
A preliminary study of synthetic magnetic resonance imaging on the changes of subcortical gray matter nuclei in obstructive sleep apnea patients
Introduction
Obstructive sleep apnea (OSA) is a sleep disorder characterized by intermittent upper respiratory collapse, leading to insufficient ventilation, hypoxia, and hypercapnia (1). OSA is estimated to affect one in seven adults globally, with China reporting the highest prevalence (2). Obesity is an important independent risk factor for OSA, and the prevalence of OSA increases in parallel with the elevation of body mass index (BMI) (3). The prevalence rate is expected to continue rising in the next few years due to increasing obesity and an aging population (4). OSA is linked to neurocognitive impairment, which may progress to dementia, which diminishes patients’ quality of life and increases the socioeconomic burden (5-7). Previous studies have linked intermittent hypoxia in OSA to brain microstructure damage (8,9), with subcortical gray matter nuclei exhibiting heightened sensitivity to hypoxia and increased susceptibility to OSA-related lesions. Subcortical gray matter nuclei are crucial for the brain’s functional connectivity, forming intricate cortical networks associated with emotions and cognition. Consequently, hypoxia-induced gray matter nuclei damage may underlie cognitive impairments observed in OSA patients. Characterizing abnormal changes in subcortical gray matter nuclei is essential for elucidating the neuropathological mechanisms underlying cognitive impairment in OSA patients. This study aimed to explore the differences in volume and relaxation values of subcortical gray matter nuclei between patients with moderate to severe OSA and healthy controls (HC) using the novel synthetic magnetic resonance imaging (SyMRI) technology. The findings will provide an objective imaging basis for quantitatively evaluating structural changes in these brain regions. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1830/rc).
Methods
Participants
Between January 2022 and December 2023, this study enrolled 30 newly diagnosed, untreated patients with moderate to severe OSA (27 males and 3 females) in the Respiratory Department of the General Hospital of TISCO. A control group of 30 (26 males and four females) age-, gender-, education-, and handedness-matched healthy individuals with no history of snoring and sleep disorders was also recruited.
The inclusion criteria for the OSA group included: (I) OSA was diagnosed by polysomnography (PSG), and the apnea-hyponea index (AHI) was ≥15 times/hour; (II) individuals aged 25–65 years; (III) right-handedness; (IV) body weight <125 kg.
The exclusion criteria for the OSA and control group included: (I) sleep disorders other than OSA; (II) neuropsychiatric disorders or substance abuse; (III) Cardiovascular metabolic diseases; (IV) brain organic diseases (cerebrovascular disease, brain injury, brain tumor); (V) contraindications for MRI examination.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of the General Hospital of TISCO (No. K202210) and informed consent was provided by all individual participants.
PSG monitor
Patients initially diagnosed with OSA underwent PSG monitoring for over 7 hours in the sleep monitoring room. Participants were not allowed alcohol, tea, coffee, sedatives, and hypnotics on the monitoring day. Sleep data were analyzed by qualified technicians.
Cognitive function evaluation
Cognitive function assessments were conducted within 1 week of completing sleep monitoring. A standardized Montreal Cognitive Assessment (MoCA) (10) was administered to all participants by a trained clinician. The MoCA total score is 30 points, with scores ≥26 indicating normal cognitive function and scores <26 suggesting cognitive dysfunction. An additional point was added to the scores if the participant’s education level was ≤12 years.
MRI data acquisition
MRI scans were performed on the same day as cognitive function assessments using a GE SIGNA Pioneer 3.0T MR scanner (GE HealthCare, Chicago, IL, USA) equipped with a 21-channel brain coil. During MRI scanning of all participants, we initially conducted conventional MRI sequences to exclude brain parenchymal lesions. These sequences included axial T2-weighted, T1-weighted, T2-fluid-attenuated inversion recovery (T2-FLAIR), and sagittal T1-weighted images. Subsequently, three-dimensional T1-weighted brain volume (3D T1-BRAVO) imaging was performed with the following parameters: repetition time (TR) =2,384 ms, echo time (TE) =2.5 ms, inversion time (TI) =1,000 ms, slice thickness =1.1 mm, number of excitations (NEX) =1, field of view (FOV) =240 mm × 240 mm, matrix =224×224, and scanning duration of 4 min 55 s. Additionally, the SyMRI sequence was obtained using the following parameters: TR =4,758 ms, TE =22.8 ms, TI =12 ms, thickness =4 mm, NEX =1, FOV =240 mm × 240 mm, matrix =320×256, and a scanning duration of 6 min 02 s.
SyMRI image processing
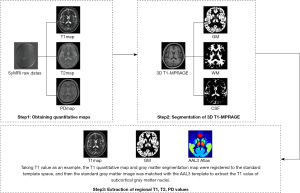
Following MRI image acquisition, the data were post-processed and analyzed. The original SyMRI image was transferred to the SyMRI8.0 post-processing workstation (https://www.syntheticmr.com/) and processed to obtain the T1, T2, and proton density (PD) quantitative atlas of the entire brain. Using the SPM12 software (http://www.fil.ion.ucl.ac.uk/spm/) based on MATLAB (R2015a, MathWorks, Natick, MA, USA), the quantitative atlas and T1-BRAVO image underwent rigid registration and segmentation. This process mapped the data to the Montreal Neurological Institute (MNI) standard template space (https://www.bic.mni.mcgill.ca/ServicesAtlases/ICBM152NLin2009) to obtain tissue probability maps. Additionally, the quantitative images (T1, T2, and PD) derived from the SyMRI sequence were also mapped to the MNI standard template space for normalization. Global quantitative parameters were calculated by averaging all voxels within corresponding tissue types (gray matter, white matter, and cerebrospinal fluid), exceeding a 90% partial volume threshold. Finally, the gray matter image in standard space and the quantitative atlas were co-registered to the automated anatomical labeling atlas 3 (AAL3) template (http://www.gin.cnrs.fr/en/tools/aal/). Overlapping areas were used as gray matter partitions to extract the volume values, along with T1, T2, and PD values of subcortical gray matter nuclei. This process is illustrated in Figure 1.
Statistical analysis
Data analysis was performed using the software SPSS 26.0 (IBM Corp., Armonk, NY, USA). Categorical variables, such as gender, were compared between groups using a chi-square test and expressed as percentages. Continuous variables were tested for normality and homogeneity of variance. Groups with normal distribution and homogeneity of variance were evaluated using the two independent samples t-test and expressed as . For data not conforming to a normal distribution or homogeneity of variance, the Mann-Whitney U rank sum test with two independent samples was used for group comparisons, expressed as median (25th percentile, 75th percentile [M (P25, P75)]. Multiple comparisons were corrected using the false discovery rate (FDR) analysis. Receiver operating characteristic (ROC) curve analysis was conducted to assess the diagnostic efficacy of SyMRI quantitative parameters that exhibited statistically significant differences after FDR correction for OSA. The area under the curve (AUC) was calculated for each parameter. After adjusting for age, BMI, and education, a partial correlation analysis method was performed to examine the correlation between abnormal brain region volume and relaxation values and MoCA scores in the OSA group. Multiple comparisons and corrections were performed using FDR. A P value <0.05 was considered statistically significant.
Results
Clinical data analysis results
There were no significant differences in sex, age, and education level between both groups (P=0.69, 0.72, 0.23); however, the OSA group exhibited a significantly higher BMI than the control group (P<0.001), as presented in Table 1.
Table 1
| Characteristics | OSA (n=30) | HC (n=30) | Statistical value | P value |
|---|---|---|---|---|
| Age (years) | 48.27±10.54 | 47.33±9.55 | 0.359† | 0.72 |
| Sex | 0.162‡ | 0.69 | ||
| Male | 27 | 26 | ||
| Female | 3 | 4 | ||
| BMI (kg/m2) | 28.39 (26.40, 29.95) | 25.20 (22.76, 26.44) | −3.874§ | <0.001* |
| Education (year) | 13.00 (11.00, 15.00) | 14.00 (11.00, 15.00) | 1.202§ | 0.23 |
†, Student’s t-test, data are presented as mean ± standard deviation, the statistical value is the t-value; ‡, Chi-squared test, data are presented as number, the statistical value is χ2 value; §, Mann-Whitney U test, data are presented as median (interquartile range), the statistical value is the z value; *, P<0.05, and the difference is statistically significant. BMI, body mass index; HC, healthy control; OSA, obstructive sleep apnea.
Comparison of MoCA scale scores between the two groups
The OSA group exhibited significantly lower scores on the total MoCA (P<0.001), visual space and executive function (P<0.001), attention (P=0.008), language (P<0.001), and delayed recall scores (P=0.006) than the control group. Detailed results are presented in Table 2.
Table 2
| Characteristics | OSA | HC | z | P value |
|---|---|---|---|---|
| Total MoCA scores | 24.00 (23.00, 25.00) | 27.00 (26.00, 28.00) | 6.490 | <0.001* |
| Visual space and executive function | 3.00 (2.00, 3.00) | 4.00 (4.00, 5.00) | 5.598 | <0.001* |
| Attention | 5.00 (3.00, 5.00) | 5.00 (5.00, 6.00) | 2.661 | 0.008* |
| Language | 2.00 (1.00, 2.00) | 3.00 (2.00, 3.00) | 4.276 | <0.001* |
| Delayed recall | 3.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | 2.766 | 0.006* |
Data are presented as median (interquartile range). *, P<0.05, and the difference is statistically significant. HC, healthy control; MoCA, Montreal Cognitive Assessment; OSA, obstructive sleep apnea.
Volume analysis results of subcortical gray matter nuclei
OSA patients exhibited a significantly reduced bilateral thalamic volume compared to HC (P=0.002, 0.003, uncorrected). This difference remained significant after applying FDR correction for multiple comparisons (Table 3).
Table 3
| Brain region | OSA (mL) | HC (mL) | t | P value | Corrected P value |
|---|---|---|---|---|---|
| Thalamus_L | 4.20±0.08 | 4.51±0.05 | −3.197 | 0.002* | 0.03* |
| Thalamus_R | 4.57±0.09 | 4.92±0.06 | −3.146 | 0.003* | 0.02* |
Data are presented as mean ± standard deviation. *, P<0.05, and the difference is statistically significant. HC, healthy control; L, left; OSA, obstructive sleep apnea; R, right.
Difference analysis results of relaxation values of subcortical gray matter nuclei
Compared to the control group, patients with moderate to severe OSA exhibited significantly increased T1 values in the left hippocampus, amygdala, caudate nucleus, right pallidum, thalamus, and bilateral putamen (P=0.02, 0.040, 0.01, <0.001, 0.03, 0.04, 0.004, uncorrected), T2 values in the left hippocampus (P =0.03, uncorrected), and PD values in the left amygdala, nucleus accumbens, right pallidum, putamen, bilateral caudate nucleus, and thalamus (P=0.02, 0.041, 0.01, 0.006, 0.007, 0.03, 0.047, 0.009, uncorrected). After FDR correction, no significant differences were observed in the T1 values of the left hippocampus, amygdala, putamen, and right thalamus, as well as in the T2 values of the left hippocampus and the PD values of the left nucleus accumbens (Tables 4-6). Figure 2 displays the image analysis results of the OSA group and the HC group, presenting the T1, T2, and PD quantitative maps of a 42-year-old male patient with moderate OSA and a 40-year-old healthy male control.
Table 4
| Brain Region | OSA (ms) | HC (ms) | Statistical value | P value | Corrected P value |
|---|---|---|---|---|---|
| Hippocampus_L | 1,285±66 | 1,245±66 | 2.351† | 0.02* | 0.08 |
| Amygdala_L | 1,198 (1,171, 1,244) | 1,178 (1,149, 1,209) | −2.055‡ | 0.040* | 0.07 |
| Caudate nucleus_L | 1,202 (1,171, 1,253) | 1,150 (1,092, 1,209) | −2.469‡ | 0.01* | 0.049* |
| Pallidum_R | 1,070 (1,032, 1,118) | 1,028 (1,010, 1,054) | −3.593‡ | <0.001* | <0.001* |
| Thalamus_R | 900±31 | 883±30 | 2.170† | 0.03* | 0.08 |
| Putamen_L | 1,014 (998, 1,045) | 998 (983, 1,014) | −2.085‡ | 0.04* | 0.09 |
| Putamen_R | 1,033±39 | 1,007±28 | 2.989† | 0.004* | 0.03* |
†, Student’s t-test, data are presented as mean ± standard deviation, the statistical value is the t-value; ‡, Mann-Whitney U test, data are presented as median (interquartile range), the statistical value is the z value; *, P<0.05, and the difference is statistically significant. HC, healthy control; L, left; OSA, obstructive sleep apnea; R, right.
Table 5
| Brain region | OSA (ms) | HC (ms) | z | P value | Corrected P value |
|---|---|---|---|---|---|
| Hippocampus_L | 91.6 (87.2, 93.8) | 88.3 (85.8, 91.2) | −2.159 | 0.03* | 0.31 |
Data are presented as median (interquartile range). *, P<0.05, and the difference is statistically significant. HC, healthy control; L, left; OSA, obstructive sleep apnea.
Table 6
| Brain region | OSA (pu) | HC (pu) | Statistical value | P value | Corrected P value |
|---|---|---|---|---|---|
| Amygdala_L | 84.2 (83.5, 84.7) | 83.4 (82.6,84.1) | −2.321‡ | 0.02* | 0.040* |
| Nucleus accumbens_L | 82.1±1.1 | 81.4±1.4 | 2.094† | 0.041* | 0.11 |
| Pallidum_R | 80.1 (79.2, 81.5) | 79.2 (78.2, 79.7) | −2.528‡ | 0.01* | 0.03* |
| Putamen_R | 79.0±1.1 | 78.3±1.0 | 2.836† | 0.006* | 0.048* |
| Caudate nucleus_L | 86.0 (84.25, 87.34) | 84.8 (83.7, 85.7) | −2.676‡ | 0.007* | 0.042* |
| Caudate nucleus_R | 82.1 (81.2, 83.0) | 81.5 (80.5, 82.2) | −2.188‡ | 0.03* | 0.044* |
| Thalamus_L | 78.3±1.4 | 77.6±1.2 | 2.025† | 0.047* | 0.03* |
| Thalamus_R | 74.8±1.1 | 74.0±1.3 | 2.695† | 0.009* | 0.04* |
†, Student’s t-test, data are presented as mean ± standard deviation, the statistical value is the t-value; ‡, Mann-Whitney U test, data are presented as median (interquartile range), the statistical value is the z value; *, P<0.05, and the difference is statistically significant. HC, healthy control; L, left; OSA, obstructive sleep apnea; PD, proton density; R, right.

ROC curve analysis
As shown in Table 7 and Figure 3, ROC curve analysis demonstrated the diagnostic utility of bilateral thalamic volume, left caudate nucleus, right putamen, and pallidum T1 values, and left amygdala, caudate nucleus, right putamen, pallidum, and thalamus PD values in OSA (P=0.002, 0.001, 0.01, 0.007, <0.001, 0.02, 0.007, 0.01, 0.01, 0.03; AUC 0.668–0.770).
Table 7
| Brain region | AUC | Threshold value | Sensitivity | Specificity | Youden’s index | P value |
|---|---|---|---|---|---|---|
| Left thalamic volume | 0.729 | 4.35 | 0.733 | 0.667 | 0.400 | 0.002 |
| Right thalamic volume | 0.739 | 4.81 | 0.767 | 0.667 | 0.434 | 0.001 |
| Left caudate nucleus T1 value | 0.686 | 1,144 | 0.867 | 0.500 | 0.367 | 0.01 |
| Right putamen T1 value | 0.702 | 1,018 | 0.667 | 0.733 | 0.400 | 0.007 |
| Right pallidum T1 value | 0.770 | 1,061 | 0.600 | 0.867 | 0.467 | <0.001 |
| Left amygdala PD value | 0.674 | 84.1 | 0.567 | 0.800 | 0.367 | 0.02 |
| Left caudate nucleus PD value | 0.701 | 86.2 | 0.467 | 0.933 | 0.400 | 0.007 |
| Right putamen PD value | 0.689 | 79.5 | 0.400 | 0.933 | 0.333 | 0.01 |
| Right pallidum PD value | 0.690 | 80.4 | 0.500 | 0.900 | 0.400 | 0.01 |
| Right thalamus PD value | 0.668 | 74.0 | 0.800 | 0.500 | 0.300 | 0.03 |
AUC, area under the curve; PD, proton density; ROC, receiver operating characteristic.

Correlation analysis between SyMRI quantitative parameters and MoCA score in abnormal brain regions of the OSA group
After adjusting for age, BMI, and education, the OSA group demonstrated negative correlations between visual space and executive function and right putamen T1 and PD values (r=−0.390, −0.449; P=0.045, 0.02) and positive correlations with the left amygdala PD values (r=0.397; P=0.04). However, these correlations were not significant in the partial correlation analysis after FDR correction (Table 8).
Table 8
| SyMRI parameter value | Brain region | Visual space and executive function | ||
|---|---|---|---|---|
| r | P value | Corrected P value | ||
| T1 | Putamen_R | −0.390 | 0.045* | 0.14 |
| PD | Amygdala_L | 0.397 | 0.04* | 0.14 |
| Putamen_R | −0.449 | 0.02* | 0.13 | |
*, at the level of 0.05 (two-tailed), the correlation is significant. L, left; MoCA, Montreal Cognitive Assessment; OSA, obstructive sleep apnea; PD, proton density; R, right; SyMRI, synthetic magnetic resonance imaging.
Discussion
This study employed SyMRI to investigate differences in subcortical gray matter nuclei volume and relaxation values between patients with moderate to severe OSA and HC. Compared to controls, patients with moderate to severe OSA exhibited reduced volume in two brain regions, increased T1 values in seven regions, increased T2 values in one region, and increased PD values in eight regions. These differences remained significant after FDR correction for multiple comparisons. ROC curve analysis revealed the diagnostic efficacy of bilateral thalamic volume, left caudate nucleus, right putamen, and pallidum T1 values, left amygdala, caudate nucleus, right putamen, and thalamus PD values in identifying gray matter nuclei damage in OSA. Of these parameters, the right pallidum T1 value had the largest AUC of 0.770, a Youden index of 0.467, a sensitivity of 0.600, and a specificity of 0.867. These findings suggest that SyMRI can effectively detect abnormal gray matter nuclei changes in OSA patients. Furthermore, the volume and relaxation value changes of specific nuclei may be expected to be potential markers for analyzing the neurological changes of OSA.
Principle and clinical application of SyMRI technology
SyMRI is a novel quantitative MRI technology capable of generating five quantitative maps and multiple contrast-weighted images from a single scan (11). Tissue T1, T2, and PD values reflect pathological changes, such as myelin sheath loss, axon degeneration, edema, and iron deposition. SyMRI measures relaxation value changes, which can quantify biophysical signals and provide relevant information for lesion evaluation (12).
SyMRI has been applied to the study of several diseases, including Alzheimer’s disease (AD) (13) and Parkinson’s disease (14). Lou et al. found that SyMRI parameters not only provide more information to distinguish AD patients from normal controls, but also believe that they may also reflect the severity of AD (13). However, the utility of this technology in investigating brain changes associated with OSA remains unexplored. Therefore, the present study employed SyMRI to measure subcortical gray matter nuclei T1, T2, and PD values in patients with moderate to severe OSA. By examining gray matter nuclei microstructure changes and their correlation with cognitive impairment, this research aimed to provide novel insights into the quantitative evaluation of brain structure in OSA.
Changes in the volume of subcortical gray matter nuclei in patients with moderate to severe OSA
Our findings indicated reduced bilateral thalamic volume in patients with moderate to severe OSA compared to the control group, suggesting a potential association with the chronic and decompensatory stages of hypoxemia, which causes neuronal damage. Previous studies have reported similar thalamic volume loss in OSA populations (15,16), suggesting that long-term recurrent apnea and hypoventilation characteristic of OSA and accompanied by intermittent hypoxia and reoxygenation may induce neuronal damage directly through hypoxia or indirectly through free radical production. Consequently, fragile brain regions may experience atrophy. However, a few previous studies have reported findings that are inconsistent with ours. For example, Taylor et al. observed increased bilateral thalamic volume in patients with moderate to severe OSA using voxel-based morphometry and attributed these changes to OSA-induced local edema and reactive cell changes (17). The current heterogeneity in findings may be related to factors such as disease severity, disease duration, and comorbidities. Future research should incorporate stratified analyses based on these variables and further observe the thalamic volume changes in OSA patients.
Changes of relaxation value of subcortical gray matter nuclei in patients with moderate to severe OSA
T1, T2, and PD values in normal brain tissue are regulated within a relatively narrow range, and the relaxation values deviate when pathological changes occur. Changes in water content, neuronal death or loss, axonal degeneration, and demyelination can change the relaxation values. In the acute pathological phase of hypoxemia in OSA patients, the mismatch between demand and supply of oxygen in the brain tissue experience oxygen supply-demand mismatch, leading to energy transfer failure, cell depolarization, and altered sodium, calcium, and potassium ion distribution into and out of the cells. This cellular dysfunction results in cytotoxic edema of the brain tissue, cellular and axonal swelling, and decreased extracellular interstitial fluid, which reduces T1, T2, and PD values (18). However, cytotoxic edema characterized by brain swelling and cerebral vascular compression can reduce cerebral blood perfusion and alter blood-brain barrier permeability. This disruption allows water molecules to leak from blood vessels into the extracellular space, transitioning to a subacute hypoxemia stage marked by aggravated angioedema. Consequently, T1, T2, and PD values increase (18). In chronic hypoxemia, extracellular fluid accumulation accelerates tissue degeneration, leading to demyelination, axonal loss, and impaired tissue barriers. The subsequent influx of ions and protein into the extracellular space exacerbates angioedema, increases extracellular fluid, and further elevates T1, T2, and PD values (18).
This study’s findings indicated significantly elevated T1 values in seven brain regions, T2 values in one region, and PD values in eight regions among OSA patients compared to controls. The significant differences in T1 values of three brain regions and PD values of seven brain regions persisted between both groups even after FDR correction. These findings suggest that the patients with moderate to severe OSA in this study are likely to represent the subacute or chronic stages of intermittent hypoxia, with brain tissue angioedema, demyelination, or axon loss, leading to extracellular fluid accumulation and increased T1 and PD values. OSA leads to varying degrees of intermittent hypoxia. Although some affected axons and neurons may recover their functions in the daytime with hypoxia relief, long-term hypoxemia causes most axons and neurons to progress to a chronic and irreversible pathological state. Conventional MRI often fails to detect this subtle brain tissue microstructural alteration. However, SyMRI has significantly superior sensitivity and can detect abnormal changes in the brain tissue relaxation value. This is of great clinical significance for early detection of OSA-related brain changes.
This study showed no significant differences in the subcortical gray matter nuclei T2 values between both groups after FDR multiple comparisons correction, possibly due to the relatively small sample size. In addition, the age-related decline in T2 values can potentially counteract the T2 value increases associated with long-term OSA-induced lesions (19). The combined effect of both factors may mask the overall changes in T2 values in certain brain regions, producing a difference in T2 values between the OSA and control groups that is not significant. Further research is needed to elucidate the complex interplay between these factors. In addition, OSA patients exhibited abnormal relaxation values in multiple subcortical gray matter nuclei; however, only bilateral thalamus volume decreased. This suggests that the thalamus may be relatively more sensitive to hypoxia, causing early thalamus damage in response to chronic intermittent hypoxia in OSA patients. As the disease progresses, the thalamus is more severely affected than other brain regions, leading to neuronal destruction and volume atrophy. These findings highlight the potential of the thalamus as a sensitive biomarker for early detection of OSA-related brain microstructure damage.
It is important to recognize that comorbidities, such as cardiovascular diseases, diabetes, and other metabolic disorders, can significantly influence the structural changes observed in patients with OSA. These conditions may exacerbate the effects of hypoxia and contribute to the overall pathology, potentially confounding our findings (20). In order to reduce confounding factors that may independently affect brain structural changes, we excluded individuals with these comorbidities from our case collection. This helped to attribute observed changes more reliably to OSA. Additionally, lifestyle factors, including physical activity levels, smoking, and dietary habits, can also play a crucial role in the health outcomes of individuals with OSA. For instance, regular physical activity is known to enhance cerebral blood flow and may mitigate some of the adverse effects of hypoxia, whereas smoking can lead to vascular damage and reduced oxygen delivery to the brain (21).
Correlation between microstructure changes of subcortical gray matter nuclei and cognitive function in patients with moderate to severe OSA
Subcortical gray matter nuclei form intricate functional and anatomical circuits with the cerebral cortex and are crucial for integrating cognition and emotion. Previous studies in OSA patients have demonstrated how long-term intermittent hypoxia affects the hippocampus, caudate nucleus, putamen, thalamus, pallidum, and nucleus accumbens, with damage in these areas causing cognitive and emotional disturbances (22-25). Our findings suggest a potential association between subcortical gray matter nuclei microstructural changes and impairments in visual space and executive function. The OSA group exhibited negative correlations between the visual space and executive function and right putamen T1 and PD values and positive correlations with left amygdala PD values. However, after FDR correction, the partial correlation analysis showed no statistical significance. This may be due to the small sample size; therefore, more data is needed for further verification.
Limitations
This study has some limitations: (I) the single-center design and relatively small sample size limit the generalizability of our findings; (II) the subcortical gray matter nucleus is small; therefore, the possibility of segmentation error cannot be completely ruled out; (III) this study included only patients with moderate to severe OSA; mild OSA was not studied. Moreover, OSA patients with variations in disease course were not included, which could limit the representativeness of the research results; (IV) in this study, patients with OSA were not stratified by age and sex, and any differences caused by these factors were neither considered nor discussed; (V) this is a cross-sectional study; thus, the relaxation value changes in OSA have not been dynamically observed over time. Future longitudinal studies are necessary to further explore the clinical utility of SyMRI in observing brain changes of OSA patients.
Conclusions
SyMRI is a novel quantitative imaging technique that effectively detects abnormal changes in subcortical gray matter nuclei among patients with moderate to severe OSA. Changes in parameter values obtained through SyMRI may help to quantitatively observe brain tissue damage. This study provides a new perspective for further exploring brain tissue structural changes in OSA patients and has good clinical value and application prospects.
Acknowledgments
We would like to thank the staff of the Respiratory Department and the Physical Examination Center of the General Hospital of TISCO for their help in the collection of research participants and Doctor Shuyi Shang for her help in the Montreal Cognitive Assessment scale evaluation.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1830/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1830/coif). K.W. is an employee of GE Healthcare. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of the General Hospital of TISCO (No. K202210) and informed consent was provided by all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, Rucker Coker T, Davidson KW, Davis EM, Donahue KE, Jaén CR, Kubik M, Li L, Ogedegbe G, Pbert L, Ruiz JM, Stevermer J, Wong JB. Screening for Obstructive Sleep Apnea in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2022;328:1945-50. [Crossref] [PubMed]
- Lyons MM, Bhatt NY, Pack AI, Magalang UJ. Global burden of sleep-disordered breathing and its implications. Respirology 2020;25:690-702. [Crossref] [PubMed]
- Messineo L, Bakker JP, Cronin J, Yee J, White DP. Obstructive sleep apnea and obesity: A review of epidemiology, pathophysiology and the effect of weight-loss treatments. Sleep Med Rev 2024;78:101996. [Crossref] [PubMed]
- Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pépin JL, Peppard PE, Sinha S, Tufik S, Valentine K, Malhotra A. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019;7:687-98. [Crossref] [PubMed]
- Seda G, Han TS. Effect of Obstructive Sleep Apnea on Neurocognitive Performance. Sleep Med Clin 2020;15:77-85. [Crossref] [PubMed]
- Lal C, Ayappa I, Ayas N, Beaudin AE, Hoyos C, Kushida CA, Kaminska M, Mullins A, Naismith SL, Osorio RS, Phillips CL, Parekh A, Stone KL, Turner AD, Varga AW. The Link between Obstructive Sleep Apnea and Neurocognitive Impairment: An Official American Thoracic Society Workshop Report. Ann Am Thorac Soc 2022;19:1245-56. [Crossref] [PubMed]
- Kinugawa K. Obstructive sleep apnea and dementia: A role to play? Rev Neurol (Paris) 2023;179:793-803. [Crossref] [PubMed]
- Chen HL, Huang CC, Lin HC, Lu CH, Chen PC, Chou KH, Su MC, Friedman M, Lin CP, Lin WC. White matter alteration and autonomic impairment in obstructive sleep apnea. J Clin Sleep Med 2020;16:293-302. [Crossref] [PubMed]
- Zhang N, Peng K, Guo JX, Liu Q, Xiao AL, Jing H. Microstructural brain abnormalities and associated neurocognitive dysfunction in obstructive sleep apnea: a pilot study with diffusion kurtosis imaging. J Clin Sleep Med 2024;20:1571-8. [Crossref] [PubMed]
- Davidescu DA, Goman A, Voita-Mekeres F, Bradacs AI, Sabina Florina SF, Csep AN, Szilagyi G, Motofelea AC, Davidescu L. Assessing Cognitive Impairments in Obstructive Sleep Apnea Patients Using Montreal Cognitive Assessment (MoCA) Scores. Cureus 2024;16:e70085. [Crossref] [PubMed]
- Gonçalves FG, Serai SD, Zuccoli G. Synthetic Brain MRI: Review of Current Concepts and Future Directions. Top Magn Reson Imaging 2018;27:387-93. [Crossref] [PubMed]
- Li CM, Chen M. Promoting actively the clinical application of synthetic MRI, improving the diagnostic efficiency of MRI. Chin J Radiol 2021;55:577-9.
- Lou B, Jiang Y, Li C, Wu PY, Li S, Qin B, Chen H, Wang R, Wu B, Chen M. Quantitative Analysis of Synthetic Magnetic Resonance Imaging in Alzheimer's Disease. Front Aging Neurosci 2021;13:638731. [Crossref] [PubMed]
- Ma WH, Wang YL, Hanjlaerbieke KK, Ding S, Jia WX. Synthetic Magnetic Resonance Combined with T1-FGRE BRAVO Sequence in Caudate Nucleus Changes in Patients with Parkinson's Disease. Chinese Journal of CT and MRI 2023;21:168-70.
- Torelli F, Moscufo N, Garreffa G, Placidi F, Romigi A, Zannino S, Bozzali M, Fasano F, Giulietti G, Djonlagic I, Malhotra A, Marciani MG, Guttmann CR. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage 2011;54:787-93. [Crossref] [PubMed]
- Alğin O, Akin B, Ocakoğlu G, Özmen E. Fully automated morphological analysis of patients with obstructive sleep apnea. Turk J Med Sci 2016;46:343-8. [Crossref] [PubMed]
- Taylor KS, Millar PJ, Murai H, Haruki N, Kimmerly DS, Bradley TD, Floras JS. Cortical autonomic network gray matter and sympathetic nerve activity in obstructive sleep apnea. Sleep 2018;41:zsx208. [Crossref] [PubMed]
- Kumar R, Chavez AS, Macey PM, Woo MA, Yan-Go FL, Harper RM. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res 2012;90:2043-52. [Crossref] [PubMed]
- Sun P, Wang W, Liu PF. Quantitative measurement of the hippocampus in healthy adults on high-resolution MRI. Journal of Harbin Medical University 2012;46:281-4, 314.
- Gleeson M, McNicholas WT. Bidirectional relationships of comorbidity with obstructive sleep apnoea. Eur Respir Rev 2022; [Crossref] [PubMed]
- Dobrosielski DA, Papandreou C, Patil SP, Salas-Salvadó J. Diet and exercise in the management of obstructive sleep apnoea and cardiovascular disease risk. Eur Respir Rev 2017; [Crossref] [PubMed]
- Zhou L, Liu G, Luo H, Li H, Peng Y, Zong D, Ouyang R. Aberrant Hippocampal Network Connectivity Is Associated With Neurocognitive Dysfunction in Patients With Moderate and Severe Obstructive Sleep Apnea. Front Neurol 2020;11:580408. [Crossref] [PubMed]
- Roy B, Sahib AK, Kang D, Aysola RS, Kumar R. Brain tissue integrity mapping in adults with obstructive sleep apnea using T1-weighted and T2-weighted images. Ther Adv Neurol Disord 2022;15:17562864221137505. [Crossref] [PubMed]
- Tahmasian M, Rosenzweig I, Eickhoff SB, Sepehry AA, Laird AR, Fox PT, Morrell MJ, Khazaie H, Eickhoff CR. Structural and functional neural adaptations in obstructive sleep apnea: An activation likelihood estimation meta-analysis. Neurosci Biobehav Rev 2016;65:142-56. [Crossref] [PubMed]
- Park KM, Kim J. Alterations of Limbic Structure Volumes in Patients with Obstructive Sleep Apnea. Can J Neurol Sci 2023;50:730-7. [Crossref] [PubMed]


