
Conventional ultrasound and high-frame-rate contrast-enhanced ultrasound characteristics of ovarian thecoma-fibroma groups
Introduction
Ovarian thecoma-fibroma groups (OTFGs) are uncommon sex cord-stromal neoplasms that account for 1–4% of all ovarian tumors (1,2). Most of OTFGs are benign masses, generally with a good prognosis. According to the 2020 World Health Organization (WHO) histological classification of ovarian tumors, fibroma and thecoma are the two main common types of OTFGs (3,4). Fibroma mostly occurs in women in their fourth decade of life and accounts for 4% of all ovarian tumors. Thecoma is mostly detected in postmenopausal women and accounts for only 1% of all primary ovarian tumors. The term “fibrothecoma” refers to rare tumors with mixed features of both fibroma and thecoma. Fibrothecoma, however, is not included in the WHO classification of ovarian tumors (5). OTFGs originate from the special mesodermal tissue of the ovarian sex cord, with different proportions of fibroblasts and thecal cells (6). Fibroma shows an abundance of fibroblasts, whereas thecoma has a high proportion of thecal cells and a low proportion of fibroblasts (7). It is sometimes difficult to differentiate the pathological type of fibromas and thecomas owing to the mixed and overlapping cellular components; therefore, they are frequently referred to as OTFGs (1,8). OTFGs with an abundance or high proportion of fibroblasts do not show hormone-mediated symptoms as fibroblasts in these tumors are inactive. Moreover, these tumors are usually asymptomatic; consequently, many patients are diagnosed only during imaging examinations. Patients with symptomatic OTFGs may show abnormal vaginal bleeding, endometrial hyperplasia, hysteromyoma, and even endometrial carcinoma as thecal cells actively can produce estrogens (9-11).
According to some studies, a large number of OTFGs are benign (1,6), and the International Ovarian Tumor Analysis (IOTA) model can help to differentiate between benign and malignant OTFGs, with an accuracy of 93% (12). Ultrasound (US) is one of the imaging modalities with frequent use to evaluate pelvic masses. It has the advantages of safety, convenience, and noninvasive diagnosis (13). Compared to conventional US, contrast-enhanced ultrasound (CEUS) uses US contrast agents to enhance the blood scattering signals, which significantly improves the detection and diagnosis of tumors (14). The frame rate of conventional contrast-enhanced ultrasound (C-CEUS) is 10–15 frames per second (fps), and that of high-frame-rate CEUS (HiFR-CEUS) is 4–10 times that of C-CEUS; this enhanced frame rate significantly improves the temporal resolution of CEUS images to better show the process of microbubble perfusion in the arterial phase, and provides more diagnostic information (15).
Since OTFGs show rapid growth, surgical excision is the preferred treatment, with laparoscopic surgery being one of the most effective treatments for OTFGs (6,10). Some studies have reported that ascites is reduced following the surgical excision of the tumor (10,16). Accordingly, it is important to diagnose OTFGs early to facilitate timely intervention for better patient outcomes. The objective of the present study was to describe the conventional US and HiFR-CEUS characteristics of OTFGs and to compare the diagnostic efficacy of these 2 methods and their combination to improve the diagnostic rate of OTFGs. We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2200/rc).
Methods
Study population
This retrospective study was conducted at the Department of Ultrasound, The First Affiliated Hospital of Shantou University Medical College from January 2021 to December 2023. A total of 126 patients with pelvic masses were recruited. Of them, 58 patients were excluded based on the following criteria: (I) presence of uterine tumors, urinary system tumors, or synchronous tumors; (II) pregnant women or age below 18 years; (III) absence of surgery or pathological diagnosis; (IV) no surgery performed within 120 days after US examination; and (V) vague US images or incomplete scanning range (Figure 1). According to the pathological diagnosis of the remaining 68 tumors, fibromas, thecomas, as well as the tumors which were difficult to pathologically differentiate regarding the type of fibroma or thecoma, were classified into OTFGs (17) and the other tumors were classified into non-OTFGs: there were 35 OTFGs and 33 non-OTFGs. All conventional US and CEUS examinations and measurements were performed by the same physician who had more than 10 years of experience in gynecological US. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of The First Affiliated Hospital of Shantou University Medical College Clinical Research Ethics Committee (No. B-2024-075) and informed consent was provided by all individual participants.
Instrument and methods
All US examinations were performed using a premium US scanner (Nuewa R9T, Mindray, Shenzhen, China). For all cases, the preoperative US examination included a transvaginal US session performed using a transvaginal US probe and a transabdominal US session performed using an abdominal probe. The frequency of the transvaginal probes varied between 3.2 and 12.8 MHz and that of the transabdominal probes varied between 1.0 and 6.0 MHz. Before the transvaginal US examination, the patient voided their bladder and assumed the bladder lithotomy position. During the transvaginal US examination, the location, size, morphology, boundary, internal echogenicity, acoustic shadows, and coloration of the OTFGs were imaged in the 2-dimensional (2D) mode. The scanner was then switched to the contrast enhancement mode, and the patient was asked to remain in the same position. Sulfur hexafluoride microbubble contrast agent SonoVue (Bracco, Milan, Italy) was used for CEUS examination. Before conducting CEUS examination, the contrast agent was mixed with 5 mL of 0.9% saline to form a microbubble suspension. A bolus of 2.0 mL of SonoVue suspension was injected through the elbow vein, followed by a bolus of 5.0 mL of saline. A timer was initiated simultaneously with the injection of SonoVue to continuously display and store tumor enhancement images in real time. CEUS was performed twice for each patient. First, a C-CEUS examination was performed. Next, HiFR-CEUS examination of the same view of the same tumor was performed 15 minutes after the first examination, after ensuring that the microbubbles from the previous injection had cleared. All CEUS examinations were performed using the same premium US scanner (Nuewa R9T, Mindray, China) and conducted after receiving informed consent from the patient.
Conventional US examination
The 2D US examinations were performed using a standardized imaging protocol, and all tumors were characterized using IOTA simple rules (SR). The benign features used to classify tumor masses in this protocol were as follows: B1, a unilocular cyst; B2, presence of solid components where the largest solid component has the largest diameter of <0.7 cm; B3, presence of acoustic shadows; B4, smooth multilocular tumor with the largest diameter of <10 cm; and B5, absence of Doppler signal. Malignant masses were also evaluated based on the following rules: M1, irregular solid tumor; M2, presence of ascites; M3, presence of at least four papillary structures; M4, irregular multilocular-solid tumor with the largest diameter of >10 cm; and M5, high Doppler signal. These rules stipulate the following: if a tumor has 1 or more benign features and does not show any malignant features, it is considered benign. In contrast, if a tumor has 1 or more malignant features and does not show any benign features, it is classified as malignant. If the tumor shows no features or has both malignant and benign features, it is considered indeterminate (18). For the indeterminate tumors, we calculated the risk of malignancy by using IOTA Assessment of Different NEoplasias in the adneXa (ADNEX) model analysis software (https://www.evidencio.com/models/show/946). The IOTA ADNEX model contains three clinical indications: age, serum carbohydrate antigen 125 (CA125) level (U/mL), type of center (oncology center vs. non-oncology center), and six US indications: maximum diameter of the lesion (mm), maximum diameter of the largest solid part of the lesion (mm), more than 10 cyst locules (yes or no), number of papillary projections (0, 1, 2, 3 or >3), presence of acoustic shadow (yes or no), and ascites (yes or no) (19,20). If the risk of malignancy ≤15%, it was considered as benign. Meanwhile, if the risk of malignancy was >15%, it was considered malignant (21,22). A color score was used to assess the amount of detectable color Doppler signals in each tumor as follows: color score 1, no color Doppler signals detected in the lesion; color score 2, a minimal amount of color Doppler signals detected in the lesion; color score 3, a moderate amount of color Doppler signals detected in the lesion; color score 4, a high amount of color Doppler signals detected in the tumors (12,16).
HiFR-CEUS examination
The following characteristics of OTFGs were observed in HiFR-CEUS: (I) the time of start of enhancement (compared to the adjacent normal myometrium): early enhancement and non-early enhancement; (II) enhancement intensity (compared to the adjacent normal myometrium): hypoenhancement and non-hypoenhancement; (III) vascularization type of OTFGs: linearity and non-linearity in the arterial phase; and (IV) rate of contrast washout (compared to the adjacent normal myometrium): quick fading and slow fading.
Statistical analysis
Statistical analysis was conducted using the software SPSS 27.0 (IBM Corp., Armonk, NY, USA) and MedCalc 22.026 (MedCalc Software, Ostend, Belgium). The three methods of diagnosing OTFG were analyzed using a 3×2 Chi-squared test. The paired Chi-squared test was used to compare any 2 of the abovementioned three methods. A P value <0.05 was considered a statistically significant difference. All reported P values were 2-sided.
Results
Overall characteristics
A total of 68 patients with ovarian tumors were identified. Based on histological diagnosis, 12 tumors were teratomas, 8 tumors were ovarian endometriomas, 7 tumors were cystadenomas, 3 tumors were cystadenocarcinomas, 2 tumors were subserosal fibroids, and 1 was fibrolipoma. All these tumors were classified as non-OTFGs. The remaining 35 patients had OTFGs. Among these 35 patients with OTFGs, fibroma and thecoma were diagnosed in 22 and 9 patients, respectively, whereas no specific histological type was identified in the remaining 4 patients.
Among the OTFGs, according to the IOTA SR, 23 tumors (23/35, 65.7%) were classified as benign tumors without any malignant features. A total of 12 tumors (12/35, 34.3%) were classified as indeterminate tumors based on the presence of ascites and other benign features; however, these tumors were classified as benign tumors according to the IOTA ADNEX model. All 35 OTFGs (35/35, 100%) occurred unilaterally, with 21 tumors (21/35, 60.0%) located at the left adnexa and the remaining 14 tumors (14/35, 40.0%) located at the right adnexa. In grayscale US imaging, the median of the largest diameter of fibromas was 111.5 mm (32–204 mm), the median of the largest diameter of thecomas was 80 mm (40–97 mm), and the median of the largest diameter of remaining OTFGs was 83 mm (66–190 mm).
Conventional US features
Among the non-OTFGs, 30 tumors (30/33, 90.9%) had well-defined borders, and the boundaries of the remaining tumors (3/33, 9.1%) were not clear. Most of the non-OTFGs had multilocular cyst (30/33, 90.9%) and the remaining 3 tumors (3/33, 9.1%) were classified as solid tumors. Among the 3 solid tumors, there were 2 masses (2/3, 66.7%) exhibiting hypoechogenicity and the remaining 1 (1/3, 33.3%) showed hyperechogenicity. Most of the non-OTFGs (30/33, 90.9%) showed as anechoic in the tumors. Further, 8 teratomas and 2 cystadenomas (10/33, 30.3%) had papillary substantial echoes in the anechoic region; 8 ovarian endometriomas (8/33, 24.2%) presented dense, small, and dotted hyperechogenicity in the anechoic area, with echo enhancement effect at the back of the tumors; 11 masses (11/33, 33.3%) showed echogenic bands in the anechoic region. In color Doppler images, only 3 tumors (3/33, 9.1%) showed rich vascularization in the cyst wall and echogenic bands, whereas most of the non-OTFGs (27/33, 81.8%) exhibited only dotted vascularization in the cyst wall. Among the 3 solid masses, 2 (2/3, 66.7%) showed considerable dotted vascularization and the third tumor (1/3, 33.3%) presented little dotted vascularization in the internal tumor.
Among the OTFGs, the border of 23 tumors was reported: most of these tumors (22/23, 95.7%) had well-defined borders, and only 1 patient showed an unclear tumor border. Most fibromas (21/22, 95.5%), thecomas (7/9, 77.8%), and the remaining OTFGs (4/4, 100%) were classified as unilocular-solid or solid tumors. However, 1 fibroma (1/22, 4.5%) and 2 thecomas (2/9, 22.2%) were multilocular-solid tumors. The results showed that 27 tumors (27/35, 77.1%) exhibited hypoechogenicity in solid parts, with granular or linear hyperechogenicity observed in grayscale US images. A total of 7 OTFGs showed anechoic cyst fluid. In color Doppler images, the vascularization of 27 tumors was reported: rich vascularization was not observed for these 27 OTFGs. Most fibromas (13/16, 81.3%), 3 thecomas (3/8, 37.5%), and 3 tumors diagnosed as OTFGs (3/3, 100%) had sparse vascularization (color score 2). Moreover, all these tumors showed little dotted or linear vascularization (color score 2). Further, 3 fibromas (3/16, 18.8%) and 5 thecomas (5/8, 62.5%) had no vascularization (color score 1, Table 1, Figure 2).
Table 1
| Characteristic | Fibroma (n=22) | Thecoma (n=9) | OTFG (n=4) |
|---|---|---|---|
| Pleural effusion | 3/22 (13.6) | 0/9 | 0/4 |
| Ascites | 10/22 (45.5) | 2/9 (22.2) | 0/4 |
| Free fluid in the pouch of Douglas | 13/22 (59.1) | 4/9 (44.4) | 4/4 (100.0) |
| Largest diameter of lesion (mm) | 111.5 [32–204] | 80 [40–97] | 83 [66–190] |
| Type of tumor | |||
| Unilocular cyst | 0/22 | 0/9 | 0/4 |
| Unilocular-solid cyst | 0/22 | 3/9 (33.3) | 1/4 (25.0) |
| Multilocular cyst | 0/22 | 0/9 | 0/4 |
| Multilocular-solid cyst | 1/22 (4.5) | 2/9 (22.2) | 0 |
| Solid tumor | 21/22 (95.5) | 4/9 (44.4) | 3/4 (75.0) |
| Number of locules in multilocular cysts or multilocular-solid masses | |||
| ≤10 | 1/1 (100.0) | 2/2 (100.0) | 0 |
| >10 | 0/1 | 0/2 | 0 |
| Echogenicity of cyst fluid in tumors not classified as solid | |||
| Anechoic | 1/1 (100.0) | 5/5 (100.0) | 1/1 (100.0) |
| Low level | 0/1 | 0/5 | 0/1 |
| Hemorrhagic | 0/1 | 0/5 | 0/1 |
| Largest diameter of solid component (mm) | 108 [32–139] | 80 [40–97] | 83 [66–190] |
| Presence of papillary projections | |||
| 0 | 22/22 (100.0) | 8/9 (88.9) | 4/4 (100.0) |
| 1 | 0/20 | 1/9 (11.1) | 0/4 |
| >3 | 0/20 | 0/9 | 0/4 |
| Color score | |||
| 1 | 3/16 (18.8) | 5/8 (62.5) | 0/3 |
| 2 | 13/16 (81.3) | 3/8 (37.5) | 3/3 (100.0) |
| 3 | 0/16 | 0/8 | 0/3 |
| 4 | 0/16 | 0/8 | 0/3 |
Results are presented as n (%) or median [range] as appropriate. OTFG, ovarian thecoma-fibroma group.
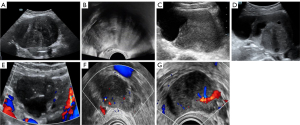
HiFR-CEUS pattern
The analysis of the performance of HiFR-CEUS for diagnosing OTFGs revealed early enhancement in all 35 OTFGs, which was identical to that in the surrounding normal ovarian tissue. Of these tumors, 33 (33/35, 94.3%) showed hypoenhancement, whereas 2 (2/35, 5.7%) showed hyper-enhancement at the peak intensity. The typical linear perfusion was observed during enhancement in all OTFGs (35/35, 100%). Compared to the adjacent normal ovarian tissue, 30 tumors (30/35, 85.7%) exhibited slow fading of contrast washout, and the remaining 5 tumors (5/35, 14.3%) exhibited quick fading of contrast washout. A total of 7 OTFGs (7/35, 20.0%) had cystic lesions with no enhancement in the cystic area (Table 2, Figures 3,4).
Table 2
| HiFR-CEUS feature | Fibroma (n=22) | Thecoma (n=9) | OTFG (n=4) |
|---|---|---|---|
| Contrast agent arrival time | |||
| Synchronous with adjacent ovarian tissue | 20 (90.9) | 7 (77.8) | 4 (100.0) |
| Earlier or later than adjacent ovarian tissue | 2 (9.1) | 2 (22.2) | 0 |
| Peak intensity | |||
| Hypo-enhancement | 21 (95.5) | 8 (88.9) | 4 (100.0) |
| Non-hypo-enhancement | 1 (4.5) | 1 (11.1) | 0 |
| Vascular type | |||
| Linearity | 22 (100.0) | 9 (100.0) | 4 (100.0) |
| Non-linearity | 0 | 0 | 0 |
| Contrast washout rate | |||
| Quick fading | 2 (9.1) | 2 (22.2) | 1 (25.0) |
| Slow fading | 20 (90.9) | 7 (77.8) | 3 (75.0) |
Results are presented as n (%) as appropriate. HiFR-CEUS, high-frame-rate contrast-enhanced ultrasound; OTFG, ovarian thecoma-fibroma group.
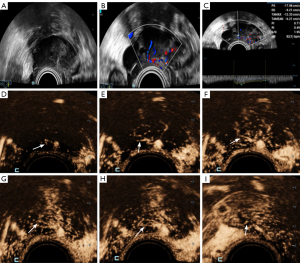
The performance of HiFR-CEUS for diagnosing non-OTFGs differed from that for diagnosing OTFGs. A total of 11 masses (11/33, 33.3%) showed contrast enhancement first in the cyst wall, followed by enhancement in the light band; no enhancement was observed in the cystic fluid. There were 10 tumors (10/33, 30.3%) that presented high enhancement of the papillary substantial echoes and no enhancement in the anechogenic area of the tumor. Early enhancement of the pedicle artery derived from the uterine artery was observed in 2 subserosal fibroids, followed by high enhancement of the tumor body (close to the enhancement level of the myometrium), and the peripheral ring showed high enhancement during the late phase. However, only 1 tumor (1/33, 3.0%) presented little linear vascularization during enhancement.
Comparison of diagnostic concordances
A comparison of the diagnostic concordances between conventional US, HiFR-CEUS, and the combination of these two methods for diagnosing OTFGs in 68 patients revealed significant differences (P<0.001, Table 3). The diagnostic concordance showed significant differences between conventional US and HiFR-CEUS (P<0.001, Table 4) and between conventional US and the combination of these 2 methods (P<0.001, Table 5). The diagnostic concordance of the combination of conventional US with HiFR-CEUS was the highest (50.0%), followed by those of HiFR-CEUS (48.5%) and conventional US (11.8%). HiFR-CEUS and the combination of conventional US and HiFR-CEUS showed higher diagnostic concordance for diagnosing OTFGs. HiFR-CEUS and the combination of conventional US and HiFR-CEUS did not show a significant difference in the diagnostic concordance (P>0.05), and the kappa value between HiFR-CEUS and the combination of the 2 methods was 0.971 (P<0.001, Table 6). No significant difference was observed between the combination of conventional US with HiFR-CEUS and histological diagnosis (P>0.05), and the kappa value between these 2 methods was 0.971 (P<0.001, Table 7). In other words, the combination of conventional US and HiFR-CEUS showed a high consistency with histological diagnosis of OTFGs.
Table 3
| Diagnostic method | Total | OTFG | OTFG concordance rate (%) | χ2 | P value |
|---|---|---|---|---|---|
| Conventional US | 68 | 8 | 11.8 | 27.453 | <0.001 |
| HiFR-CEUS | 68 | 33 | 48.5 | ||
| Conventional US combined with HiFR-CEUS | 68 | 34 | 50.0 |
HiFR-CEUS, high-frame-rate contrast-enhanced ultrasound; OTFG, ovarian thecoma-fibroma group; US, ultrasound.
Table 4
| Conventional US | HiFR-CEUS | Total | P value | |
|---|---|---|---|---|
| OTFG | Non-OTFG | |||
| OTFG | 8 | 0 | 8 | <0.001 |
| Non-OTFG | 25 | 35 | 60 | |
| Total | 33 | 35 | 68 | |
HiFR-CEUS, high-frame-rate contrast-enhanced ultrasound; OTFG, ovarian thecoma-fibroma group; US, ultrasound.
Table 5
| Conventional US | Conventional US combined with HiFR-CEUS | Total | P value | |
|---|---|---|---|---|
| OTFG | Non-OTFG | |||
| OTFG | 8 | 0 | 8 | <0.001 |
| Non-OTFG | 26 | 34 | 60 | |
| Total | 34 | 34 | 68 | |
HiFR-CEUS, high-frame-rate contrast-enhanced ultrasound; OTFG, ovarian thecoma-fibroma group; US, ultrasound.
Table 6
| HiFR-CEUS | Conventional US combined with HiFR-CEUS | Total | P value | Kappa | P valuea | |
|---|---|---|---|---|---|---|
| OTFG | Non-OTFG | |||||
| OTFG | 33 | 0 | 33 | >0.99 | 0.971 | <0.001 |
| Non-OTFG | 1 | 34 | 35 | |||
| Total | 34 | 34 | 68 | |||
a, for the comparison of the kappa value between HiFR-CEUS and the combination of conventional US with HiFR-CEUS. HiFR-CEUS, high-frame-rate contrast-enhanced ultrasound; OTFG, ovarian thecoma-fibroma group; US, ultrasound.
Table 7
| Conventional ultrasound combined with HiFR-CEUS | Histological diagnosis | Total | P value | Kappa | P valuea | |
|---|---|---|---|---|---|---|
| OTFG | Non-OTFG | |||||
| OTFG | 34 | 0 | 34 | >0.99 | 0.971 | <0.001 |
| Non-OTFG | 1 | 33 | 34 | |||
| Total | 35 | 33 | 68 | |||
a, for the comparison of the kappa value between conventional US combined with HiFR-CEUS and histological diagnosis. HiFR-CEUS, high-frame-rate contrast-enhanced ultrasound; OTFG, ovarian thecoma-fibroma group; US, ultrasound.
The sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of conventional US for diagnosing OTFGs were 23%, 100%, 60%, 100%, and 55%, respectively. These values were 94%, 97%, 96%, 97%, and 94%, respectively, for HiFR-CEUS and 97%, 100%, 99%, 100%, and 97%, respectively, for the combination. The area under the receiver operating characteristic curve (AUC) of the combination method [0.99, 95% confidence interval (CI): 0.92–1.00] was higher than those of HiFR-CEUS (0.96, 95% CI: 0.88–0.99) and conventional US (0.61, 95% CI: 0.49–0.73) (Table 8).
Table 8
| Diagnostic method | Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) | NPV (%) | AUC |
|---|---|---|---|---|---|---|
| Conventional US | 23 (8/35) [10, 40] |
100 (33/33) [89, 100] |
60 (41/68) [48, 72] |
100 (8/8) [63, 100] |
55 (33/60) [51, 59] |
0.61 [0.49, 0.73] |
| HiFR-CEUS | 94 (33/35) [81, 99] |
97 (32/33) [84, 100] |
96 (65/68) [88, 99] |
97 (33/34) [83,100] |
94 (32/34) [81, 98] |
0.96 [0.88, 0.99] |
| Conventional US combined with HiFR-CEUS | 97 (34/35) [85, 100] |
100 (33/33) [89, 100] |
99 (67/68) [92, 100] |
100 (34/34) [90, 100] |
97 (33/34) [83, 100] |
0.99 [0.92, 1.00] |
Data in square brackets are 95% CIs, with numbers of nodules in parentheses. AUC, area under the receiver operating characteristic curve; CI, confidence interval; HiFR-CEUS, high-frame-rate contrast-enhanced ultrasound; NPV, negative predictive value; OTFG, ovarian thecoma-fibroma group; PPV, positive predictive value; US, ultrasound.
Discussion
Our study indicates that the diagnostic accuracy of conventional US is not high (60%), which ties well with a previous study (12), and that of HiFR-CEUS is 96%. The combination of conventional US and HiFR-CEUS has better diagnostic efficacy (sensitivity: 97%, specificity: 100%, accuracy: 99%, PPV: 100%, NPV: 97%, AUC: 0.99, P<0.001) than either conventional US or HiFR-CEUS alone; this combination shows great potential to diagnose OTFGs, leading to improved accuracy, efficiency, and early detection, which can ultimately provide better patient outcomes.
In the present study, all OTFG tumors (35/35, 100%) were benign, which agreed with the reported literature (23). OTFGs commonly occur as endocrine tumors, some of them can secrete estrogen, resulting in irregular vaginal bleeding. In addition, these tumors grow faster than other tumors, which could lead to adnexal torsion, similar to previous reports (17,24,25). Some studies have reported that, in patients diagnosed with OTFGs associated with ascites or Meigs syndrome, the abdominal fluid shows regression after tumor removal (10,16). Therefore, to reduce patient symptoms and improve patient outcome, the accurate and early diagnosis of OTFGs through the combination of conventional US and HiFR-CEUS is critical.
In grayscale US imaging, most of OTFGs were found to have well-defined borders, smooth contours, solid or unilocular-solid mass, and irregular internal echogenicity. Therefore, these tumors mainly presented as heterogeneously hypoechoic lesions, with posterior acoustic enhancement or attenuation, as reported earlier (26). Most thecomas or OTFGs with high proportion of thecal cells showed posterior acoustic enhancement. Several fibromas or OTFGs with a high proportion of fibroblasts mainly showed posterior acoustic attenuation, as reported earlier (9,27). Doppler US revealed hypovascularity of all OTFGs, with sparse vascularization in the tumor or in the area surrounding the tumor. In tumors with complications of hemorrhage or cystic rupture, the regions were mainly found to be anechoic. In the present study, 7 OTFG tumors showed cystic degeneration. Previous studies have shown a positive correlation between tumor size and the occurrence of cystic degeneration, and the central necrosis region might be present due to insufficient blood supply (28,29).
The characteristics of OTFGs in HiFR-CEUS examination were as follows. After the contrast microbubbles were injected, the tumors began to show enhancement in the early phase of HiFR-CEUS examination (in synchronization with the ovarian tissue) and exhibited low enhancement or slightly lower enhancement at the peak intensity. In the late phase of enhancement, the contrast agent slowly washed out. With the increase in tumor size, hemorrhage, necrosis, or cystic degeneration becomes more common, resulting in no enhancement in these tumor areas. This finding is consistent with a previous report (11). The most characteristic finding during enhancement was linear vascularization of the OTFGs. This characteristic could be explained by the insufficient vascularization of OTFGs and the high content of densely arranged fibrous tissues, which results in slow perfusion of the US contrast agent.
A total of 7 patients with OTFGs were misdiagnosed with uterine fibroids or broad ligament fibroids based on conventional US images. This might have been due to the similar internal echogenicity of OTFGs and fibroids (12,13,30). In addition, 2 patients were misdiagnosed with endometriomas. The diagnostic sensitivity of grayscale US imaging for diagnosing OTFGs was low (only 23%). However, the diagnostic sensitivity increased to 97% for the combination of conventional US with HiFR-CEUS. Therefore, the clinical implementation of this combination is important to enable the accurate diagnosis of OTFGs. Most of the OTFGs were large in size, and the largest diameter of the lesion was 88 mm (32–204 mm), which could likely cause adnexal torsion (13,25). Therefore, to prevent the tumor from becoming too large and inducing adnexal torsion, early diagnosis and timely treatment are critical for patients with OTFGs. Therefore, the combination of 2D US with HiFR-CEUS is an important approach to diagnose OTFGs.
In the present study, we found that OTFGs mostly occurred in perimenopausal and postmenopausal women. This finding agreed with previous studies (13,28,29,31). Some 60.0% (21/35) of the lesions occurred after menopause, and 74.3% (26/35) of the lesions were detected in women aged 45–76 years. Only 2 patients had Meigs syndrome and elevated CA125 levels. In a previous study, OTFGs with atypical conventional US images combined with Meigs syndrome and CA125 elevated levels were easily misdiagnosed with ovarian malignancy (11). Hence, it is essential to combine conventional US with HiFR-CEUS examination to differentiate OTFGs from ovarian malignancy, as this combination approach showed high diagnostic efficiency (AUC =0.99).
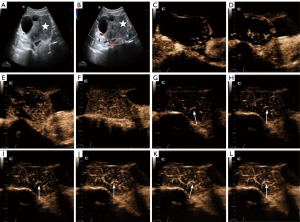
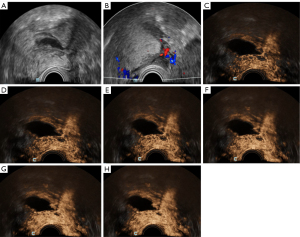
In our study, 1 patient underwent this combination examination 3 times after a tumor was detected in her left adnexa during a physical examination. At the first examination on 14 December 2022, we diagnosed the OTFG tumor with the largest diameter of only 30 mm. After 3.5 months, there was no considerable change in tumor size at the second examination. At the third examination on 7 November 2023, the lesion diameter increased to 40 mm. Based on this case, we assumed that if this patient would not have undergone the combination method, she would have probably been misdiagnosed using conventional US imaging. With the increase in tumor size, hemorrhage or even adnexal torsion might have occurred in the patient (Figure 5).
As discussed earlier, OTFGs are common endocrine tumors, and some of them can secrete estrogen, resulting in irregular vaginal bleeding. Some OTFG patients can also have co-existing endometrium- or myometrium-associated diseases. Endometrial lesions such as endometrial hyperplasia, polyps, and cancer and myometrial lesions such as uterine fibroids have been detected in OTFG patients (6,11,32). In the present study, endometrial hyperplasia was detected in 2 patients, endometrial polyps in 1 patient, and uterine fibroids in 11 patients. Therefore, patients with OTFGs should be carefully examined to confirm whether their tumors are associated with endometrial or myometrial lesions. Additionally, patients with surgical excision of the OTFG tumor should undergo regular monitoring of the estrogen levels during their follow-up period.
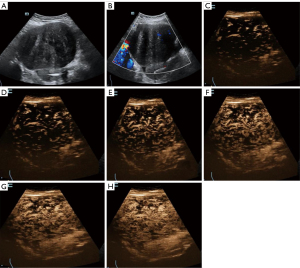
In the present study, another patient could have been misdiagnosed with an OTFG tumor if they had only undergone HiFR-CEUS examination, yet histological diagnosis confirmed fibrolipoma. Conventional US showed a unilocular-solid tumor located at the left adnexa, with smooth contours, well-defined borders, and an internal heterogeneous echo pattern. Most of the solid mass presented hyperechogenicity and a small part presented hypoechogenicity. In color Doppler US, sparse vascularization was noted in the hypoechoic solid lesion. These findings indicated non-OTFG. However, linear perfusion was unexpectedly observed in the HiFR-CEUS examination, which indicated OTFG. Therefore, to improve the accuracy of OTFG diagnosis, the combination of conventional US and HiFR-CEUS is crucial during routine patient examination (Figure 6).
The prompt and appropriate diagnosis is critical in patients with OTFGs. This is because early diagnosis and timely intervention could avoid more severe symptoms while as the tumor continues to grow. Therefore, the combination of conventional US and HiFR-CEUS has significant clinical value. Currently, there is not much research on the features of OTFGs in HiFR-CEUS. The present study analyzed the features of OTFGs in both conventional US and HiFR-CEUS to improve diagnostic accuracy. Our results suggest that the combination of conventional US and HiFR-CEUS could be used as the first step to detect and locate OTFGs. It is important to note that the most typical HiFR-CEUS feature of OTFGs is the linear vascularization, which can be used to diagnose OTFGs more accurately and provide better guidance for clinical treatment. Moreover, the findings of the combination method showed high consistency with the results of the histological diagnosis of OTFGs. To the best of our knowledge, there is a great deal of recent evidence that artificial intelligence technology can greatly improve accuracy and efficacy of diagnosing gynecological tumors (33-36). We firmly believe that the combination of conventional US and HiFR-CEUS could also have a significant impact on gynecologic oncology.
The strength of this study is that 68 patients included study all underwent surgery within 120 days and all of them had a pathological diagnosis which provided us a reliable reference. Nevertheless, there are still several potential limitations of the research. First, this study included a small sample size, which might have led to bias in the study results, and a larger sample size of the specific subgroup may be required to validate our findings. Second, this was a retrospective study; some of the conventional US images were static, which made it difficult to perform a comprehensive assessment of the entire lesion; further prospective studies can be carried out in the future. Third, the majority of masses were large and examiners could select only the most abnormal section to perform CEUS; this hindered the simultaneous examination of the entire mass, inevitably resulting in omissions as well as deviations. Besides, this study did not involve the combination of other imaging examinations; the combined application of different imaging examinations in the diagnosis of OTFGs should be explored future research.
Conclusions
Most of the OTFGs showed characteristic linear perfusion in HiFR-CEUS. The combination of conventional US and HiFR-CEUS greatly improved the rate of diagnosing OTFGs. In summary, the combination of conventional US and HiFR-CEUS has significant value in the accurate diagnosis of OTFGs.
Acknowledgments
The authors would like to express their gratitude to all the clinicians at the Gynecology, Pathology Department of The First Affiliated Hospital of Shantou University Medical College who provided professional advice and guidance. Additionally, the authors thank the patients and their families for their participation in this study.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-2200/rc
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2200/coif). Y.W. is an employee of Shenzhen Mindray Bio-medical Electronics Co., Ltd. This employment had no influence on the study design, data analysis, result, or decision to publish. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of The First Affiliated Hospital of Shantou University Medical College Clinical Research Ethics Committee (No. B-2024-075) and informed consent was provided by all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang S, Li Y, Wang M, Liu H, Meng F, Hua G, Hu Q, Zhao X. Pelvic effusion in patients with ovarian thecoma-fibroma associated with the tumor size and plaste CA-125 level: A retrospective magnetic resonance imaging study. Clin Imaging 2022;81:62-6. [Crossref] [PubMed]
- Shang W, Wu L, Xu R, Chen X, Yao S, Huang P, Wang F. Clinical laboratory features of Meigs' syndrome: a retrospective study from 2009 to 2018. Front Med 2021;15:116-24. [Crossref] [PubMed]
- Fang SG, Wei JG, Chen ZW. WHO (2020) Classification of female reproductive system tumors. J Diag Pathol 2021;28:142-8. [Crossref]
- McCluggage WG, Singh N, Gilks CB. Key changes to the World Health Organization (WHO) classification of female genital tumours introduced in the 5th edition (2020). Histopathology 2022;80:762-78.
- Javadi S, Ganeshan DM, Jensen CT, Iyer RB, Bhosale PR. Comprehensive review of imaging features of sex cord-stromal tumors of the ovary. Abdom Radiol (NY) 2021;46:1519-29. [Crossref] [PubMed]
- Zhou XX, Zhong XF, Chang WL, Mao XJ. The value of MRI in diagnosing and differentiation of ovarian theca fibroma and brenner tumor. Radiol Practice 2023;38:593-7.
- Chen J, Wang J, Chen X, Wang Y, Wang Z, Li D. Computed tomography and magnetic resonance imaging features of ovarian fibrothecoma. Oncol Lett 2017;14:1172-8. [Crossref] [PubMed]
- Foti PV, Attinà G, Spadola S, Caltabiano R, Farina R, Palmucci S, Zarbo G, Zarbo R, D'Arrigo M, Milone P, Ettorre GC. MR imaging of ovarian masses: classification and differential diagnosis. Insights Imaging 2016;7:21-41. [Crossref] [PubMed]
- Lu BL, Huang BJ, Qi JL, Fan YL, Wang WP. Clinical and sonographic features of ovarian sex cord stromal tumors. Fudan Univ J Med Sci 2020;47:882-7.
- Cao YY, Niu JM, Wang H, Chen J, Shi LY, Yang T, Zhou LP. Ultrasonic manifestations and clinical features of ovarian follicular cell tumor. J Clin Ultrasound in Med 2016;18:642-3.
- Wang YT, Cao XS, Chen XF, Fang YH, Chen YB, Chen Y. Ovarian Fibrothecomas: MRI findings and clinicalpathology. J Med Imaging 2020;30:1457-60.
- Jiang MJ, Le Q, Yang BW, Yuan F, Chen H. Ovarian sex cord stromal tumours: analysis of the clinical and sonographic characteristics of different histopathologic subtypes. J Ovarian Res 2021;14:53. [Crossref] [PubMed]
- Chen H, Liu Y, Shen LF, Jiang MJ, Yang ZF, Fang GP. Ovarian thecoma-fibroma groups: clinical and sonographic features with pathological comparison. J Ovarian Res 2016;9:81. [Crossref] [PubMed]
- Ma JX, Ma YH, Gao SS, Wu J, Zhu M, Duan ZL. Value of Intravenous Contrast-enhanced Ultrasound in Differential Diagnosis of Adenomyosis and Uterine Fibroids. Journal of Kunming Medical University 2020;41:114-8.
- Fei X, Li N, Zhu L, Han P, Jiang B, Tang W, Sang M, Zhang X, Luo Y. Value of high frame rate contrast-enhanced ultrasound in distinguishing gallbladder adenoma from cholesterol polyp lesion. Eur Radiol 2021;31:6717-25. [Crossref] [PubMed]
- Paladini D, Testa A, Van Holsbeke C, Mancari R, Timmerman D, Valentin L. Imaging in gynecological disease (5): clinical and ultrasound characteristics in fibroma and fibrothecoma of the ovary. Ultrasound Obstet Gynecol 2009;34:188-95. [Crossref] [PubMed]
- Zhang Z, Wu Y, Gao J. CT diagnosis in the thecoma-fibroma group of the ovarian stromal tumors. Cell Biochem Biophys 2015;71:937-43. [Crossref] [PubMed]
- Carballo EV, Maturen KE, Li Z, Patel-Lippmann KK, Wasnik AP, Sadowski EA, Barroilhet LM. Surgical outcomes of adnexal masses classified by IOTA ultrasound simple rules. Sci Rep 2022;12:21848. [Crossref] [PubMed]
- Hiett AK, Sonek JD, Guy M, Reid TJ. Performance of IOTA Simple Rules, Simple Rules risk assessment, ADNEX model and O-RADS in differentiating between benign and malignant adnexal lesions in North American women. Ultrasound Obstet Gynecol 2022;59:668-76. [Crossref] [PubMed]
- Kadooka M, Suemitsu T, Ashimoto K, Takesawa A, Matsui H, Otsuka I, Tajima A. Validation of the IOTA ADNEX Model Among Japanese Women Performed by Gynecology Trainees and Ultrasound Specialists: A Retrospective Diagnostic Accuracy Study. J Ultrasound Med 2024;43:1857-68. [Crossref] [PubMed]
- Araujo KG, Jales RM, Pereira PN, Yoshida A, de Angelo Andrade L, Sarian LO, Derchain S. Performance of the IOTA ADNEX model in preoperative discrimination of adnexal masses in a gynecological oncology center. Ultrasound Obstet Gynecol 2017;49:778-83. [Crossref] [PubMed]
- Zhao W, Li Q. Clinical application of simplified flowchart-based O-RADS combined with ADNEX model in evaluating the benign and malignant nature of adnexal tumors in middle-aged and elderly women. China Journal of Modern Medicine 2024;34:14-9.
- Nagawa K, Kishigami T, Yokoyama F, Murakami S, Yasugi T, Takaki Y, Inoue K, Tsuchihashi S, Seki S, Okada Y, Baba Y, Hasegawa K, Yasuda M, Kozawa E. Diagnostic utility of a conventional MRI-based analysis and texture analysis for discriminating between ovarian thecoma-fibroma groups and ovarian granulosa cell tumors. J Ovarian Res 2022;15:65. [Crossref] [PubMed]
- Zhang Y, Liu L. Clinical characteristics and MRI diagnosis of fibrothecoma and ovarian fibroma. J Pract Radiol 2024;40:764-7.
- Zhang R, Chen JW, Liu XF, Li JL, Yan C. Comparative analysis of ultrasonographic features and pathology of the thecoma‐fibroma group of ovarian tumors. Shanxi Med J 2016;45:760-2.
- Zhu LH, Y XM, Li N, Su J. Comparison of ultrasound signs of ovarian follicular membrane - fibroma and ovarian granulosa cell tumor. Journal of Clinical and Experimental Medicine 2020:2453-6.
- Ruan HS. Value of ultrasound in the diagnosis of ovarian follicle membrane-fibroma. Medical Equipment 2020;33:12-3.
- Qin LL, Hong Y, Fu XY, Zhang M, Wu TN, Hu J. Clinical and Ultrasonic Characteristics of Ovarian Thecoma‐fibroma Groups with Meigs Syndrome. Chinese J Ultrasound Med 2021;37:194-7.
- Zhu H, Lin P, Li Z, Deng Z. Magnetic Resonance Imaging Features of Ovarian Thecoma. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2020;42:651-7. [PubMed]
- Bian M, Li Q, Wang ZY, Liu JQ. Clinical and ultrasonic characteristics compared with pathology in the patients of ovarian thecoma--fibroma groups J Clin Pathol Res 2018;38:1698-701.
- Zhang H, Zhang H, Gu S, Zhang Y, Liu X, Zhang G. MR findings of primary ovarian granulosa cell tumor with focus on the differentiation with other ovarian sex cord-stromal tumors. J Ovarian Res 2018;11:46. [Crossref] [PubMed]
- Wang H. The value of MRI in distinguishing ovarian thecoma-fibroma tumors from subserous uterine leiomyomas and malignant solid ovarian tumors. Tianjin Medical University; 2019.
- Jiang Y, Wang C, Zhou S. Artificial intelligence-based risk stratification, accurate diagnosis and treatment prediction in gynecologic oncology. Semin Cancer Biol 2023;96:82-99. [Crossref] [PubMed]
- Garg P, Mohanty A, Ramisetty S, Kulkarni P, Horne D, Pisick E, Salgia R, Singhal SS. Artificial intelligence and allied subsets in early detection and preclusion of gynecological cancers. Biochim Biophys Acta Rev Cancer 2023;1878:189026. [Crossref] [PubMed]
- Gao Y, Zeng S, Xu X, Li H, Yao S, Song K, et al. Deep learning-enabled pelvic ultrasound images for accurate diagnosis of ovarian cancer in China: a retrospective, multicentre, diagnostic study. Lancet Digit Health 2022;4:e179-87. [Crossref] [PubMed]
- Akazawa M, Hashimoto K. Artificial intelligence in gynecologic cancers: Current status and future challenges - A systematic review. Artif Intell Med 2021;120:102164. [Crossref] [PubMed]



