
Right atrial cystic-solid capillary hemangioma: an overview of a rare case description and differential diagnosis of cardiac tumors
Introduction
Cardiac hemangioma is an exceptionally rare benign primary cardiac tumor, accounting for only 1–2% of all primary cardiac tumors (1). These tumors can develop in various cardiac layers, including the endocardium, myocardium, or epicardium (including the pericardium), with initiation in the epicardium being particularly rare (2). Despite ongoing research, the exact etiology and pathogenesis of cardiac hemangiomas are not fully understood; genetic factors, developmental abnormalities, and environmental influences may play a role. Clinical manifestations can vary depending on the tumor’s location, size, and growth rate (3). Some patients may remain asymptomatic for long periods, whereas others may present with a range of cardiac symptoms, including arrhythmias, chest pain, heart failure, dyspnea, angina pectoris, and heart murmurs. These symptoms are often correlated with the tumor location and its impact on cardiac function. In this case report, we present a middle-aged female patient diagnosed with an asymptomatic right atrial capillary hemangioma, which was confirmed by surgical pathology.
Case presentation
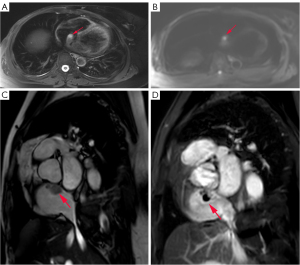
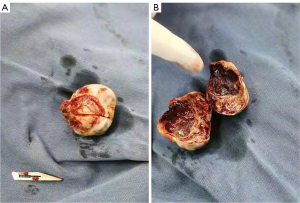
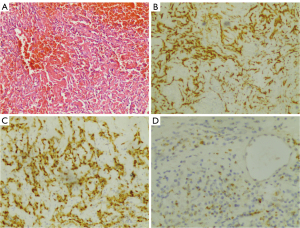
A 47-year-old woman, who underwent radical surgery for right breast cancer, followed by four cycles of adjuvant chemotherapy with a docetaxel (TXT, 110 mg) and cyclophosphamide (CTX, 0.9 g) regimen, was reviewed 8 months after surgery and shown to have right atrial space occupancy. Preoperative and postoperative electrocardiograms did not show any abnormal changes, and all showed sinus rhythm. The echocardiogram revealed an oval nodule with regular margins, measuring approximately 2.7 cm × 2.0 cm, located at the apex of the right atrium, adjacent to the interatrial septum (Figure 1). The nodule had a wide base, medium echogenicity, moderate blood supply, and showed no activity or internal flow abnormalities. Remarkably, the patient remained asymptomatic, with no chest pain, palpitations, or dyspnea. Cardiac magnetic resonance imaging (MRI) showed a wide-based lesion in the apical right atrium adjacent to the interatrial septum. The lesion was characterized by heterogeneous signals and a cystic-solid lesion. The solid component exhibited isosignal intensity on the phase-sensitive inversion recovery-turbo field echo (PSIR-TFE) sequence and high signal intensity on the T2-weighted spectral presaturation with inversion recovery (T2-SPIR) sequence and diffusion-weighted imaging (DWI) sequence, described as the “bubble-light sign”. Conversely, the cystic component displayed low signal intensity on the PSIR-TFE sequence, high signal intensity on the T2-SPIR sequence, and no significant diffusion restriction on the DWI sequence. Late gadolinium enhancement (LGE) sequences showed marked enhancement of the solid component, with no enhancement in the cystic component (Figure 2). The patient underwent surgical intervention involving a transthoracic median incision, aortic cannulation, and establishment of extracorporeal circulation with aortic root perfusion using blood-containing cold-crystal arrest fluid, leading to cardiac arrest. The cephalic end of the extracorporeal internal jugular vein cannula is usually placed in the lower third of the superior vena cava, close to the entrance to the right atrium, to ensure that blood can flow smoothly back into the right atrium and provide proper return flow to the extracorporeal circulatory system. A right atrial incision revealed normal anatomy of the two major arteries with an aorta to pulmonary artery (AO:PA) ratio of approximately 1:1. The tumor was attached to the apex of the right atrium and the upper portion of the interatrial septum. It appeared as a lobular oval mass with complete encapsulation. It involved the base of the superior vena cava and the tricuspid septal annulus on the right side. The tumor was carefully excised using a circular knife without penetrating the atrial septum. The resulting atrial septal defect was repaired with 0.6% glutaraldehyde-fixed autologous pericardium using continuous sutures with 5-0 Prolene and spacers. Upon release of the aortic cross clamp, cardiac activity resumed spontaneously with no evidence of blood leakage at the patch site. The right atrial incision was closed with continuous sutures using 5-0 Prolene. Following the withdrawal of extracorporeal circulation, the chest was closed in layers. Postoperative histopathological analysis revealed a single gray polypoid tissue measuring 2.0 cm × 1.6 cm × 1.0 cm, with a dark red profile with focal grayish-yellow areas. The tissue had a medium texture and a cystic-solid composition, including a thin-walled cystic cavity measuring 0.8 cm in diameter containing dark red fluid (Figure 3). Microscopic examination revealed a prominent meshwork of capillary-sized vessels surrounding larger “supply” vessels, characterized by a monolayer of endothelial cells and a significant presence of erythrocytes within the vessels. These findings were consistent with a diagnosis of cardiac capillary hemangioma. Immunohistochemical staining was positive for CD31, CD34, and FLI-1 markers (Figure 4). Following a 21-month follow-up period, the patient remained free of hemangioma recurrence and associated symptoms.

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Cardiac hemangiomas require careful differentiation from other cardiac pathologies that may present with similar clinical features or imaging characteristics. Here is a concise comparison of key conditions to consider: (I) mucinous tumors, the most common primary cardiac tumor, may present as cystic or solid masses with irregular, often lobulated morphology. Although frequently located in the left atrium (4), they can also occur in any cardiac chamber, and may be near cardiac valves. (II) Cardiac papillary fibroma, consisting primarily of fibroblasts and collagen fibers. These tumors appear as dense connective tissue on imaging and typically show as homogeneous, dense masses with well-defined borders, often found in heart valves, particularly the aortic valve. They are the most prevalent primary tumor of the heart valves (5). (III) Cardiac angiosarcoma represents a malignant tumor originating from endothelial cells and may present as an irregular, ill-defined mass on imaging. It can exhibit infiltrative growth and local tissue destruction. (IV) Right atrial thrombus, a clot formed by the aggregation of fibrin and platelets during coagulation, presents a distinguishing feature from hemangiomas. Hemangiomas are richly vascularized and demonstrate marked enhancement on imaging, whereas thrombi typically lack vascularity and do not enhance with contrast. In cases where clinical presentation does not allow for the exclusion of thrombus, observation of mass regression under anticoagulation or antiplatelet therapy may lead to a retrospective diagnosis (6). Although these diseases may exhibit some imaging overlap, they differ significantly in terms of patient history, clinical presentation, imaging features, and histological examination. Therefore, a comprehensive analysis and differential diagnosis are essential to establish the correct diagnosis and treatment plan. Li et al. conducted a study involving 200 patients with cardiac hemangiomas, revealing that hemangiomas of the left heart accounted for 39.5% of cases, with the left ventricle comprising 23.1% and the aortic valve 1.0%. Conversely, hemangiomas of the right heart accounted for 44.1% of cases, with the right atrium comprising 26.2%. Therefore, anatomically, the right atrium is the most common site of cardiac hemangiomas (7). Gender does not significantly influence the occurrence of cardiac hemangiomas, as they can develop in both males and females. However, they tend to be more prevalent in females compared to males (5). Cardiac hemangiomas can present at any age, including among infants, children, adolescents, and adults (5,7). Hemangiomas can be classified into several subtypes: capillary hemangiomas, which are characterized by capillary composition and typically appear as red or purplish-red lesions on the skin or mucous membranes; cavernous hemangiomas, consisting of dilated, spongy vascular tissues forming a honeycomb structure of varying sizes, commonly found in the skin, muscles, or internal organs; arteriovenous hemangiomas, which are composed of dysplastic, malformed arterioles and veins; and mixed hemangiomas, containing various types of vascular tissue such as capillaries, small arteries, and veins. Notably, cavernous hemangiomas are the most prevalent type, followed by capillary hemangiomas (5). Furthermore, the natural progression of cardiac hemangiomas remains somewhat uncertain, as they may either degenerate, stabilize, or grow over time (8,9). Most patients experience stability, with no significant changes in tumor size observed during clinical follow-up. For example, Chrissos reported a case where the cardiac hemangioma size remained unchanged over a 22-year follow-up period (10). Palmer et al. documented a case of an unresectable hemangioma in the right ventricle that spontaneously regressed after 2 years (11). In conclusion, primary cardiac hemangiomas lack specific clinical presentations and have unpredictable natural histories. Hence, early and effective diagnosis and treatment of patients with cardiac hemangiomas are of paramount importance. The echocardiogram stands out as one of the most frequently utilized diagnostic modalities for assessing cardiac hemangiomas, offering insights into their location, dimensions, shape, and other pertinent characteristics. Computed tomography (CT) imaging, on the other hand, yields three-dimensional views of the vascular architecture within cardiac structures, aiding in precise evaluations of hemangioma location, size, shape, and their spatial relationship with neighboring tissues. Additionally, CT scans are instrumental in excluding extracardiac metastases stemming from malignant cardiac tumors. Cardiac MRI elevates diagnostic precision further by furnishing comprehensive anatomical and functional insights into cardiac physiology, particularly delineating the intricate vascular distribution within hemangiomas. Meanwhile, cardiac angiography emerges as a pivotal tool, offering clear visualization of both the structural aspects of cardiac hemangiomas and the dynamic patterns of blood flow, thereby facilitating accurate diagnosis and elucidating their interplay with peripheral blood vessels. Each of these diagnostic tools offers distinct advantages and utility in assessing the location, size, and morphology of cardiac hemangiomas, as well as their effects on surrounding tissues. Nevertheless, achieving precise preoperative diagnosis remains challenging, with the majority of cases relying on postoperative histopathological examination for definitive confirmation (12). Surgical resection stands out as the preferred treatment approach (13).
Conclusions
Primary capillary hemangioma of the right atrium is a rare and typically asymptomatic condition. Preoperative diagnosis is challenging, often requiring postoperative histopathological examination for definitive confirmation. Surgical resection is the preferred treatment approach for symptomatic patients, offering favorable postoperative recovery and a low risk of recurrence.
Acknowledgments
We would like to express our deep gratitude to Professor Congying Yang for her guidance and critiques for the case report. We would also like to extend our gratitude to the all the radiology and the pathology technicians in our hospital for the high-quality images in this case.
Footnote
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1788/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xie T, Masroor M, Chen X, Liu F, Zhang J, Yang D, Liu C, Xiang M. Rheumatism as a cause of cardiac hemangioma: a rare case report and review of literature with special focus on etiology. BMC Cardiovasc Disord 2023;23:203. [Crossref] [PubMed]
- Abuharb MYI, Bian XM, He J. Epicardial cardiac cavernous Haemangioma-a case report. BMC Cardiovasc Disord 2019;19:179. [Crossref] [PubMed]
- Bernal-Gallego B, Hernández-Jiménez V, Castillo L, González-Davia R, De Antonio-Antón N, Reyes-Copa G. Unexpected diagnosis: large hemangioma in the interatrial septum. J Cardiothorac Surg 2024;19:305. [Crossref] [PubMed]
- Irani AD, Estrera AL, Buja LM, Safi HJ. Biatrial myxoma: a case report and review of the literature. J Card Surg 2008;23:385-90. [Crossref] [PubMed]
- Miao H, Yang W, Zhou M, Zhu Q, Jiang Z. Atrial Hemangioma: A Case Report and Review of the Literature. Ann Thorac Cardiovasc Surg 2019;25:71-81. [Crossref] [PubMed]
- Louali FE, Tamdy A, Soufiani A, Oukerraj L, Omari D, Bounjoum F, Mekouar F, Tazi-Mezalek Z, Bouhouch R, Zarzur J, Cherti M. Cardiac thrombosis as a manifestation of Behçet syndrome. Tex Heart Inst J 2010;37:568-71. [PubMed]
- Li W, Teng P, Xu H, Ma L, Ni Y. Cardiac Hemangioma: A Comprehensive Analysis of 200 Cases. Ann Thorac Surg 2015;99:2246-52. [Crossref] [PubMed]
- Tse TS, Tsui KL, Ling LC, Chui WH, Choi MC, Li SK, Chiu CS. Necrotic cardiac haemangioma masquerading as sepsis with disseminated intravascular coagulation. Hong Kong Med J 2005;11:308-10. [PubMed]
- Eftychiou C, Antoniades L. Cardiac hemangioma in the left ventricle and brief review of the literature. J Cardiovasc Med (Hagerstown) 2009;10:565-7. [Crossref] [PubMed]
- Chrissos DN, Agelopoulos NG, Garyfallos DJ, Stergiopoulou PD. Follow-Up of an Unresectable Hemangioma in the Heart. Echocardiography 1998;15:239-42. [Crossref] [PubMed]
- Palmer TE, Tresch DD, Bonchek LI. Spontaneous resolution of a large, cavernous hemangioma of the heart. Am J Cardiol 1986;58:184-5. [Crossref] [PubMed]
- Liu F, Dong M, Li Q. Lobulated Hemangioma as a Rare Cause of Tricuspid Regurgitation. Clin Med Insights Case Rep 2024;17:11795476241274699. [Crossref] [PubMed]
- Anbardar MH, Soleimani N, Mohammadzadeh S. Two cases of cardiac hemangioma in different anatomical locations presenting with chest pain and palpitation. Clin Case Rep 2022;10:e05495. [Crossref] [PubMed]


