, with CT and/or magnetic resonance imaging (MRI) evidence of cerebral infarction (7). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Board of Beijing Tiantan Hospital, Capital Medical University (approval No. KY2022-015-04), and informed consent was obtained from all individual participants.
To be eligible for inclusion in the study, the patients had to meet the following inclusion criteria: (I) have suffered from an anterior circulation ischemic stroke within 30 days; and (II) have CT and/or MRI evidence of cerebral infarction. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had a prior history of radiotherapy to the neck; (II) had a diagnosis of cardiogenic stroke, lacunar stroke, or cryptogenic stroke based on the TOAST classification; (III) had impaired consciousness preventing their cooperation in the completion of ultrasound examination; (IV) had inadequate quality or non-measurable ultrasound images due to the presence of calcified plaques causing extensive acoustic shadowing; (V) had a prior history of carotid endarterectomy or carotid stenting; and/or (VI) declined to participate in the study
Instruments and methods
The ultrasound images were acquired by a sonographer with over 20 years of experience and analyzed by two physicians, each with more than 5 years of experience, using a double-blind method. Ultrasound equipment (Epiq 7, Philips Healthcare, Amsterdam, Netherlands) and a phased array probe with transducer frequencies of 1.0–5.0 and 5–14 MHz were used. For the comprehensive scanning of the cervical and intracranial vasculature, each patient was placed in a supine position, with a thin pillow supporting their neck to optimally expose the head and neck area.
Baseline information
Using a blinded approach, the following information was gathered: (I) demographic data, including age and gender; (II) medical history, including hypertension, diabetes mellitus, dyslipidemia, prior ischemic cerebrovascular accidents, and coronary artery disease; (III) history of smoking and alcohol consumption; (IV) history of substance use; (V) anthropometric data, including height, weight, and body mass index; and (VI) ischemic cerebrovascular event etiology.
ICCUS scanning of the cervical artery
The observational indicators of the bilateral carotid artery (CA), vertebral artery (VA), and subclavian artery (SA) included: plaque thickness and length, plaque echogenicity, plaque surface morphology, plaque composition [i.e., intraplaque hemorrhage (IPH), lipid-rich necrotic core (LRNC), and calcification], stenosis number, lateral stenosis, stenosis location, and stenosis degree. For patients with multiple carotid plaques and stenoses, the largest plaque and stenosis were collected.
The echogenicity of the plaques was categorized based on grayscale ultrasound imagery as follows: type 1, homogeneous hypoechoic; type 2, hypoechoic predominant; type 3, hyperechoic predominant; and type 4, homogeneous hyperechoic (8,9). Plaque ulceration was defined as the presence of an echogenic defect (at least 2 mm × 2 mm) on the plaque surface with colored flow signal filling visible on color Doppler flow imaging (CDFI) (10). IPH was defined as the presence of an anechoic zone in the plaque (11). The LRNC was defined as the presence of a hypoechoic zone in the plaque. Calcification was defined as calcium deposits in the plaque, manifesting as hyperechoic areas with weak or insignificant acoustic shadows. The degree of ICA stenosis was determined using the North American Symptomatic Carotid Endarterectomy Trial criteria (12) (Table S1). VA stenosis and SA stenosis were assessed as per the criteria in the Chinese Guidelines for Vascular Ultrasound Detection of Stroke (13-15) (Tables S2-S4).
ICCUS scanning of the intracranial artery
Abnormal indicators of the following vessels were collected: anterior cerebral artery (ACA), middle cerebral artery (MCA), posterior cerebral artery (PCA), terminal internal carotid artery (TICA), carotid siphon (CS), vertebral artery V4 (VA-V4), basilar artery (BA), and the opening of the cerebral collaterals, including the anterior communicating artery (AComA), posterior communicating artery (PComA), ophthalmic artery (OA), leptomeningeal collaterals (LMCs), and neovascularization.
The criteria in the Chinese Guidelines for Vascular Ultrasound in Stroke and the Chinese Guidelines for the Clinical Application of Cerebrovascular Ultrasound for intracranial artery stenosis and cerebral collaterals opening were used in this study (13,16). Intracranial artery stenosis (≥50%) was diagnosed if the CDFI showed the thinning of localized blood flow bundles and colorful mosaic-like blood flow signals. Pulse Wave (PW) Doppler demonstrates an elevated blood flow velocity at the stenotic segment, while distal to the stenosis, velocities may remain within normal limits, exhibit mild reduction, or demonstrate a significant decrease. Compensatory hemodynamic augmentation is observed in adjacent arterial pathways. The opening of the following cerebral collaterals presented as follows: (I) AComA: the affected side of the ACA showed a reversed flow signal with compensatory increased blood flow velocity in the contralateral ACA, and the compression of the contralateral common CA resulted in decreased blood flow velocity in both the affected MCA and ACA; (II) PComA: the PCA flow velocity in the affected side was elevated, and had a higher Pulsatility Index (PI) than the ipsilateral MCA and ACA, but a lower PI than the contralateral PCA. This elevation increased when compressing the healthy common CA, but only if the AComA was open. If not, the compression test was ineffective; (III) OA: in PW Doppler, it is manifested as reverse or biphasic blood flow signals, and had a low-velocity, low-resistance blood flow signal; (IV) LMCs: the ACA or PCA had a high-velocity, low-resistance flow diversion blood flow signa (17); and (V) neovascularization: the breath-holding test was employed to assess cerebrovascular reactivity, which reflects the compensatory capacity of the cerebral vasculature, thus indirectly indicating tertiary collateral circulation function. Patients were instructed to hold their breath for 30 seconds, and bilateral MCA blood flow velocities were monitored. The breath-holding duration, pre-breath-hold baseline mean blood flow velocity (Vm baseline), and post-breath-hold mean blood flow velocity (Vm post-breath-hold) were recorded to compute the breath-holding index (BHI). A BHI <0.69 indicated an abnormality, and a BHI ≥0.69 indicated neovascularization opening and adequate tertiary collateral circulation compensation. The BHI was calculated as follows: BHI = (Vm post-breath-hold – Vm baseline)/(Vm baseline × breath-holding duration).
Statistical analysis
SPSS 21.0 software was used for the statistical analysis. The continuous variables are expressed as the mean ± standard deviation, and the t-test or the Mann-Whitney U test was used to compare groups. The χ2 test or Fisher’s exact test was used to compare the classification variables. A forward stepwise approach was employed to conduct the multivariate logistic regression analysis to examine the association between the ICCUS risk biomarkers and ischemic stroke to identify independent predictors of ischemic stroke. The predictive performance was assessed through the application of the receiver operating characteristic (ROC) curves and the area under the curve (AUC). Statistical significance was defined as a P value <0.05. The overall sample size was assessed as 5–10 times the number of variables in the multifactor logistic regression analysis. Patients with missing data were not included in the study.
Results
Univariate analysis
Patient characteristics
From July 2023 to April 2024, 289 patients who underwent ICCUS examinations were included in the study. Of these patients, 162 were excluded (4 for CA entrapment, 15 for aortitis, 8 for intracranial aneurysm, 1 for smog disease, 1 for myofibrillar dysplasia, 2 for intracranial vascular balloon dilatation, 2 for cerebral hemorrhage, 30 for carotid endarterectomy or carotid stenting, and 99 for incomplete clinical information). Thus, ultimately, 127 patients with complete clinical and ultrasound data were included in the analysis. Of these patients, 96 (75.6%) were male and 31 (24.4%) were female. The patients had a mean age of 64.1±8.4 years. CAs showed stenosis in 114 patients and plaque in 119 patients. VAs showed stenosis in 30 patients and plaque in 29 patients. SAs showed stenosis in 46 patients and plaque in 116 patients. The intracranial arteries showed stenosis in 77 patients and open collateral circulation in 94 patients. Table 1 displays the baseline characteristics of the patients. In this study, sex, dyslipidemia, and a history of ischemic cerebrovascular events were statistically significantly associated with ischemic stroke (all P<0.05) (Figure 1).
Table 1
| Variables | Stroke group | Non-stroke group | χ2/t/Z | P |
|---|---|---|---|---|
| Age (years), mean ± SD | 64.10±9.41 | 64.07±7.95 | –0.33 | 0.745 |
| Sex (male) | 38 | 58 | 7.54 | 0.008* |
| Body mass index (kg/m2), mean ± SD | 24.89±3.28 | 24.13±2.93 | 0.42 | 0.520 |
| Smoking history | 18 | 35 | 0.03 | >0.99 |
| Alcohol drinking history | 16 | 23 | 1.61 | 0.225 |
| Hypertension | 32 | 58 | 0.86 | 0.411 |
| Diabetes mellitus | 15 | 25 | 0.52 | 0.544 |
| Dyslipidemia | 15 | 47 | 4.31 | 0.041* |
| History of stroke | 13 | 12 | 5.04 | 0.033* |
| Coronary heart disease | 7 | 17 | 0.20 | 0.811 |
| Anti-platelet drugs | 18 | 41 | 0.33 | 0.578 |
| Anti-hypertensive | 27 | 53 | 0.05 | >0.99 |
| Statin | 17 | 45 | 1.75 | 0.194 |
*, P<0.05. χ2, Chi-square test; t, Student’s t-test; Z, Z-test; SD, standard deviation.
CA
The average thickness and length of the CA plaques were 3.8±1.3 and 22.8±13.0 mm, respectively. IPH and plaque echogenicity were significantly associated with ischemic stroke (P=0.001, P=0.048, respectively). In relation to the CAs, 1 patient had <50% stenosis, 23 patients had 50–69% stenosis, 72 patients had 70–99% stenosis, 17 patients had occlusions, and 14 patients had no stenosis. A statistically significant difference was observed between the non-stenotic, non-occlusive (0–99% stenosis) and occlusive groups (P=0.043) (Table S5).
VA
The average thickness and length of the VA plaques were 0.5±1.0 and 1.6±3.5 mm, respectively. In relation to the VAs, 5 patients had <50% stenosis, 12 patients had 50–69% stenosis, 8 patients had 70–99% stenosis, 5 patients had occlusions, and 97 patients had no stenosis (Table S6).
SA
The average thickness and length of the SA plaques were 3.0±1.3 and 12.7±7.0 mm, respectively. In relation to the SAs, 3 patients had <50% stenosis, 37 patients had 50–69% stenosis, 5 patients had 70–99% stenosis, 1 patient had an occlusion, and 81 patients had no stenosis (Table S7).
Intracranial arteries
Stenosis was present in the intracranial arteries of 77 patients, and open collateral circulation was observed in 94 patients. In ICCUS scanning, ACA, PCA, and TICA multiple stenosis, as well as lateralization of the stenoses, were statistically different from ischemic stroke. The VA-V4 multiple stenoses were also statistically different from ischemic stroke (all P<0.05). No statistically significant difference was found between stenoses of the MCA and BA, and ischemic stroke (Table S8).
The opening of the AComA and OA in the cerebral collaterals was associated with the occurrence of ischemic stroke. The neovascularization and number of LMCs were not statistically different from the occurrence of stroke. However, the occurrence of stroke was found to be associated with the total number of cerebral collaterals. The total number of cerebral collaterals predicted stroke occurrence with an AUC of 0.635 [95% confidence interval (CI): 0.534–0.736, P=0.014]. Thus, the number of total cerebral collaterals can be used to predict the occurrence of ischemic stroke with a certain degree of accuracy. Further analysis revealed that the optimal cut-off value for the number of total cerebral collaterals was 2.5, with a sensitivity of 45.2% and a specificity of 76.5%. The total number of cerebral collaterals was subsequently categorized using cut-off values by ≤2 and >2, and the results remained statistically different after categorization (P=0.021).
Multifactorial logistic regression analysis
The factors with P values <0.1 in the univariate analysis were considered independent variables (Table 2). The multivariate logistic regression analysis showed that ischemic stroke was positively correlated with IPH, multiple stenoses of the ACA and PCA, and the opening of AComA in the cerebral collaterals (all P<0.05) as detected by ICCUS (Table 3 and Figure 2). The predictive accuracy of a model that combined multiple parameters was examined, and it had an AUC of 0.802 (95% CI: 0.71–0.89, P<0.001). Thus, the above-mentioned ICCUS parameters were found to be independent predictors of ischemic stroke and showed favorable diagnostic performance (Figure 3). In addition, if the IPH predictor for cervical vessels was combined with any of the one to three predictors for the intracranial vessels, the diagnostic accuracies increased progressively to 68.1–71.4%, 74.5–76.0%, and 80.2%, respectively (Table 4).
Table 2
| Variables | χ2/t/Z | P |
|---|---|---|
| Patients characteristics | ||
| Sex (male) | 7.54 | 0.008* |
| Dyslipidemia | 4.31 | 0.041* |
| History of stroke | 5.04 | 0.033* |
| CA | ||
| Plaque echogenicity | 9.59 | 0.048* |
| Intraplaque hemorrhage | 10.63 | 0.002* |
| ACA | ||
| Stenosis number | 13.19 | 0.001* |
| Stenosis lateral | 12.37 | 0.002* |
| Stenosis synthesis | 13.67 | 0.008* |
| PCA | ||
| Stenosis number | 9.90 | 0.007* |
| Stenosis lateral | 9.55 | 0.008* |
| Stenosis synthesis | 11.63 | 0.040* |
| TICA | ||
| Stenosis number | 6.30 | 0.043* |
| Stenosis lateral | 6.30 | 0.043* |
| VA-V4 | ||
| Stenosis number | 6.89 | 0.032* |
| AComA | ||
| Open | 5.49 | 0.023* |
| OA | ||
| Open | 7.07 | 0.029* |
| Total collaterals | 6.41 | 0.011* |
| Total collaterals† | 7.74 | 0.021* |
*, P<0.05; †, categorize the data; χ2, Chi-square test; t, Student’s t-test; Z, Z-test. ACA, anterior cerebral artery; AComA, anterior communicating artery; CA, carotid artery; OA, ophthalmic artery; PCA, posterior cerebral artery; TICA, terminal internal carotid artery; VA-V4, vertebral artery V4.
Table 3
| Variables | B | SE | Wald test | P | OR (95% CI) |
|---|---|---|---|---|---|
| IPH | 1.649 | 0.542 | 9.251 | 0.002 | 5.20 (1.80–15.05) |
| ACA multiple stenoses | 3.074 | 1.220 | 6.352 | 0.004 | 21.63 (1.98–236.17) |
| PCA multiple stenoses | 2.989 | 0.965 | 9.601 | 0.002 | 19.87 (3.00–131.62) |
| AComA opening | 1.523 | 0.498 | 9.345 | 0.001 | 4.58 (1.73–12.17) |
AComA, anterior communicating artery; ACA, anterior cerebral artery; B, regression coefficient; 95% CI, 95% confidence interval; IPH, intraplaque hemorrhage; OR, odds ratio; PCA, posterior cerebral artery; SE, standard error.

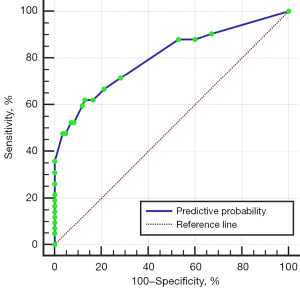
Table 4
| Variables | AUC | SD | P | 95% CI |
|---|---|---|---|---|
| IPH | 0.626 | 0.055 | 0.021 | 0.52–0.73 |
| ACA† | 0.636 | 0.055 | 0.013 | 0.53–0.74 |
| PCA† | 0.608 | 0.056 | 0.049 | 0.50–0.72 |
| AComA‡ | 0.609 | 0.054 | 0.046 | 0.50–0.71 |
| IPH + ACA† | 0.708 | 0.052 | <0.001 | 0.61–0.81 |
| IPH + PCA† | 0.714 | 0.051 | <0.001 | 0.61–0.82 |
| IPH + AComA‡ | 0.681 | 0.052 | 0.001 | 0.58–0.78 |
| IPH + ACA† + PCA† | 0.745 | 0.050 | <0.001 | 0.65–0.84 |
| IPH + ACA† + AComA‡ | 0.754 | 0.049 | <0.001 | 0.66–0.85 |
| IPH + PCA† + AComA‡ | 0.760 | 0.046 | <0.001 | 0.67–0.85 |
| IPH + ACA† + PCA† + AComA‡ | 0.802 | 0.045 | <0.001 | 0.71–0.89 |
†, multiple stenoses; ‡, opening. ACA, anterior cerebral artery; AComA, anterior communicating artery; AUC, area under the curve; 95% CI, 95% confidence interval; IPH, intraplaque hemorrhage; PCA, posterior cerebral artery; ROC, receiver operating characteristic; SD, standard deviation.
Discussion
Carotid plaque size and the carotid stenosis it causes are popular research topics in ischemic cardiovascular disease (18). Our univariate analysis showed that carotid plaque thickness was associated with the occurrence of ischemic stroke; however, in the multivariate analysis, there was no statistical difference. Following improvements in the ability to image atherosclerosis in vivo, particularly using MRI, research has shown that atherosclerotic plaque susceptibility is more influenced by their composition than their size or the degree of luminal stenosis (3,19,20). The present study analyzed plaque composition and concluded that IPH (but not plaque size) was an independent predictor of ischemic stroke, which is in line with previous studies (19,20).
IPH is one of the culprits of unstable plaques. Hypoxia and inflammatory stimuli lead to the development of thin-walled neovascularization in atherosclerotic plaque. Neovascularization rupture leads to intravascular erythrocyte exudation, which attracts macrophages into the plaque, and creates a highly reactive microenvironment that further promotes microvessel formation, leading to the formation of IPH, which destabilizes the plaque (21,22). Artery-to-artery embolization occurs when an embolus formed by dislodged instability plaque flows distally into the skull and becomes embedded in a smaller vessel downstream. This causes a sustained reduction in local cerebral blood flow, ultimately leading to ischemic stroke (23). The presence of IPH increases the likelihood of ischemic stroke by 5.2-fold. Moreover, IPH is associated with a two-fold increase risk of stroke at the 5-year follow-up (24). Unexpectedly, we found no correlation between the presence of LRNC or calcification and the occurrence of ischemic stroke. The size rather than the presence of LRNC has been proposed to be a predictor of ischemic stroke (25,26). In addition, calcification may increase plaque stability (27,28).
In addition to the aforementioned ischemic stroke caused by unstable plaque dislodgment that blocks intracranial blood vessels, the accumulation of lipids in intracranial arteries causes stenosis of the blood vessels and can also lead to ischemic stroke (29). One study of patients with acute ischemic stroke concluded that transcranial color-coded Doppler provides reasonably accurate diagnostic information to rule in or rule out the occlusion or stenosis of intracranial arteries with a summary sensitivity of 95% and specificity of 95% (30). In this study, ICCUS was used to scan the intracranial arteries, and multiple stenoses of the ACA were found to be an independent predictor of ischemic stroke. Compared to the patients without ACA stenosis, those with multiple stenoses of the ACA were 21.6 times more likely to experience ischemic stroke. A large sample size study of stroke in an Asian population noted that patients with ACA infarcts were more likely to suffer from ischemic strokes than those with MCA and PCA infarcts, and that ACA infarcts may be associated with stroke recurrence (31). Besides, multiple stenoses of the PCA is also an independent predictor of ischemic stroke. When multiple stenoses of the PCA were present on the ICCUS scanning, the odds of ischemic stroke increased 19.9-fold. Surprisingly, the MCA was not found to be associated with ischemic stroke in either the univariate or multivariate analyses. This may be due to the watershed penetrating arterioles originating from collateral circulation, which maintain constant blood flow (32).
When ischemic stroke occurs, the body provides additional blood supply to the regions supplied by the narrowed or occluded intracranial artery through the collaterals. Adequate blood supply from the collaterals is beneficial in maintaining brain tissue viability (29,32), and plays an important protective role in preventing and relieving brain tissue damage. In this study, we also investigated the predictive value of collateral circulation for ischemic stroke. The overall number of collaterals was found to be associated with ischemic stroke in the univariate analysis, and the cut-off value analysis indicated that >3 collaterals increased the likelihood of ischemic stroke, but no statistical significance was found in multivariate analysis. TICA occlusion was found to affect the development and formation of collaterals (33). In the univariate analysis, it was also associated with ischemic stroke. Both were eliminated as confounders in the multivariate analysis, which might explain the negative results obtained.
Further, we concluded that the opening of the AComA in the cerebral collateral on ICCUS scanning was an independent predictor of ischemic stroke, and ischemic stroke was 4.6 times more likely to occur when the AComA was opened than when it was unopened. When occlusion or stenosis of intracranial vessels occurs, the AComA participates in cerebral blood flow redistribution as a pivotal primary collateral pathway. In addition, it has been proposed that its flow alleviates recurrent stroke-induced neurovascular injury, and improves neurobehavioral outcomes by promoting the establishment of collateral circulation (34). This may account for the predictive role of the AComA cerebral collateral opening for ischemic stroke.
In this study, the Carotid Artery System and the Vertebrobasilar Artery System were comprehensively scanned by ICCUS, and IPH, multiple stenoses of the ACA, multiple stenoses of the PCA, and the opening of the AComA in the cerebral collateral were identified as predictors of ischemic stroke. We further analyzed the prediction accuracy of these factors, and found that the use of only one predictor had an accuracy of approximately 60% (60.8–63.6%), the use of IPH and any one intracranial predictor had an accuracy of 70% (68.1–71.4%), the use of IPH and any two intracranial predictors had an accuracy of 74.5–6.0%, and the use of all four risk factors had an overall accuracy if up to 80.2%. Therefore, for patients with leading risk factors for stroke [i.e., high systolic blood pressure, a high body mass index, high fasting plasma glucose, and a history of smoking (35)], ICCUS should be performed regularly, as the more independent predictors present, the higher the likelihood of ischemic stroke.
For patients with acute ischemic stroke, the therapeutic time window, especially after they present with symptoms of neurological deficits and before the occurrence of cerebral infarction, is crucial. Using ICCUS, sonographers can comprehensively scan the ICA and vertebrobasilar artery systems of patients in minutes. Immediately after scanning, ICCUS provides information about the stenosis and occlusion of vessels, as well as about the hemodynamic status of the cerebral circulation, which in combination with predictors for stroke occurrence, facilitates rapid clinical decision making. Further, ICCUS can be performed simultaneously as emergency personnel conduct neurological assessments, maintain vital signs, and perform other resuscitation maneuvers, thereby gaining considerable time to adopt subsequent therapeutic measures. In summary, ICCUS holds significant value in the rescue and treatment of ischemic stroke.
This study had some limitations. First, because blood circulation in the brain is an interacting and integrally regulated whole, no distinction was made between the right and left cerebral hemispheres in this study. Second, as ICCUS is not a clinical universal stroke test and requires concurrent CT or MRA, patients with missing data were excluded from the study, which resulted in a small study sample size. Third, the VA-V3 segment was not examined because of its deep location and the difficulty of ultrasound scanning. Finally, the diagnostic advantages of MRI in IPH and the LRNC are well documented, but it has been suggested that experienced sonographers can improve their recognition of both conditions (36,37). In this study, ultrasonographers working for up to 20 years were used as operators to minimize the diagnostic gap between US and MRI. Considering the advantages and disadvantages of the aforementioned ultrasound examinations, we believe that ICCUS is a better choice for the comprehensive scanning of cervical and intracranial vascular status, including carotid plaque, in the diagnosis of ischemic stroke.
Conclusions
Given that cervicocerebral circulation is an inseparable whole, this study used comprehensive, accurate, efficient, and reproducible ICCUS scanning, co-incorporated the Carotid Artery System and the Vertebrobasilar Artery System to conduct a comprehensive analysis, and identified more comprehensive and reliable independent predictors of ischemic stroke. Our findings may have significant guiding value for the early prevention and timely treatment of patients.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1763/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1763/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Board of Beijing Tiantan Hospital, Capital Medical University (approval No. KY2022-015-04), and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018;137:e67-e492. [Crossref] [PubMed]
- Saba L, Cau R, Kopczak A, Schindler A, Saam T. Reply: Carotid Plaque-RADS: A Novel Stroke Risk Classification System. JACC Cardiovasc Imaging 2024;17:227. [Crossref] [PubMed]
- Bos D, Arshi B, van den Bouwhuijsen QJA, Ikram MK, Selwaness M, Vernooij MW, Kavousi M, van der Lugt A. Atherosclerotic Carotid Plaque Composition and Incident Stroke and Coronary Events. J Am Coll Cardiol 2021;77:1426-35. [Crossref] [PubMed]
- Mendelson SJ, Prabhakaran S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. JAMA 2021;325:1088-98. [Crossref] [PubMed]
- Zhang L, Pu T, Xu X. Raynald, Zheng S, Fu J, Yong Q, Zhang W, He W. Diagnostic feasibility of middle cerebral artery stenosis or occlusion evaluated by TCCS and CEUS: Repeatability, reproducibility, and diagnostic agreement with DSA. J Stroke Cerebrovasc Dis 2024;33:107575. [Crossref] [PubMed]
- Hou C, Li S, Zhang L, Zhang W, He W. The differences between carotid web and carotid web with plaque: based on multimodal ultrasonic and clinical characteristics. Insights Imaging 2024;15:78. [Crossref] [PubMed]
- Huang Z, Cheng XQ, Liu YN, Bi XJ, Deng YB. Value of Intraplaque Neovascularization on Contrast-Enhanced Ultrasonography in Predicting Ischemic Stroke Recurrence in Patients With Carotid Atherosclerotic Plaque. Korean J Radiol 2023;24:338-48. [Crossref] [PubMed]
- Arnold JA, Modaresi KB, Thomas N, Taylor PR, Padayachee TS. Carotid plaque characterization by duplex scanning: observer error may undermine current clinical trials. Stroke 1999;30:61-5. [Crossref] [PubMed]
- Gray-Weale AC, Graham JC, Burnett JR, Byrne K, Lusby RJ. Carotid artery atheroma: comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg (Torino) 1988;29:676-81. [PubMed]
- Cheng L, Zheng S, Zhang J, Wang F, Liu X, Zhang L, Chen Z, Cheng Y, Zhang W, Li Y, He W. Multimodal ultrasound-based carotid plaque risk biomarkers predict poor functional outcome in patients with ischemic stroke or TIA. BMC Neurol 2023;23:13. [Crossref] [PubMed]
- Nishi T, Kume T, Yamada R, Neishi Y, Uemura S. Variation of the Appearance of Intraplaque Hemorrhage on Optical Coherence Tomography and Intravascular Ultrasound. JACC Cardiovasc Interv 2023;16:997-9. [Crossref] [PubMed]
- North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke 1991;22:711-20. [Crossref] [PubMed]
- Hua Y, Hui PJ, Xing YQ, et al. Guidelines for ultrasound examination of stroke vessels in China. Chinese Journal of Medical Ultrasound 2015;12:599-610. (Electronic Edition).
- Hua Y, Meng XF, Jia LY, Ling C, Miao ZR, Ling F, Liu JB. Color Doppler imaging evaluation of proximal vertebral artery stenosis. AJR Am J Roentgenol 2009;193:1434-8. [Crossref] [PubMed]
- Hua Y, Jia L, Li L, Ling C, Miao Z, Jiao L. Evaluation of severe subclavian artery stenosis by color Doppler flow imaging. Ultrasound Med Biol 2011;37:358-63. [Crossref] [PubMed]
- Chinese Society of Neurology, Cerebrovascular Disease Group of Chinese Society of Neurology, Neuroimaging Cooperative Group of Chinese Society of Neurology. Chinese Guidelines for the Clinical Application of Cerebrovascular Ultrasound. Chinese Journal of Neurology 2016;7:507-18.
- Chinese Society of Cerebral Blood Flow and Metabolism. The Chinese guidelines for the evaluation and management of cerebral collateral circulation in ischemic stroke (2017). Zhonghua Nei Ke Za Zhi 2017;56:460-71. [PubMed]
- Saba L, Cau R, Murgia A, Nicolaides AN, Wintermark M, Castillo M, et al. Carotid Plaque-RADS: A Novel Stroke Risk Classification System. JACC Cardiovasc Imaging 2024;17:62-75. [Crossref] [PubMed]
- Homssi M, Saha A, Delgado D. RoyChoudhury A, Thomas C, Lin M, Baradaran H, Kamel H, Gupta A. Extracranial Carotid Plaque Calcification and Cerebrovascular Ischemia: A Systematic Review and Meta-Analysis. Stroke 2023;54:2621-8. [Crossref] [PubMed]
- van Dam-Nolen DHK, Truijman MTB, van der Kolk AG, Liem MI, Schreuder FHBM, Boersma E, Daemen MJAP, Mess WH, van Oostenbrugge RJ, van der Steen AFW, Bos D, Koudstaal PJ, Nederkoorn PJ, Hendrikse J, van der Lugt A, Kooi ME. Carotid Plaque Characteristics Predict Recurrent Ischemic Stroke and TIA: The PARISK (Plaque At RISK) Study. JACC Cardiovasc Imaging 2022;15:1715-26. [Crossref] [PubMed]
- Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 2005;25:2054-61. [Crossref] [PubMed]
- Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316-25. [Crossref] [PubMed]
- Feske SK. Ischemic Stroke. Am J Med 2021;134:1457-64. [Crossref] [PubMed]
- Alkhouli M, Holmes D, Klaas JP, Lanzino G, Benson JC. Carotid Intraplaque Hemorrhage: An Underappreciated Cause of Unexplained Recurrent Stroke. JACC Cardiovasc Interv 2021;14:1950-2. [Crossref] [PubMed]
- Sun YM, Xu HY, Wang S, Wang ZJ, Zhou Y, Yu W. Carotid massive intraplaque hemorrhage, lipid-rich necrotic core, and heavy circumferential calcification were associated with new ipsilateral ischemic cerebral lesions after carotid artery stenting: high-resolution magnetic resonance vessel wall imaging study. Cardiovasc Diagn Ther 2023;13:355-66. [Crossref] [PubMed]
- Cui Z, Xu S, Miu J, Tang Y, Pan L, Cao X, Zhang J. Development and Validation of a Fusion Model Based on Carotid Plaques and White Matter Lesion Burden Imaging Characteristics to Evaluate Ischemic Stroke Severity in Symptomatic Patients. J Magn Reson Imaging 2025;61:648-60. [Crossref] [PubMed]
- Karlöf E, Seime T, Dias N, Lengquist M, Witasp A, Almqvist H, Kronqvist M, Gådin JR, Odeberg J, Maegdefessel L, Stenvinkel P, Matic LP, Hedin U. Correlation of computed tomography with carotid plaque transcriptomes associates calcification with lesion-stabilization. Atherosclerosis 2019;288:175-85. [Crossref] [PubMed]
- Huo R, Yuan W, Xu H, Yang D, Qiao H, Han H, Wang T, Liu Y, Yuan H, Zhao X. Investigating the Association of Carotid Atherosclerotic Plaque MRI Features and Silent Stroke After Carotid Endarterectomy. J Magn Reson Imaging 2024;60:138-49. [Crossref] [PubMed]
- Liu WJ. The diagnosis of intracranial artery stenosis in patients with stroke by transcranial Doppler ultrasound: A meta-analysis. Technol Health Care 2024;32:639-49. [Crossref] [PubMed]
- Mattioni A, Cenciarelli S, Eusebi P, Brazzelli M, Mazzoli T, Del Sette M, Gandolfo C, Marinoni M, Finocchi C, Saia V, Ricci S. Transcranial Doppler sonography for detecting stenosis or occlusion of intracranial arteries in people with acute ischaemic stroke. Cochrane Database Syst Rev 2020;2:CD010722. [Crossref] [PubMed]
- Cho H, Kim T, Kim YD, Na S, Choi YH, Song IU, Chung SW, Koo J, Kwon H, Park JH, Im H. A clinical study of 288 patients with anterior cerebral artery infarction. J Neurol 2022;269:2999-3005. [Crossref] [PubMed]
- Uniken Venema SM, Dankbaar JW, van der Lugt A, Dippel DWJ, van der Worp HB. Cerebral Collateral Circulation in the Era of Reperfusion Therapies for Acute Ischemic Stroke. Stroke 2022;53:3222-34. [Crossref] [PubMed]
- Wiegers EJA, Mulder MJHL, Jansen IGH, Venema E, Compagne KCJ, Berkhemer OA, Emmer BJ, Marquering HA, van Es ACGM, Sprengers ME, van Zwam WH, van Oostenbrugge RJ, Roos YBWEM, Majoie CBLM, Roozenbeek B, Lingsma HF, Dippel DWJ, van der Lugt A. Clinical and Imaging Determinants of Collateral Status in Patients With Acute Ischemic Stroke in MR CLEAN Trial and Registry. Stroke 2020;51:1493-502. [Crossref] [PubMed]
- Wen Z, Jiang Y, Zhang L, Xu X, Zhao N, Xu X, Wang F, Gao J, Yang GY, Liu X. The effect of anterior communicating artery flow on neurovascular injury and neurobehavioral outcomes in mice with recurrent stroke. Brain Res 2019;1724:146440. [Crossref] [PubMed]
- Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021;20:795-820. [Crossref] [PubMed]
- Nicolaides AN, Kakkos SK, Kyriacou E, Griffin M, Sabetai M, Thomas DJ, Tegos T, Geroulakos G, Labropoulos N, Doré CJ, Morris TP, Naylor R, Abbott AL; . Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg 2010;52:1486-1496.e1-5.
- Kakkos SK, Griffin MB, Nicolaides AN, Kyriacou E, Sabetai MM, Tegos T, Makris GC, Thomas DJ, Geroulakos GAsymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group. The size of juxtaluminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J Vasc Surg 2013;57:609-618.e1; discussion 617-8. [Crossref] [PubMed]



