
Ultrasound super-resolution imaging and shear wave elastography for the non-invasive diagnosis of non-proliferative diabetic retinopathy: a pilot study
Introduction
Diabetic retinopathy (DR) is a chronic, progressive, and blinding eye disease. According to the International Diabetes Federation, the global number of diabetes cases increased from 151 million in 2000 to 463 million in 2019 and is projected to reach 700 million by 2045. The projected number of cases accounts for 10.9% of the world’s population, with most cases originating in Asia (1,2). DR is one of the most common microvascular complications of diabetes and is characterized by ischemic microvascular lesions and retinal neurodegeneration (3). Clinically, DR is classified into non-proliferative and proliferative types on the basis of fundus examination results. Non-proliferative DR (NPDR) typically represents the early stage of the disease, during which patients often have no noticeable symptoms. During this stage, retinal lesions are limited to hemorrhages without significant neovascularization, and the lack of effective diagnostic methods often leads to delayed treatment initiation (4).
Routine ophthalmic exams, such as fundus photography and fluorescein angiography (FA), are the standard methods for detecting fundus lesions, though early microvascular leakages can be missed (5). However, these methods can be invasive, cause discomfort, and involve contrast agents that may cause adverse reactions. Conventional imaging techniques like optical coherence tomography (OCT) provide valuable insights into retinal anatomy and pathology but have limitations in their ability to quantitatively assess tissue biomechanics and microcirculation (6). While conventional ultrasound is a non-invasive alternative for the detection of DR, its use for the evaluation of NPDR has several limitations. Firstly, its poor resolution fails to detect microvascular changes and subtle retinal lesions (7). Additionally, it lacks the ability to provide quantitative measurements of blood flow and tissue stiffness, which are essential for early diagnosis and monitoring. Lastly, conventional ultrasound inadequately assesses microcirculation in the retinal and choroidal layers, a limitation resulting in the absence of critical early NPDR markers (8,9).
Recent advancements in ultrasound technology, specifically super-resolution imaging (SRI) and shear wave elastography (SWE), address these limitations and offer new opportunities for the early detection and comprehensive assessment of NPDR.
SRI is a cutting-edge imaging technique that combines several advanced methodologies to achieve high-resolution visualization of microvascular structures. This innovative approach combines contrast-enhanced ultrasound (CEUS), ultrafast frame rate imaging, advanced clutter filtering, and novel central positioning techniques. By leveraging CEUS, SRI enhances the contrast between blood vessels and surrounding tissues, providing clear differentiation (10). Ultrafast frame rate imaging captures rapid changes in blood flow, offering detailed temporal resolution. Advanced clutter filtering removes noise and artifacts from the images, ensuring high-quality visualization of minute blood vessels. Microbubble localization and accumulation enables the rendering of microvasculature below the diffraction limit imposed by the frequency of the ultrasound (11,12). Collectively, these advancements allow for the visualization of the microcirculation in the fundus using SRI, providing detailed images of capillary networks and blood flow dynamics that are undetectable via conventional ultrasound. Animal experiments have confirmed the effectiveness of SRI in identifying early lesions in the posterior ocular vasculature (13,14), emphasizing its potential for the early detection of NPDR.
In addition, SWE is a technique that measures the stiffness of tissues by tracking the propagation of shear waves generated by focused ultrasound pulses. Recent studies have reported that SWE reflects biomechanical changes through quantitative assessment and dynamic monitoring of optic nerve stiffness. Increased stiffness detected by SWE indicates early optic nerve damage, which is a precursor to more severe stages of DR (15,16).
These advanced ultrasound techniques enhance the detection of microvascular changes and tissue stiffness, and provide a non-invasive, patient-friendly approach for monitoring DR progression. By integrating SRI and SWE, clinicians can obtain comprehensive data regarding vascular and neural components of the retina, facilitating early diagnosis and timely interventions.
Therefore, this study aimed to comprehensively assess early changes in patients with NPDR using SRI and SWE, and to evaluate their application value in NPDR. By addressing the limitations of conventional methods, SRI and SWE improve the early, non-invasive detection of NPDR. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1442/rc).
Methods
Participants
This prospective study included 66 patients who visited the Department of Ultrasound at Renmin Hospital of Wuhan University from January to April 2024. The severity of the lesions was classified according to the International Clinical Diabetic Retinopathy Disease Severity Scale [2003] (17), fundus examination results as the gold standard. Thirty-four patients with NPDR were allocated to an observation group, and 32 patients with non-DR (NDR) were allocated to a control group.
To ensure a representative sample, specific inclusion and exclusion criteria were rigorously applied. Patients were included if they had undergone a fundus examination with a clinical diagnosis consistent with the NPDR grading standard, had no contraindications for CEUS examination, were able to cooperate with the required examinations, and provided informed consent. Patients were excluded if they had hypertension or heart failure affecting hemodynamics, proliferative DR, a history of other ophthalmic diseases, or a history of eye surgery. Additionally, patients with systemic conditions that could influence retinal blood flow, such as advanced kidney disease, or those on medications affecting hemodynamics, were excluded to minimize potential confounding factors.
A power analysis was conducted to determine the appropriate sample size needed to detect significant differences between the NPDR and NDR groups. Based on previous studies and an estimated effect size, the study was calculated with 66 participants to achieve 80% power at a 0.05 significance level. This ensures sufficient power to reliably detect differences in the measured parameters between the two groups.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Clinical Research Ethics Committee of Renmin Hospital of Wuhan University (No. WDRM2024-K109) and all patients provided informed consent.
Baseline acquisition
Demographic and clinical data, including sex, age, comorbidities, and the progression of illness, were collected from the patients’ medical records. Hemodynamic parameters, such as heart rate, body mass index (BMI), disease course, systolic blood pressure (SBP), and diastolic blood pressure (DBP), were continuously monitored. Laboratory values, including fasting blood glucose (FBG), hemoglobin A1c (HbAlc), cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and intraocular pressure (IOP), were evaluated.
Ultrasound image acquisition
The ultrasound examinations were conducted using a Mindray Resona A20 Pro (Mindray Bio-Medical Electronics, Shenzhen, China) ultrasound diagnostic instrument equipped with an LM18-5WU probe. Two-dimensional (2D) images were routinely collected, and color Doppler flow imaging of the eye was performed. The measurements included the axial length of the eye and blood flow parameters of the posterior ocular vessels, such as the peak systolic velocity (PSV), end-diastolic velocity (EDV), and resistance index (RI) of the central retinal artery (CRA) and posterior ciliary artery (PCA).
To ensure consistency in the 2D imaging plane across patients, a standardized procedure was implemented. All examinations were performed by a single experienced operator to minimize inter-operator variability. Each patient Each patient’s head was stabilized and they were instructed to fixate on a designated target point to minimize eye movement. The operator aligned the probe to visualize the optic disc in a uniform orientation, selecting the center of the optic disc or a reference axis on the ultrasound machine as the primary landmark to replicate the imaging angle. Multiple images were acquired per patient, and only those with optimal clarity and alignment of the target structures were included in the analysis. In cases where any misalignment was detected, the scan was repeated until a consistent imaging plane was verified.
Ultrasound SWE acquisition
In 2D mode, the plane that showed the optic nerve most clearly was selected with the probe position fixed and the SWE color scale set to 0–140 kPa. A 10 mm × 10 mm sample box with a 1 mm circular region of interest (ROI) was used. Sound touch elastography (STE) high-quality mode was applied, and the sample box was placed in the retrobulbar optic nerve area. Three to five seconds were allowed for the image to stabilize before the elastography image was frozen. An image where the sample box was more than 90% filled with color and the motion stability index (M-STB index) was at least four stars was selected. Along the optic nerve direction, the same horizontal optic nerve region 3 mm from the optic disc was selected for three measurements to obtain the elastic Young’s modulus mean (Emean) of the optic nerve.
Ultrasound SRI acquisition
Following the SWE examination, the SL10-3U probe (Mindray Bio-Medical Electronics) was positioned over the patient’s eyelid to clearly display the posterior part of the eye. SonoVue (Bracco, Milan, Italy) was administered as a 0.5 mL bolus injection into the cubital vein, followed by a 5 mL saline flush. The machine’s microvascular imaging mode was activated. When the contrast enhancement reached its peak, the patient was instructed to keep his or her eyes still and the probe was held still to obtain dynamic images over a course of 3 seconds. The machine acquired 1,500 frames of images at a frame rate of 500 frames/second and automatically generated a super-resolution ultrasound image of the fundus, including a microvascular display map and a color-coded vascular velocity map.
To ensure reproducibility and accuracy in the quantitative analysis of fundus microvascular density, the following strict quality control and ROI selection criteria were implemented:
ROI selection criteria
A fixed circular ROI with an area of 0.07 cm2 was used in all images. The selected ROI size was based on the fact that this size almost completely encompasses the microvascular structures at the optic disc in healthy individuals, making it ideal for quantifying microvascular density in the posterior segment. The ROI was positioned based on anatomical landmarks, specifically the center of the optic disc, ensuring consistent placement and size across images from different patients, thus reducing potential subjective bias during manual ROI selection.
Image quality control and exclusion criteria
Multiple SRI images were acquired and initially assessed for the clarity of microvascular structures. If an image did not clearly display the microvascular structures or exhibited a low signal-to-noise ratio, repeated scans were performed until an image meeting the minimum quality standards was obtained. Only images with clearly visible microvascular structures and adequate signal intensity were included in the final analysis. Images not meeting these quality standards after multiple attempts were excluded. In cases where images partially displayed analyzable microvascular structures, those images were retained to preserve sample representativeness.
Blinding protocol
To ensure the objectivity of the results and minimize potential bias, all SRI imaging data were anonymized and coded to conceal patients’ group information during analysis. Two experienced sonographers, blinded to group allocation, independently performed the measurements. Group information was revealed only after all analyses, ensuring an unbiased evaluation.
Statistical analysis
Statistical analyses were performed using SPSS (version 27.0). Normally distributed continuous variables were expressed as mean ± standard deviation (SD) and were compared between the two groups using independent samples t-tests, after confirming equal variances with Levene’s test. Non-normally distributed continuous variables were presented as median [interquartile range (IQR)] and were compared using Mann-Whitney U tests. Categorical variables are reported as counts (percentages) and were compared using Pearson’s Chi-squared test or Fisher’s exact test, depending on expected cell frequencies. Receiver operating characteristic (ROC) curve analysis was performed to assess the diagnostic performance of significant ultrasound parameters, with the optimal cutoff value determined by maximizing the Youden index (sensitivity + specificity − 1). The area under the ROC curve (AUC) with a 95% confidence interval (CI) was reported. Correlations between variables were assessed using Pearson’s correlation coefficient (for normally distributed data) or Spearman’s rank correlation (for non-normally distributed data). A two-sided P value <0.05 was considered statistically significant.
Results
Baseline characteristics and laboratory test indices
There were no statistically significant differences between the two groups regarding sex, age, BMI and heart rate, SBP, DBP, HDL, HbAlc, or IOP. However, the disease course and FBG, cholesterol, and LDL were significantly different between the two groups (Table 1).
Table 1
| Variables | NDR (n=32) | NPDR (n=34) | t/χ2/U | P value |
|---|---|---|---|---|
| Sex (male/female) | 18/14 | 19/15 | χ2=0.001 | 0.976 |
| Age (years) | 48.00±9.40 | 46.56±8.14 | t=0.667 | 0.437 |
| BMI (kg/m2) | 25.36±3.39 | 27.06±3.93 | t=−1.873 | 0.424 |
| Heart rate (bpm) | 88.84±10.13 | 88.12±11.04 | t=0.278 | 0.782 |
| SBP (mmHg) | 110.06±9.99 | 114.76±10.14 | t=−1.901 | 0.062 |
| DBP (mmHg) | 72.50±5.83 | 73.82±6.90 | t=−0.842 | 0.401 |
| Course (years) | 4.00 (2.00, 7.25) | 8.00 (4.25, 9.00) | U=361 | 0.018 |
| Laboratory test index | ||||
| FBG (mmol/L) | 8.93±1.54 | 9.97±1.78 | t=−2.533 | 0.014 |
| HbAlc (%) | 7.25±0.79 | 7.33±0.85 | t=−0.421 | 0.675 |
| Cholesterol (mmol/L) | 4.47±0.84 | 5.52±1.36 | t=−3.770 | <0.001 |
| LDL (mmol/L) | 2.62±0.54 | 3.78±0.62 | t=−8.091 | <0.001 |
| HDL (mmol/L) | 1.20±0.15 | 1.22±0.11 | t=−0.492 | 0.625 |
| IOP (mmHg) | 15.22±3.77 | 15.85±3.14 | t=−0.744 | 0.459 |
Data are expressed as number, mean ± standard deviation or median (interquartile range). NDR, non-diabetic retinopathy; NPDR, non-proliferative diabetic retinopathy; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; HbAlc, hemoglobin A1c; LDL, low-density lipoprotein; HDL, high-density lipoprotein; IOP, intraocular pressure.
US parameters
Conventional ultrasound appearances of eyeballs
The ocular axial length (22.29±0.71 vs. 22.54±0.58 mm), PSV of the PCA (11.40±0.79 vs. 11.59±0.50 cm/s), EDV (4.02±0.39 vs. 4.01±0.17 cm/s), RI (0.65±0.01 vs. 0.64±0.01), and RI of the CRA (0.66±0.01 vs. 0.67±0.01) were not significantly different between the NDR and NPDR groups. However, the PSV [9.72 (9.40, 10.19) vs. 9.25 (8.98 9.59) cm/s] and EDV (3.18±0.23 vs. 2.99±0.18 cm/s) of the CRA were significantly different between the two groups (P<0.05) (Figures 1,2 and Table 2).
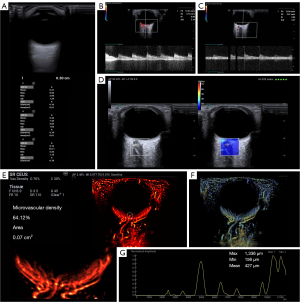

Table 2
| Variables | NDR (n=32) | NPDR (n=34) | t/U | P value |
|---|---|---|---|---|
| Axial length (mm) | 22.29±0.71 | 22.54±0.58 | t=−1.617 | 0.111 |
| CRA | ||||
| PSV (cm/s) | 9.72 (9.40, 10.19) | 9.25 (8.98, 9.59) | U=839 | 0.001 |
| EDV (cm/s) | 3.18±0.23 | 2.99±0.18 | t=3.466 | 0.001 |
| RI | 0.66±0.01 | 0.67±0.01 | t=−1.671 | 0.112 |
| PCA | ||||
| PSV (cm/s) | 11.40±0.79 | 11.59±0.50 | t=1.176 | 0.244 |
| EDV (cm/s) | 4.02±0.39 | 4.01±0.17 | t=0.001 | 0.991 |
| RI | 0.65±0.01 | 0.64±0.01 | t=1.770 | 0.082 |
| Emean of optic nerve (kPa) | 6.36 (5.59, 7,61) | 8.50 (7.81, 9.76) | U=160 | <0.001 |
| Microvascular density (%) | 50.64 (48.09, 58.86) | 28.67 (25.63, 32.89) | U=1,052 | <0.001 |
Data are expressed as mean ± standard deviation or median (interquartile range). NDR, non-diabetic retinopathy; NPDR, non-proliferative diabetic retinopathy; CRA, central retinal artery; PSV, peak systolic velocity; EDV, end-diastolic velocity; RI, resistance index; PCA, posterior ciliary artery.
Evaluation of optic nerve stiffness via SWE
The optic nerve Emean in the NPDR group [8.50 (7.81, 9.76) kPa] was greater than that in the NDR group [6.36 (5.59, 7.61) kPa] (Figures 1,2 and Table 2).
Evaluation of microcirculation via SRI
The microvascular density of the optic disc in the NPDR group [28.67% (25.63%, 32.89%)] was significantly lower than that in the NDR group [50.64% (48.09%, 58.86%)] (P<0.001) (Figures 1,2 and Table 2). The microvascular density of the optic disc was significantly and negatively correlated with the Emean of the optic nerve (R=−0.83; P<0.001), as shown in Figure 3.

ROC curve analysis
ROC analysis of the ultrasound parameters revealed statistically significant differences between the two groups in the optimal cutoff values for distinguishing NPDR: CRA PSV at 9.34 cm/s, EDV at 3.18 cm/s, optic nerve stiffness at 7.33 kPa, and fundus vascular density at 42.99%. NPDR should be highly suspected when the CRA PSV is <9.34 cm/s, EDV is <3.18 cm/s, optic nerve stiffness is >7.33 kPa, and fundus vascular density is <42.99%. Among these parameters, the fundus vascular density had the highest diagnostic value (sensitivity: 97%; specificity: 94%) (Figure 4 and Table 3).
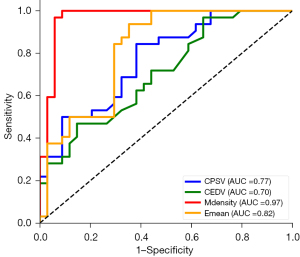
Table 3
| Parameters | AUC (95% CI) | Cutoff value | Sensitivity | Specificity | P value |
|---|---|---|---|---|---|
| CPSV | 0.77 (0.65–0.87) | 9.34 | 0.84 | 0.62 | 0.001 |
| CEDV | 0.70 (0.59–0.83) | 3.18 | 0.47 | 0.85 | 0.001 |
| Emean | 0.82 (0.76–0.95) | 7.33 | 0.94 | 0.70 | <0.001 |
| Microvascular density | 0.97 (0.92–0.99) | 42.99 | 0.97 | 0.94 | <0.001 |
ROC, receiver operating characteristic; US, ultrasound; NPDR, non-proliferative diabetic retinopathy; AUC, area under the curve; CI, confidence interval; CPSV, central retinal artery peak systolic velocity; CEDV, central retinal artery end-diastolic velocity; Emean, elastic Young’s modulus mean.
Discussion
In recent years, the incidence of diabetes has increased annually due to changes in lifestyle (18). Vascular lesions are major complications of diabetes and severely affect the quality of life of patients with diabetes. Among these complications, ocular vascular lesions are common and can lead to retinopathy, often resulting in visual impairment or blindness (19). Patients with NPDR typically do not experience significant visual impairment and are asymptomatic (20). Most patients are diagnosed with DR after it has progressed to the middle or late stages, resulting in poor clinical outcomes (21). This underscores the importance of early detection and proactive measures in preventing DR progression.
The results of this study suggest that patients with NPDR generally exhibit normal intraocular structures, which limits the diagnostic effectiveness of 2D ultrasound. When color Doppler was used to assess the posterior ocular vasculature, we found that the CRA had the highest detection rate. Compared with patients without retinopathy, those with NPDR presented with lower blood flow velocity in the CRA, indicating retinal damage. Although the PCA also supplies blood to the posterior eye, no significant differences in the PCA parameters were observed between the groups, which may be attributed to measurement errors and suboptimal vascular display in some patients. These findings support the conclusion that color Doppler can detect certain blood flow alterations in NPDR, but its clinical utility is constrained in some cases.
This study is the first clinical application of SRI technology to visualize retinal microcirculation. For fundus microvasculature distributions that are not visible via conventional ultrasound or CEUS, SRI technology has significant advantages. This technology captures high-speed images within seconds and tracks microbubble trajectories, automatically creating images of the fundus microvasculature. This efficient imaging capability enables SRI technology to clearly visualize fundus microcirculation with a spatial resolution of up to 100 micrometers, surpassing traditional imaging methods (22). Furthermore, the potential of 3D SRI technology, which is under development, could further enhance SRI by offering three-dimensional visualization of retinal microvasculature. This advancement could improve diagnostic accuracy and treatment planning, allowing for more detailed assessments of the retinal microvascular network (23,24).
Our findings of this study highlight a key early indicator of DR: a reduction in microvascular density in the fundus. In patients without DR, the microvascular network in the fundus was dense and intricately branched, which indicated healthy vascular function. Conversely, even in the early stages of DR, microvascular density significantly decreases, with vessels appearing sparser and more fragmented. One of the major advantages of SRI over traditional imaging techniques, such as color Doppler ultrasound, is its superior diagnostic efficiency. The high-resolution capability of SRI facilitates the precise detection of early vascular changes, allowing for earlier diagnosis and intervention. The observed differences in blood flow parameters between healthy and diseased retinas were attributed to the pathological conditions associated with diabetes (25). Specifically, the high-glucose and high-inflammatory environment in the diabetic retina leads to ischemia and hypoxia, which subsequently stimulates endothelial cell dysfunction and damages vascular structures. These pathological changes result in altered vessel diameter and reduced vascular elasticity (26). Furthermore, the mechanical stretching of vessel walls due to decreased elasticity may lead to vascular disruption. In the early stages of diabetes mellitus (DM), elevated expression of adhesion factor-1 and transient leukocyte aggregation contribute to capillary occlusion. These processes affect normal blood flow, worsening ischemic and hypoxic conditions, and further compromising the integrity of the microvascular network (27,28).
Recent studies indicate that retinal neurodegeneration and vascular lesions coexist in the early stages of diabetes, contributing to the occurrence and development of DR (29,30). Optic nerve damage may occur prior to microvascular lesions in patients with diabetes (31). Our study revealed that the stiffness of the optic nerve in the posterior eye during the early stages of DR tends to increase, and changes in stiffness are significantly and negatively correlated with the microvascular density at the fundus. The optic nerve, which is formed by the axons of retinal ganglion cells, is highly sensitive to ischemia, hypoxia, and metabolic disorders. These signs may be due to high glucose levels causing insufficient perfusion and damage to the small vessels in the preoptic nerve, leading to optic disc edema, ischemic optic neuropathy, or optic neuritis. After edema subsides, it ultimately leads to optic nerve atrophy, increasing optic nerve stiffness (32,33). Furthermore, low perfusion of the optic nerve generates excitotoxic substances, inducing the production of free radicals and causing ganglion cell apoptosis, further leading to secondary optic nerve damage (34).
Given the potential for early detection and assessment of these pathological changes, advanced imaging techniques like SRI and SWE offer advantages in managing NPDR. These methods provide more detailed, non-invasive insights compared to traditional diagnostic modalities such as fluorescein angiography (FA) or OCT angiography (OCT-A). Unlike FA, which is invasive and requires contrast agents, SRI offers a non-invasive way to visualize retinal microcirculation at high resolution. Similarly, OCT-A can image retinal vessels but cannot quantify tissue stiffness, an important indicator of early structural changes (35). SWE uniquely quantifies optic nerve stiffness, providing diagnostic value for assessing disease progression and guiding treatment. Integrating SRI and SWE allows clinicians to comprehensively assess retinal health, combining high-resolution microvascular imaging with quantitative biomechanical measurements to enhance early detection, risk stratification, and treatment monitoring in NPDR patients.
This study had several limitations, including a small sample size and suboptimal vascular display in some patients, which may have affected the results. Additionally, quantitative analysis was limited to specific fundus regions, which may not fully represent overall blood flow changes. These limitations could have led to an underestimation or overestimation of vascular parameters, potentially affecting the reliability of the findings. To address these issues in future research, a larger sample size will be used to enhance statistical reliability and minimize biases from patient variability. Imaging techniques will also be improved by incorporating higher-resolution ultrasound probes or combining ultrasound with other imaging modalities to enhance image clarity and precision. Additionally, stricter inclusion criteria will be applied to reduce factors that may compromise vascular imaging quality, thereby improving the consistency and accuracy of data.
Conclusions
In summary, the advanced ultrasound techniques SRI and SWE significantly improve the non-invasive diagnosis of NPDR. These methods enable detailed monitoring of hemodynamic changes and direct visualization of fundus microcirculation. The combination of SRI (to assess microvascular density) and SWE (to measure optic nerve stiffness) provides a comprehensive evaluation of ocular lesions in patients with NPDR, facilitating early diagnosis and interventions, which may improve the clinical outcomes and prevent the progression of DR. Future larger multi-center studies will further validate these findings and ensure their broader applicability.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1442/rc
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1442/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Clinical Research Ethics Committee of Renmin Hospital of Wuhan University (No. WDRM2024-K109) and all patients provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abou Taha A, Dinesen S, Vergmann AS, Grauslund J. Present and future screening programs for diabetic retinopathy: a narrative review. Int J Retina Vitreous 2024;10:14. [Crossref] [PubMed]
- Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, Unwin N, Wild SH, Williams R. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 2020;162:108086. [Crossref] [PubMed]
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 2022;183:109119. [Crossref] [PubMed]
- Sohn EH, Han IC, Abramoff MD. Diabetic Retinal Neurodegeneration-Should We Redefine Retinopathy From Diabetes? JAMA Ophthalmol 2019;137:1132-3. [Crossref] [PubMed]
- Zhang Q, Rezaei KA, Saraf SS, Chu Z, Wang F, Wang RK. Ultra-wide optical coherence tomography angiography in diabetic retinopathy. Quant Imaging Med Surg 2018;8:743-53. [Crossref] [PubMed]
- Lam C, Wong YL, Tang Z, Hu X, Nguyen TX, Yang D, Zhang S, Ding J, Szeto SKH, Ran AR, Cheung CY. Performance of Artificial Intelligence in Detecting Diabetic Macular Edema From Fundus Photography and Optical Coherence Tomography Images: A Systematic Review and Meta-analysis. Diabetes Care 2024;47:304-19. [Crossref] [PubMed]
- Ul Banna H, Mitchell B, Chen S, Palko J. Super-Resolution Ultrasound Localization Microscopy Using High-Frequency Ultrasound to Measure Ocular Perfusion Velocity in the Rat Eye. Bioengineering (Basel) 2023;10:689. [Crossref] [PubMed]
- Kadakia A, Zhang J, Yao X, Zhou Q, Heiferman MJ. Ultrasound in ocular oncology: Technical advances, clinical applications, and limitations. Exp Biol Med (Maywood) 2023;248:371-9. [Crossref] [PubMed]
- Yamaguchi T. Basic concept and clinical applications of quantitative ultrasound (QUS) technologies. J Med Ultrason (2001) 2021;48:391-402. [Crossref] [PubMed]
- Li Y, Foo LL, Wong CW, Li J, Hoang QV, Schmetterer L, Ting DSW, Ang M. Pathologic myopia: advances in imaging and the potential role of artificial intelligence. Br J Ophthalmol 2023;107:600-6. [Crossref] [PubMed]
- Bodard S, Denis L, Chabouh G, Battaglia J, Anglicheau D, Hélénon O, Correas JM, Couture O. Visualization of Renal Glomeruli in Human Native Kidneys With Sensing Ultrasound Localization Microscopy. Invest Radiol 2024;59:561-8. [Crossref] [PubMed]
- Denis L, Bodard S, Hingot V, Chavignon A, Battaglia J, Renault G, Lager F, Aissani A, Hélénon O, Correas JM, Couture O. Sensing ultrasound localization microscopy for the visualization of glomeruli in living rats and humans. EBioMedicine 2023;91:104578. [Crossref] [PubMed]
- Qian X, Huang C, Li R, Song BJ, Tchelepi H, Shung KK, Chen S, Humayun MS, Zhou Q. Super-Resolution Ultrasound Localization Microscopy for Visualization of the Ocular Blood Flow. IEEE Trans Biomed Eng 2022;69:1585-94. [Crossref] [PubMed]
- Lei S, Zhang C, Zhu B, Gao Z, Zhang Q, Liu J, Li Y, Zheng H, Ma T. In vivo ocular microvasculature imaging in rabbits with 3D ultrasound localization microscopy. Ultrasonics 2023;133:107022. [Crossref] [PubMed]
- İnal M, Tan S, Yumusak EM, Şahan MH, Alpua M, Örnek K. Evaluation of the optic nerve using strain and shear wave elastography in patients with multiple sclerosis and healthy subjects. Med Ultrason 2017;19:39-44. [Crossref] [PubMed]
- Zakrzewski J, Zakrzewska K, Pluta K, Nowak O, Miłoszewska-Paluch A. Ultrasound elastography in the evaluation of peripheral neuropathies: a systematic review of the literature. Pol J Radiol 2019;84:e581-91. [Crossref] [PubMed]
- Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677-82. [Crossref] [PubMed]
- Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023;402:203-34. [Crossref] [PubMed]
- Kour V, Swain J, Singh J, Singh H, Kour H. A Review on Diabetic Retinopathy. Curr Diabetes Rev 2024;20:e201023222418. [Crossref] [PubMed]
- Santos AR, Ribeiro L, Bandello F, Lattanzio R, Egan C, Frydkjaer-Olsen U, et al. Functional and Structural Findings of Neurodegeneration in Early Stages of Diabetic Retinopathy: Cross-sectional Analyses of Baseline Data of the EUROCONDOR Project. Diabetes 2017;66:2503-10. [Crossref] [PubMed]
- Cao D, Yang D, Yu H, Xie J, Zeng Y, Wang J, Zhang L. Optic nerve head perfusion changes preceding peripapillary retinal nerve fibre layer thinning in preclinical diabetic retinopathy. Clin Exp Ophthalmol 2019;47:219-25. [Crossref] [PubMed]
- Bodard S, Denis L, Hingot V, Chavignon A, Hélénon O, Anglicheau D, Couture O, Correas JM. Ultrasound localization microscopy of the human kidney allograft on a clinical ultrasound scanner. Kidney Int 2023;103:930-5. [Crossref] [PubMed]
- Coudert A, Denis L, Chavignon A, Bodard S, Naveau M, Sistiaga PP, Saulnier R, Orset C, Vivien D, Chappard C, Couture O 3D. Transcranial ultrasound localization microscopy reveals major arteries in the sheep brain. IEEE Trans Ultrason Ferroelectr Freq Control. ;
- Chabouh G, Denis L, Bodard S, Lager F, Renault G, Chavignon A, Couture O. Whole Organ Volumetric Sensing Ultrasound Localization Microscopy for Characterization of Kidney Structure. IEEE Trans Med Imaging 2024;43:4055-63. [Crossref] [PubMed]
- Huang B, Yan J, Morris M, Sinnett V, Somaiah N, Tang MX. Acceleration-Based Kalman Tracking for Super-Resolution Ultrasound Imaging In Vivo. IEEE Trans Ultrason Ferroelectr Freq Control 2023;70:1739-48. [Crossref] [PubMed]
- Meng C, Gu C, He S, Su T, Lhamo T, Draga D, Qiu Q. Pyroptosis in the Retinal Neurovascular Unit: New Insights Into Diabetic Retinopathy. Front Immunol 2021;12:763092. [Crossref] [PubMed]
- Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, Aiello LP, Ogura Y, Adamis AP. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A 1999;96:10836-41. [Crossref] [PubMed]
- Aschauer J, Pollreisz A, Karst S, Hülsmann M, Hajdu D, Datlinger F, Egner B, Kriechbaum K, Pablik E, Schmidt-Erfurth UM. Longitudinal analysis of microvascular perfusion and neurodegenerative changes in early type 2 diabetic retinal disease. Br J Ophthalmol 2022;106:528-33. [Crossref] [PubMed]
- Singh AD, Kulkarni YA. Vascular adhesion protein-1 and microvascular diabetic complications. Pharmacol Rep 2022;74:40-6. [Crossref] [PubMed]
- Callan A, Jha S, Valdez L, Tsin A. Cellular and Molecular Mechanisms of Neuronal Degeneration in Early-Stage Diabetic Retinopathy. Curr Vasc Pharmacol 2024;22:301-15. [Crossref] [PubMed]
- Simó R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia 2018;61:1902-12. [Crossref] [PubMed]
- Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol 2012;11:521-34. [Crossref] [PubMed]
- Chakravarthy H, Devanathan V. Molecular Mechanisms Mediating Diabetic Retinal Neurodegeneration: Potential Research Avenues and Therapeutic Targets. J Mol Neurosci 2018;66:445-61. [Crossref] [PubMed]
- Khalilpour S, Latifi S, Behnammanesh G, Majid AMSA, Majid ASA, Tamayol A. Ischemic optic neuropathy as a model of neurodegenerative disorder: A review of pathogenic mechanism of axonal degeneration and the role of neuroprotection. J Neurol Sci 2017;375:430-41. [Crossref] [PubMed]
- Javed A, Khanna A, Palmer E, Wilde C, Zaman A, Orr G, Kumudhan D, Lakshmanan A, Panos GD. Optical coherence tomography angiography: a review of the current literature. J Int Med Res 2023;51:3000605231187933. [Crossref] [PubMed]


