
First-trimester diagnosis of Meckel syndrome by ultrasonography with suspected mutation of CC2D2A: a case description
Introduction
Meckel syndrome (MKS) is a hereditary autosomal recessive condition that is rare, often fatal, and associated with various severe malformations. The incidence of MKS is low, and the worldwide incidence of MKS has been estimated at 1 in 135,000 live births (1), but higher incidences of MKS are observed in endogamous populations, such as Gujarati Indians, Tatars, and Hutterites, and in Finland where 1 in every 9,000 live births is affected (2). The main manifestations of MKS are abnormalities of the central nervous system, renal cystic changes and limb deformities, and intracranial, facial, and congenital cardiovascular features. Encephalocele can be diagnosed as early as 11 weeks of pregnancy (3). The first-trimester diagnosis of MKS is crucial, as many patients may elect pregnancy termination. Early termination of pregnancy can reduce the physical and psychological damage to pregnant women. Here, we used ultrasound to detect MKS in a fetus in the first trimester.
Case presentation
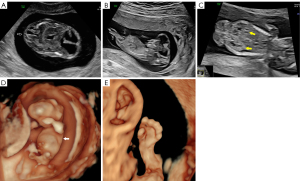
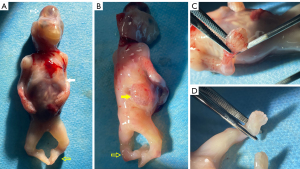
A 28-year-old nulliparous pregnant woman of Chinese descent, in a nonconsanguineous union, arrived at Gansu Provincial Maternity and Child-care Hospital at 12 weeks and 2 days pregnancy for a targeted fetal examination due to the detection of suspected encephalocele at another ultrasound center. Prenatal ultrasound examination showed fetal ventricular dilatation with severe occipital encephalocele (Figure 1A,1B), bilateral polycystic kidney (Figure 1C), chest hypoplasia (Figure 1D), polydactyly of the hands and feet, and equinovarus (Figure 1E). The triad of encephalocele, polydactyly, and cystic renal dysplasia constitute a clinical diagnosis of MKS. The patient was informed of the fatal outcome of MKS, and the pregnant woman and her family elected termination of the pregnancy. Fetal external dysmorphology exam was performed, and the family granted permission to acquire images, which confirmed severe occipital encephalocele, thoracic dysplasia, polydactyly of hands and feet, and equinovarus (Figure 2A-2D). The patient declined a full pathological autopsy, and no genetic testing of the fetus was completed. The diagnosis of MKS was determined according to prenatal and postnatal characteristics. After 8 months, the CC2D2A gene mutation was detected in both the mother and father. The mother had a CC2D2A c.1465C>T p.Arg489* heterozygous mutation. The father had a CC2D2A c.3688C>T p.Arg1230* heterozygous mutation. The genetic test confirmed a 25% chance of recurrence. Two months later, the mother spontaneously conceived again. Unfortunately, this second conception resulted in spontaneous abortion.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardians for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
MKS is a lethal autosomal recessive congenital anomaly syndrome caused by mutations in genes encoding proteins that are structural or functional components of the primary cilium (2). Ciliopathies are a group of disorders caused by mutations in ciliary genes, with MKS being the most severe among them. The primary cilium is a microtubule-supported structure that projects from the apical surface of vertebrate cells. It serves as an “antenna”, sensing and transmitting chemical and mechanical signals while also controlling key signaling pathways crucial for proper embryonic development (2). Ciliopathies exhibit a wide genetic spectrum, in which a single gene mutation may lead to different phenotypes (allelism), or multiple gene mutations can produce overlapping clinical presentations (4). To date, 14 genes have been linked to MKS (2), with research showing that MKS3 (TMEM67) mutations account for 16% of cases, making it the leading genetic cause of the condition (5). In one study, CC2D2A mutations were identified as a key contributor to MKS, accounting for 10% of cases in a cohort of 120 fetuses with MKS (6). Other studies have reported allelism between MKS and Joubert syndrome (JBS) for the genes TMEM67/MKS3, CEP290/MKS4, and RPGRIP1L/MKS5. This suggests that MKS and JBS may be different expressions of the same condition, with the severity depending on the mutation. Missense mutations are typically observed in JBS, while null mutations that disrupt protein function are linked to the more severe MKS phenotype (6). The initial clinical description of JBS included ataxia, intellectual disability, abnormal eye movements, agenesis of the cerebellar vermis, and, in some cases, hyperpnea (7).
This case was diagnosed at 12 weeks and 2 days pregnancy. Because MKS is characterized by encephalocele, polycystic kidney disease, and polydactyly in early pregnancy (8), it can be clinically diagnosed in early pregnancy by ultrasound. This case’s findings were consistent with the classic presentation, but there were some distinguishing factors. Specifically, while encephalocele is a hallmark feature of MKS, its location and severity can vary. In our case, there was severe occipital encephalocele, which is frequently observed in MKS, However, there are other encephalocele in MKS such as frontal encephalocele or parietal encephalocele. Our case had polydactyly of both the hands and feet, which aligns with descriptions in the literature (9), although related study (10) has noted that polydactyly may be present in either the upper or lower limbs but not both. Bilateral polycystic kidneys were identified in this case, which is another classic sign of MKS. This is consistent with related study (2) indicating that renal involvement is almost always bilateral in MKS, which can lead to renal failure early in life. Chest hypoplasia is an unusual finding in this case and does not commonly appear in the literature on MKS. Related study (11) suggests that MKS can have a range of other congenital anomalies (e.g., cardiac or pulmonary issues), but chest hypoplasia is not feature widely documented in the literature. It is still critical to perform molecular testing to counsel families regarding a 25% risk for recurrence with confidence and to allow for reproductive planning and decision-making. In this patient’s case, the carrier status for CC2D2A-related MKS was confirmed in both the pregnant woman and her partner, which is consistent with previously published reports (6). The specific mutations in this case, c.1465C>T, p.Arg489*, c.3688C>T, and p.Arg1230*, are novel in the sense that different mutations within the same gene can present with varying phenotypic severity, but both parents being carriers is typical of an autosomal recessive inheritance pattern, which has also been observed in other familial cases.
The differential diagnosis for MKS includes trisomy 13, Smith-Lemli-Opitz Syndrome (SLOS), JBS, and ciliopathies, and these diseases have overlapping features and even the same gene mutations, with the same gene manifesting with varying phenotypic severities. For example, JBS can also be caused by mutations in the CC2D2A gene. Diagnosis of MKS often requires genetic testing and detailed imaging studies to differentiate it from these other disorders, with both the clinical presentation and underlying genetic mutations needing to be considered (12,13).
The ultrasonographic prenatal diagnosis of MKS was first accomplished in 1982 (14). Given the severe prognosis and the potential for recurrence in future pregnancies, an early and precise antenatal diagnosis is crucial (15). Detection of MKS is possible in the first trimester, and ultrasound features can provide diagnostic clues for clinical diagnosis. The identification of mutant genes via family analysis can provide a basis for the prenatal diagnosis or preimplantation genetic testing of the family.
Acknowledgments
None.
Footnote
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1943/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardians for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Auber B, Burfeind P, Herold S, Schoner K, Simson G, Rauskolb R, Rehder H. A disease causing deletion of 29 base pairs in intron 15 in the MKS1 gene is highly associated with the campomelic variant of the Meckel-Gruber syndrome. Clin Genet 2007;72:454-9. [Crossref] [PubMed]
- Hartill V, Szymanska K, Sharif SM, Wheway G, Johnson CA. Meckel-Gruber Syndrome: An Update on Diagnosis, Clinical Management, and Research Advances. Front Pediatr 2017;5:244. [Crossref] [PubMed]
- Jones D, Fiozzo F, Waters B, McKnight D, Brown S. First-trimester diagnosis of Meckel-Gruber syndrome by fetal ultrasound with molecular identification of CC2D2A mutations by next-generation sequencing. Ultrasound Obstet Gynecol 2014;44:719-21. [Crossref] [PubMed]
- Braun DA, Hildebrandt F. Ciliopathies. Cold Spring Harb Perspect Biol 2017;9:a028191. [Crossref] [PubMed]
- Iannicelli M, Brancati F, Mougou-Zerelli S, Mazzotta A, Thomas S, Elkhartoufi N, et al. Novel TMEM67 mutations and genotype-phenotype correlates in meckelin-related ciliopathies. Hum Mutat 2010;31:E1319-31. [Crossref] [PubMed]
- Mougou-Zerelli S, Thomas S, Szenker E, Audollent S, Elkhartoufi N, Babarit C, et al. CC2D2A mutations in Meckel and Joubert syndromes indicate a genotype-phenotype correlation. Hum Mutat 2009;30:1574-82. [Crossref] [PubMed]
- Jayarajan RO, Chakraborty S, Raghu KG, Purushothaman J, Veleri S. Joubert syndrome causing mutation in C2 domain of CC2D2A affects structural integrity of cilia and cellular signaling molecules. Exp Brain Res 2024;242:619-37. [Crossref] [PubMed]
- Chen CP. Meckel syndrome: genetics, perinatal findings, and differential diagnosis. Taiwan J Obstet Gynecol 2007;46:9-14. [Crossref] [PubMed]
- Mittermayer C, Lee A, Brugger PC. Prenatal diagnosis of the Meckel-Gruber syndrome from 11th to 20th gestational week. Ultraschall Med 2004;25:275-9. [Crossref] [PubMed]
- Al-Belushi M, Al Ibrahim A, Ahmed M, Ahmed B, Khenyab N, Konje JC. A review of Meckel-Gruber syndrome--incidence and outcome in the state of Qatar. J Matern Fetal Neonatal Med 2016;29:2013-6. [Crossref] [PubMed]
- Barisic I, Boban L, Loane M, Garne E, Wellesley D, Calzolari E, Dolk H, Addor MC, Bergman JE, Braz P, Draper ES, Haeusler M, Khoshnood B, Klungsoyr K, Pierini A, Queisser-Luft A, Rankin J, Rissmann A, Verellen-Dumoulin C. Meckel-Gruber Syndrome: a population-based study on prevalence, prenatal diagnosis, clinical features, and survival in Europe. Eur J Hum Genet 2015;23:746-52. [Crossref] [PubMed]
- Baala L, Audollent S, Martinovic J, Ozilou C, Babron MC, Sivanandamoorthy S, et al. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet 2007;81:170-9. [Crossref] [PubMed]
- Baala L, Romano S, Khaddour R, Saunier S, Smith UM, Audollent S, et al. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am J Hum Genet 2007;80:186-94. [Crossref] [PubMed]
- Schmidt W, Kubli F. Early diagnosis of severe congenital malformations by ultrasonography. J Perinat Med 1982;10:233-41. [Crossref] [PubMed]
- Nyberg DA, Hallesy D, Mahony BS, Hirsch JH, Luthy DA, Hickok D. Meckel-Gruber syndrome. Importance of prenatal diagnosis. J Ultrasound Med 1990;9:691-6. [Crossref] [PubMed]


