
The changes in 3D bone morphology mediate the association between meniscal extrusion and radiographic progression of osteoarthritis: data from the Osteoarthritis Initiative
Introduction
Osteoarthritis (OA) is a leading cause of pain, activity restriction, and chronic disability, with a global prevalence of >7% (528 million people) (1). Although there are significant differences in the incidence and years lived with disability of individuals with OA across different counties, the burden is increasing in most of the world (2). The knee is one of the most commonly affected sites of OA, and the pathological process of the occurrence and development of knee osteoarthritis (KOA) is complex. Moreover, the pathogenesis of KOA remains unclear due to the numerous risk factors that contribute to KOA (3).
The meniscus plays a role in transferring and absorbing loads, reducing stress and friction, and maintaining knee joint stability during knee movements (4). Early studies found that meniscus lesions are strongly associated with KOA and that meniscus injury or degeneration may initiate or accelerate the pathological cascade leading to OA (5). After axial loading, the meniscus flattens, converting axial stress into annular tension, and in this process, the meniscus is squeezed to produce a certain degree of mobility. Meniscal extrusion (ME) occurs when the meniscus body margin (middle meniscus) exceeds 3 mm or beyond the outside of the tibial plateau margin (ME grade ≥2) (6,7). Studies have shown that medial meniscal extrusion (MME) is closely related to the progression of KOA, which may accelerate the occurrence and development of OA (8), and that MME can predict the structural progression of KOA (9). In studies with a follow-up of >30 months, meniscus volume and degree of extrusion at baseline were associated with radiological OA in middle-aged obese women (10,11). Thus, MME may be a target for the prevention of KOA progression (12).
MME decreases the tibiofemoral contact area, resulting in increased localized stress, which contributes to pathological changes in cartilage and subchondral bone (13-15). Etiologically, changes in subchondral bone structure may respond to both mechanical load and adaptation to endochondral ossification. These processes will lead to the expansion and shape alteration of the metaphysis of the proximal tibia and distal femur (16). One study reported that MME can predict an increase in subchondral bone lesions and tibial plateau bone expansion in KOA, with subchondral bone changes being an early consequence of MME (17). The authors of the study reported that MME leads to cartilage defects, with endochondral ossification being associated with cartilage loss (17). MME may be a structural risk factor for the development of tibiofemoral osteophytes (18). It is worth noting that a few recent studies have proposed MME to be caused by the stretching and displacement of the coronary ligament compressed by osteophytes (15,19). However, these studies were all cross-sectional in design, and there is no direct evidence that osteophytes cause MME (19). Although magnetic resonance imaging (MRI) studies have reported increases in tibial plateau size and alterations in bone surface profiles, these findings do not comprehensively capture the spectrum and complexity of bone shape changes that occur in KOA (20). Three-dimensional (3D) bone morphology has the potential to extract more detailed shape features as compared with the two-dimensional (2D) version (21). Statistical shape modeling (SSM) is a form of supervised machine learning that helps parameterize complex 3D anatomical shapes, such as the shape of the knee (22). The 3D bone morphology constructed using SSM can calculate subtle shape changes related to KOA, thereby improving sensitivity and enabling quantitative changes in the knee joint after the onset of KOA (21). The changes in 3D bone morphology have been shown to predict the radiographic onset of KOA (21) and to discriminate knees with OA from non-OA knees (23); consequently, they have been employed as endpoints in clinical trials on disease-modifying OA drugs (24). Accordingly, in this study, we hypothesized that bone morphology changes mediate the association between MME and KOA radiographic progression. To eliminate any potential influence of these bone morphological changes on MME, we maintained the severity of MME across the control group and case group. Three-dimensional bone morphology changes may serve as a valuable indicator for assessing the necessity of early intervention of KOA in cases of MME.
Specifically, the purpose of our study was to evaluate the potential mediating effect of bone morphology at baseline and the changes in bone morphology from baseline to 24 months of follow-up on the association between MME and KOA progression. Additionally, we sought to clarify the relationship between MME and bone morphological changes during KOA progression. Mediation analysis was used to investigate the causal sequence through which MME causes changes in 3D bone morphology and through which changes in 3D bone morphology cause KOA progression. Finally, we assessed the total mediating effect of the changes in 3D bone morphology on the association between MME and the progression of KOA from baseline to 24, 36, and 48 months. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1056/rc).
Methods
Study participants
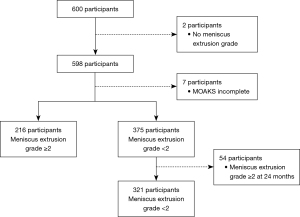
The Osteoarthritis Initiative (OAI) study included men and women aged 45–79 years with or at risk of symptomatic knee OA (25). The Foundation for the National Institute of Health (FNIH) Osteoarthritis Biomarkers Consortium is a subset of the OAI database that is a publicly available and includes the demographics, health history, subjective knee symptoms, biochemical biomarkers and imaging measurements of knee Kellgren-Lawrence grade (KLG), joint space, bone morphology, and cartilage injury from a series of 600 prospectively enrolled participants (26) (Appendix 1). The participants of our study were obtained from the FNIH Osteoarthritis Biomarkers Consortium database. Figure 1 shows the representative knee magnetic resonance (MR) images with different severities of MME. The inclusion criterion for the control group for this study was participants with MME grade <2 at baseline and at the 24-month follow-up according to MRI (Figure 2). Meanwhile, the exclusion criteria were as follows: (I) no evaluation of ME and (II) no complete MRI Osteoarthritis Knee Score (MOAKS) available at baseline (Figure 2). The MOAKS of participants in this study can be freely obtained from the FNIH database online (https://nda.nih.gov/oai). The clinical variables of the participants at baseline included age, sex, trauma history, body mass index (BMI), the minimum joint space width (minJSW) of the medial tibiofemoral joint compartment, KLG, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score, and WOMAC disability score (16,26). Radiographic progression was defined as a loss in medial minJSW of ≥0.7 mm from baseline to 24, 36, or 48 months (Figure 3). This threshold was established based on the distribution of changes in minJSW observed over a 12-month period in normal knees from healthy reference participants in the OAI study. It was estimated to correspond to a 10% probability of change attributable to measurement error (27). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Medical Ethics Review Board of the University of California, San Francisco (approval No. 10-00532), and the four clinical centers of Osteoarthritis Initiative Project recognized this study as Health Insurance Portability and Accountability Act (HIPAA)-compliant. All participants provided written informed consent.

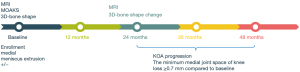
MR image acquisition and quantitative measurement
MRI was performed using 3.0-Tesla MRI systems (Trio; Siemens Healthineers, Erlangen, Germany). The pulse sequence protocol included 2D coronal intermediate-weighted (IW) turbo spin echo, 3D sagittal dual-echo steady-state (DESS), coronal and axial multiplane recombination, and sagittal IW fatty saturated turbo spin echo (TSE) sequences (28). MOAKS is a semiquantitative scoring tool that was developed from the Whole Organ Magnetic Resonance Imaging Score (WORMS) and Boston Leeds Osteoarthritis Knee Score (BLOKS) measures. MOAKS has been shown to have very good to excellent reliability (6). MOAKS is used to evaluate the cartilage, bone marrow, meniscus, osteophytes, synovitis, edema, and the cruciate ligament of patients with KOA (6). The acquired images were assessed through MOAKS at baseline and 24 months and consisted of items including the meniscus injury score, whether the meniscus was extruded, whether the posterior root of the meniscus was torn, and whether the bone marrow was injured (6,29). There were a total of 12 parameters for bone morphology, including the area of the medial/lateral femur, medial/lateral tibia, medial/lateral femoral trochlea, medial/lateral patella, and notch (intercondylar spine); the shape vectors of the tibia, femur, and patella at baseline; and the changes in bone morphology from baseline to 24 months.
The measurement of 3D bone morphology has been previously described (16). The bone morphology on 3D MRI was reconstructed using active appearance models (AAMs). AAMs are a form of the SSM method and learn the changes in object shape and gray texture from the training set and encode shape and appearance as principal components. AAMs of the femur, tibia, and patella were constructed by using a training set of 96 knee MRI DESS water-excitation sequences including various KLGs. In the process of model training, the principal components are added to make the model account for 98% of variance in the shape data from the training set. In order to achieve this goal, the final femoral shape included 69 principal components, tibia shape included 66, and patella shape included 59. Using linear discriminant analysis (LDA) to identify the principal components in this shape space could distinguish vectors of different groups (such as non-OA and OA). The distance along the vector was normalized by treating the mean non-OA shape as –1 and the mean OA shape as +1. Extreme examples of OA and non-OA shape were created by finding the points at –3 standard deviation (SD) of the OA and +3 SD of the non-OA groups on a line passing through the means of the OA and non-OA groups and were examined using a 3D viewer (Imorphics Ltd., Manchester, UK). AAMS analysis was applied to generate bone morphology, which was represented by the principal components from 3D MR images of the participants in this study. The femur, tibia, and principal components were sequentially projected onto LDA vectors, and the vectors are obtained based on the distance from the mean OA or mean non-OA (21,26) (Figure S1).
Statistical analysis
Continuous variables are expressed as the mean ± SD, while categorical variables are expressed as numbers and percentages. The demographic and clinical characteristics of the participants at baseline were compared using the independent samples t-test or the Mann-Whitney test and Chi-squared test. Logistic regression was used to evaluate the risk factors for KOA progression (no radiographic progression =0, radiographic progression =1), adjusted for age, sex, BMI, KLG, injury, minJSW, WOMAC pain score, and WOMAC disability score (16,26). Responsiveness was measured using the standardized response mean (SRM; SRM = mean change/standard deviation of change) of 3D bone morphology. The R “mediation” package was employed to assess the simple mediation effects of each bone morphology on the association between MME and KOA progression. The R “lavaan” package was employed to assess the multiple mediating effects of bone morphology on the association between MME and KOA progression. Statistical significance was set at a two-sided P value <0.05. Data analysis was conducted via SPSS version 22.0 (IBM Corp., Armonk, NY, USA), Empower (R) (X & Y Solutions, Inc., Boston, MA, USA; http://www.empowerstats.com), and R 4.2.2 (http://www.Rproject.org).
Results
Participants characteristics and MOAKS analysis at baseline
A total of 537 of the initial 600 participants were included in the study, with 321 and 216 participants in the control and case groups, respectively (Figure 2). The baseline characteristics are provided in Table 1. Participants in the control and case cohort were matched in terms of their baseline characteristics including BMI [mean (SD): 30.48 (4.76) vs. 30.70 (4.73) kg/m2; P=0.802] and female gender [n (%): 196 (61.06%) vs. 120 (55.56%); P=0.204]. In participants with MME, there was a higher WOMAC pain score [mean (SD): 9.79 (11.27) vs. 7.93 (10.29); P=0.021] and WOMAC disability score [mean (SD): 14.05 (16.85) vs. 10.93 (14.79); P=0.037], higher prevalence of knee injury (42.59% vs. 31.46%; P=0.005), higher KLG (KLG 3; 64.81% vs. 20.87%; P<0.001), and higher minJSW [mean (SD): 3.13 (0.94) vs. 4.20 (1.09) mm; P<0.001]. In the analysis of the baseline MOAKS, participants with MME had a greater number of subregions with at least one focal abnormality in the assessment of cartilage surface area [mean (SD): 5.90 (2.22) vs. 4.15 (2.09); P<0.001], greater number of subregions with at least one lesion [mean (SD): 1.58 (0.59) vs. 1.39 (0.49); P<0.001], and greater number of osteophytes [mean (SD): 7.72 (3.37) vs. 5.88 (3.72); P<0.001]. Participants in the case group showed severe radiological OA characteristics (Table S1).
Table 1
| Parameter | Control (n=321) | Case (n=216) | P value |
|---|---|---|---|
| BMI (kg/m2) | 30.48±4.76 | 30.70±4.73 | 0.802 |
| Age (years) | 60.45±8.70 | 63.33±9.11 | <0.001 |
| WOMAC pain score | 7.93±10.29 | 9.79±11.27 | 0.021 |
| WOMAC disability score | 10.93±14.79 | 14.05±16.85 | 0.037 |
| minJSW (mm) | 4.20±1.09 | 3.13±0.94 | <0.001 |
| KLG | <0.001 | ||
| 1 | 48 (14.33) | 12 (5.56) | |
| 2 | 208 (64.80) | 64 (29.63) | |
| 3 | 67 (20.87) | 140 (64.81) | |
| Knee injury | 101 (31.46) | 92 (42.59) | 0.005 |
| Female | 196 (61.06) | 120 (55.56) | 0.204 |
| Right knee | 182 (56.70) | 109 (50.46) | 0.155 |
Data are presented as the mean ± standard deviation or n (%). BMI, body mass index; KLG, Kellgren-Lawrence grade; minJSW, minimum joint space width; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Association between MME and KOA progression
During the follow-up period, KOA progression in the case group was significantly higher than that in the control group (61.11% vs. 34.89%; P<0.001). Univariate regression analysis adjusted for clinical factors showed that MME at baseline was significantly corelated with KOA progression [odds ratio (OR) =2.60; 95% confidence interval (CI): 1.72–3.93]. Multivariate regression analysis also showed that MME correlated with KOA progression (OR =1.61; 95% CI: 1.01–2.57) (Tables S2,S3).
Association between bone morphological changes and KOA progression
Adjusted logistic regression analysis showed that the medial patellar area (OR =1.29; 95% CI: 1.02–1.64), femoral vector (OR =1.30; 95% CI: 1.05–1.61), tibial vector (OR =1.31; 95% CI: 1.07–1.59), and patellar vector (OR =1.31, 95% CI:1.08–1.59) were correlated with the progression of KOA at baseline. Only changes in the lateral femoral area (OR =1.17; 95% CI: 0.98–1.40) and patellar vector (OR =1.16, 95% CI: 0.97–1.40) were not corelated with KOA progression, while the other bone morphological changes were (OR =1.28–2.15) (Table 2).
Table 2
| Bone morphology | Baseline | 24 months | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Medial area | |||||||
| Femur | 1.09 | 0.83–1.45 | 0.531 | 2.15 | 1.70–2.71 | <0.001 | |
| Tibia | 1.05 | 0.77–1.94 | 0.771 | 1.57 | 1.29–1.91 | <0.001 | |
| Patella | 1.29 | 1.02–1.64 | 0.036 | 1.28 | 1.06–1.55 | 0.009 | |
| Trochlea | 1.05 | 0.80–1.38 | 0.708 | 1.79 | 1.44–2.20 | <0.001 | |
| Lateral area | |||||||
| Femur | 1.08 | 0.80–1.45 | 0.612 | 1.17 | 0.98–1.40 | 0.090 | |
| Tibia | 0.98 | 0.73–1.31 | 0.869 | 1.64 | 1.34–1.91 | <0.001 | |
| Patella | 1.26 | 0.99–1.60 | 0.061 | 1.37 | 1.13–1.67 | <0.001 | |
| Trochlea | 0.97 | 0.74–1.27 | 0.820 | 1.45 | 1.20–1.75 | <0.001 | |
| Notch | 1.02 | 0.81–1.30 | 0.859 | 1.49 | 1.23–1.81 | <0.001 | |
| Shape (vector) | |||||||
| Femur | 1.30 | 1.05–1.61 | 0.015 | 2.11 | 1.69–2.64 | <0.001 | |
| Tibia | 1.31 | 1.07–1.59 | 0.008 | 1.58 | 1.30–1.92 | <0.001 | |
| Patella | 1.31 | 1.08–1.59 | 0.006 | 1.16 | 0.97–1.40 | 0.106 | |
Bone morphology includes the surface areas of the medial/lateral tibia, medial/lateral femur, medial/lateral patella, medial/lateral trochlea, and notch; bone shape vector includes the femur, tibia, and patella. OR adjusted for age, sex, BMI, history of injury, minJSW, KLG, WOMAC pain score, and WOMAC disability score. BMI, body mass index; CI, confidence interval; KLG, Kellgren-Lawrence grade; KOA, knee osteoarthritis; minJSW, minimum joint space width; OR, odds ratio; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Association between MME and bone morphological changes
At baseline, except for the area of the lateral trochlea and notch, there was no statistical difference between the case group and the control group. The bone surface area of the case group was significantly higher than that of the control group, while the shape vector of the case group was significantly lower than that of the control group (P<0.05). At 24-month follow-up, except for the changes in lateral/medial patella area and lateral tibia area, all bone morphological changes in the case group were higher than those in the control group (P<0.05). The changes in the femoral vector and tibial vector observed in the case group were significantly lower than those recorded in the control group. (Table 3). The lower the vector value was, the more pronounced the severity of KOA (Figure S1). The SRMs of bone morphology in the case group were higher than those in the control group.
Table 3
| Bone morphology | Baseline | Change at 24 months | SRM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Case | P value | Control | Case | P value | Control vs. case | |||
| Medial area (mm2) | |||||||||
| Femur | 2,400.14±365.57 | 2,504.71±416.96 | 0.004 | 19.39±32.01 | 40.49±45.69 | <0.001 | 0.61 vs. 0.89 | ||
| Tibia | 1,164.04±187.53 | 1,215.82±207.01 | 0.003 | 10.39±18.17 | 19.06±22.51 | <0.001 | 0.57 vs. 0.85 | ||
| Patella | 541.49±85.03 | 563.13±94.92 | 0.019 | 4.72±14.82 | 6.83±17.33 | 0.133 | 0.32 vs. 0.39 | ||
| Trochlea | 682.38±102.06 | 706.44±114.12 | 0.014 | 7.36±9.98 | 13.07±14.77 | <0.001 | 0.74 vs. 0.88 | ||
| Lateral area (mm2) | |||||||||
| Femur | 1,710.82±292.37 | 1,772.65±320.27 | 0.034 | 3.86±31.56 | 10.46±38.59 | 0.031 | 0.12 vs. 0.27 | ||
| Tibia | 915.61±146.07 | 945.70±162.24 | 0.047 | 7.19±13.03 | 8.86±15.60 | 0.180 | 0.55 vs. 0.57 | ||
| Patella | 692.17±113.39 | 719.34±123.68 | 0.024 | 6.41±19.71 | 9.09±20.50 | 0.128 | 0.33 vs. 0.44 | ||
| Trochlea | 1,472.09±202.53 | 1,509.91±241.41 | 0.131 | 6.72±15.90 | 9.44±16.97 | 0.038 | 0.42 vs. 0.56 | ||
| Notch area (mm2) | 1,261.64±183.55 | 1,287.07±199.42 | 0.206 | 7.30±20.41 | 17.06±26.40 | <0.001 | 0.36 vs. 0.65 | ||
| Shape (vector) | |||||||||
| Femur | 0.12±1.15 | −0.55±1.24 | <0.001 | −0.14±0.22 | −0.27±0.31 | <0.001 | 0.64 vs. 0.87 | ||
| Tibia | 0.13±1.07 | −0.68±1.24 | <0.001 | −0.18±0.38 | −0.27±0.43 | 0.013 | 0.47 vs. 0.63 | ||
| Patella | −0.05±1.66 | −0.59±1.66 | <0.001 | −0.16±0.64 | −0.24±0.67 | 0.179 | 0.25 vs. 0.36 | ||
Data are presented as the mean ± standard deviation. KOA, knee osteoarthritis; SRM, standardized response mean (SRM = mean change/standard deviation of change).
The mediation effect of 3D bone morphology between MME and KOA progression
The estimation of mediating effects showed that bone morphology at baseline did not mediate the effect of MME on KOA progression (Table S4). The mediating proportion of the change in the medial femur area was 22.53% (95% CI: 10.30–24.66%), that in the medial tibial area was 13.02% (95% CI: 4.39–28.67%), that in the medial femoral trochlea region was 18.55% (95% CI: 7.85–36.49%), that in the notch area was 10.20% (95% CI: 2.06–23.25%), that in the in the femoral bone shape vector was 26.67% (95% CI: 9.82–42.90%), and that in the tibial bone shape vector was 8.59% (95% CI: –0.33% to 21.37%); however, the P value was 0.054 (Table 4 and Table S4). Multiple mediation analyses indicated that changes in bone morphology accounted for 31.2% of the total effect on the progression of KOA.
Table 4
| Bone morphology | Proportion mediated (%) | P value | |
|---|---|---|---|
| Estimate | 95% CI | ||
| Medial femur (area) | 22.53 | 10.30 to 24.66 | <0.001 |
| Medial tibia (area) | 13.02 | 4.39 to 28.67 | 0.004 |
| Medial patella (area) | 3.81 | −0.82 to 12.54 | 0.126 |
| Medial trochlea (area) | 18.55 | 7.85 to 36.49 | <0.001 |
| Lateral femur (area) | 1.75 | −1.23 to 8.18 | 0.318 |
| Lateral tibia (area) | 6.57 | −2.75 to 16.87 | 0.144 |
| Lateral patella (area) | 3.89 | −1.54 to 13.16 | 0.172 |
| Lateral trochlea (area) | 4.37 | −2.95 to 14.22 | 0.270 |
| Notch (area) | 10.20 | 2.06 to 23.25 | 0.004 |
| Femur (vector) | 26.67 | 9.82 to 42.90 | <0.001 |
| Tibia (vector) | 8.59 | −0.33 to 21.37 | 0.054 |
| Patella (vector) | 2.08 | −1.23 to 9.36 | 0.260 |
Proportion mediated (%) = indirect effect/total effect ×100%. CI, confidence interval; KOA, knee osteoarthritis; MME, medial meniscal extrusion.
Discussion
After adjusting for potential confounding factors, we found that MME was associated with the radiological progression of KOAs. Participants in the case group had more severe OA imaging features. Moreover, multiple mediation analysis demonstrated that bone morphology changes mediated approximately one-third of the association between MME and the radiological progression of KOA. Therefore, our findings suggest that MME associated KOA progression is partially associated with the changes in bone morphology.
Studies have demonstrated that MME is associated with OA progression. MME is an important risk factor for early KOP and has become a diagnostic tool for predicting early OA (30,31). Lee et al. reported MME to be correlated with OA severity (32). In our study, analysis of KLG and MOAKS at baseline showed that KLG, osteophyte number, bone marrow lesions, meniscus score, and cartilage injury were higher in the case group than in the control group. Our results further confirmed that MME is correlated with KOA severity.
In this study, the radiographic progression rate of KOA in the case group was 61.11%. The adjusted multivariate regression analysis confirmed that MME was a risk factor for the progression of KOA. The mechanism through which MME accelerates KOA progression is complex. MME not only accelerates cartilage injury and subchondral bone marrow lesions but also accelerates the formation of osteophytes and expansion of bone (17,18,33). Changes in bone morphology are considered an early feature of OA pathogenesis, which occurs before radiological changes in KOA (21). Compared with radiological assessment of KOA, bone morphology changes over time are more sensitive (21,34). Bone morphology changes are mainly corelated with the radiographic progression of KOA and weakly correlated with the longitudinal progression of pain (16,35). We calculated changes in bone morphology in the case group after 24 months of follow-up and found that bone morphological changes in the case group were higher than those in the control group. In this study, except for the lateral femoral area and patellar vector, all bone morphological changes were associated with the progression of KO. The odds ratio for the area and vector changes of the medial femur and medial tibia were higher than those of the lateral and patellar regions, and the correlation between bone biomarkers (area and vector) for the medial femur and KOA progression was stronger compared to those for the medial tibia. A few studies have examined the relationship between MME and the changes in bone morphology. MME may simulate a condition similar to complete meniscectomy (36). The loss of meniscus function leads to an increase in biomechanical stress in the subchondral bone (37). This change in biomechanical load may lead to bone remodeling and changes in bone shape (18). In their study, Wang et al. found that MME indicated an increase in subchondral bone injury and tibial plateau bone expansion in KO and that subchondral bone changes were an early consequence of MME (17).
According to other studies, MME is associated with increased tibiofemoral cartilage volume loss (38,39). Changes in subchondral bone structure during OA may also respond to endochondral osteogenesis. Endochondral ossifications can lead to metaphyseal expansion and shape alteration (40). MME accelerates cartilage damage and then promotes endochondral ossification to generate osteophytes, which may be another reason for the bone morphological changes caused by MME. Using T2 mapping, Hada et al. found that medial tibial osteophytes were closely related to MME in patients with early OA (15). The width of the osteophyte was closely related to the MME, and the absolute value of the correlation coefficient was 0.76 (41). Other research suggests that MME may be caused by the stretching and displacement of the meniscotibial ligament (coronary ligament) being compressed by osteophytes (15,19). However, most of the related studies were cross-sectional in design, so no direct evidence indicating that osteophytes cause MME has been obtained. The prevalence of osteophytes in patients with early-stage KOA and the association between osteophyte formation and MME remain unclear (19). MME seems to a structural risk factor for the development of tibiofemoral osteophytes (18). Osteophytes only partially explain the increase in bone area (42); therefore, further research is needed regarding the relationship between MME and bone morphology.
In our longitudinal study, the grading of MME in our case group and control group participants remained unchanged, and the relationship between MME and bone morphology during the progression of KOA was analyzed. Our study further confirmed the association between MME and bone morphological changes during KOA progression. To the best of our knowledge, this study is the first to examine whether changes in bone morphology mediate the association between MME and KOA progression. In this study, a simple mediation effect analysis showed that bone morphology changes were significantly different between MME and KOA progression and that bone morphology at baseline had no moderating effect between MME and KOA progression. Multiple mediation effects analysis showed that changes in bone morphology accounted for 31.2% of the total effect of KOA progression. The total change in bone morphology was a strong mediator of KOA progression.
This involved several limitations which should be addressed. First, the definition of MME in this study was based on a meniscus displacement of ≥3 mm, which does not consider the differences in knee joint size. Therefore, the proportion of meniscus displacements was not considered. Define extrusion by dividing the displacement of the outer edge of the tibial plateau by the percentage of meniscus width may be more sensitive (43). Second, MRI of the knee was performed under standard non–load-bearing conditions, meaning that the load-bearing position, which affects the degree of MME (44) and may exert a certain impact on the grouping of meniscus displacement, was not considered. Third, the 3D bone shape in this study was the shape change of the entire bone, but changes in local areas may be more sensitive (16).
Conclusions
Changes in bone morphology partially mediate the association between MME and KOA progression. Our findings indicate that MME contributes to KOA progression by accelerating 3D bone morphological changes.
Acknowledgments
Data in this manuscript were obtained and analyzed from controlled access datasets distributed by OAI, a data repository housed within the NIMH Data Archive (NDA). OAI is a collaborative informatics system created by the National Institute of Mental Health and the National Institute of Arthritis, Musculoskeletal, and Skin Diseases (NIAMS) to provide a worldwide resource to accelerate the pace of biomarker identification, scientific investigation, and OA drug development. This research does not represent the opinions or views of the OAI investigators, the National Institutes of Health, or private funding partners.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1056/rc
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1056/coif). C.D. and Y.S. report that this work was supported by Liaoning Provincial Natural Science Foundation Joint Fund (No. 2023011988-JH3/4600). X.L. reports this work was supported by The Basic Research Program of Liaoning Province (No. 2022JH2/101300030). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Medical Ethics Review Board of the University of California, San Francisco (approval No. 10-00532) and the four clinical centers of osteoarthritis initiative project recognized the project as HIPAA-compliant. All participants provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leifer VP, Katz JN, Losina E. The burden of OA-health services and economics. Osteoarthritis Cartilage 2022;30:10-6. [PubMed]
- Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA, Hoy D, Ashrafi-Asgarabad A, Sepidarkish M, Almasi-Hashiani A, Collins G, Kaufman J, Qorbani M, Moradi-Lakeh M, Woolf AD, Guillemin F, March L, Cross M. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis 2020;79:819-28. [PubMed]
- Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019;393:1745-59. [PubMed]
- Seedhom BB, Dowson D, Wright V. Ann Rheum Dis 1974;33:111. [PubMed]
- Rai MF, Brophy RH, Rosen V. Molecular biology of meniscus pathology: Lessons learned from translational studies and mouse models. J Orthop Res 2020;38:1895-904. [PubMed]
- Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, Roemer FW. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage 2011;19:990-1002. [PubMed]
- Swamy N, Wadhwa V, Bajaj G, Chhabra A, Pandey T. Medial meniscal extrusion: Detection, evaluation and clinical implications. Eur J Radiol 2018;102:115-24. [PubMed]
- Papalia R, Papalia G, Russo F, Diaz LA, Bressi F, Sterzi S, Denaro V. Meniscal extrusion as booster of osteoarthritis. J Biol Regul Homeost Agents 2017;31:33-44. [PubMed]
- Chiba D, Sasaki E, Ota S, Maeda S, Sugiyama D, Nakaji S, Ishibashi Y. US detection of medial meniscus extrusion can predict the risk of developing radiographic knee osteoarthritis: a 5-year cohort study. Eur Radiol 2020;30:3996-4004. [Crossref] [PubMed]
- van der Voet JA, Runhaar J, van der Plas P, Vroegindeweij D, Oei EH, Bierma-Zeinstra SMA. Baseline meniscal extrusion associated with incident knee osteoarthritis after 30 months in overweight and obese women. Osteoarthritis Cartilage 2017;25:1299-303. [Crossref] [PubMed]
- Xu D, van der Voet J, Waarsing JH, Oei EH, Klein S, Englund M, Zhang F, Bierma-Zeinstra S, Runhaar J. Are changes in meniscus volume and extrusion associated to knee osteoarthritis development? A structural equation model. Osteoarthritis Cartilage 2021;29:1426-31. [Crossref] [PubMed]
- Yoshizuka H, Taniguchi T, Fukuta K, Mitsutake T, Honda S. Decrease in medial meniscal extrusion after physical therapy to improve knee pain and range of motion in patients with knee osteoarthritis: A retrospective study. PLoS One 2022;17:e0277628. [Crossref] [PubMed]
- Bloecker K, Wirth W, Guermazi A, Hunter DJ, Resch H, Hochreiter J, Eckstein F. Relationship Between Medial Meniscal Extrusion and Cartilage Loss in Specific Femorotibial Subregions: Data From the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2015;67:1545-52. [Crossref] [PubMed]
- Svensson F, Felson DT, Zhang F, Guermazi A, Roemer FW, Niu J, Aliabadi P, Neogi T, Englund M. Meniscal body extrusion and cartilage coverage in middle-aged and elderly without radiographic knee osteoarthritis. Eur Radiol 2019;29:1848-54. [Crossref] [PubMed]
- Hada S, Ishijima M, Kaneko H, Kinoshita M, Liu L, Sadatsuki R, Futami I, Yusup A, Takamura T, Arita H, Shiozawa J, Aoki T, Takazawa Y, Ikeda H, Aoki S, Kurosawa H, Okada Y, Kaneko K. Association of medial meniscal extrusion with medial tibial osteophyte distance detected by T2 mapping MRI in patients with early-stage knee osteoarthritis. Arthritis Res Ther 2017;19:201. [Crossref] [PubMed]
- Hunter D, Nevitt M, Lynch J, Kraus VB, Katz JN, Collins JE, Bowes M, Guermazi A, Roemer FW, Losina E. FNIH OA Biomarkers Consortium. Longitudinal validation of periarticular bone area and 3D shape as biomarkers for knee OA progression? Data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis 2016;75:1607-14. [Crossref] [PubMed]
- Wang Y, Wluka AE, Pelletier JP, Martel-Pelletier J, Abram F, Ding C, Cicuttini FM. Meniscal extrusion predicts increases in subchondral bone marrow lesions and bone cysts and expansion of subchondral bone in osteoarthritic knees. Rheumatology (Oxford) 2010;49:997-1004. [Crossref] [PubMed]
- Snoeker BAM, Ishijima M, Kumm J, Zhang F, Turkiewicz AT, Englund M. Are structural abnormalities on knee MRI associated with osteophyte development? Data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2021;29:1701-8. [Crossref] [PubMed]
- Negishi Y, Kaneko H, Aoki T, Liu L, Adili A, Arita H, et al. Medial meniscus extrusion is invariably observed and consistent with tibial osteophyte width in elderly populations: The Bunkyo Health Study. Sci Rep 2023;13:22805. [Crossref] [PubMed]
- Reichenbach S, Guermazi A, Niu J, Neogi T, Hunter DJ, Roemer FW, McLennan CE, Hernandez-Molina G, Felson DT. Prevalence of bone attrition on knee radiographs and MRI in a community-based cohort. Osteoarthritis Cartilage 2008;16:1005-10. [Crossref] [PubMed]
- Neogi T, Bowes MA, Niu J, De Souza KM, Vincent GR, Goggins J, Zhang Y, Felson DT. Magnetic resonance imaging-based three-dimensional bone shape of the knee predicts onset of knee osteoarthritis: data from the osteoarthritis initiative. Arthritis Rheum 2013;65:2048-58. [Crossref] [PubMed]
- Heimann T, Meinzer HP. Statistical shape models for 3D medical image segmentation: a review. Med Image Anal 2009;13:543-63. [Crossref] [PubMed]
- Barr AJ, Dube B, Hensor EM, Kingsbury SR, Peat G, Bowes MA, Sharples LD, Conaghan PG. The relationship between three-dimensional knee MRI bone shape and total knee replacement-a case control study: data from the Osteoarthritis Initiative. Rheumatology (Oxford) 2016;55:1585-93. [Crossref] [PubMed]
- McGuire D, Bowes M, Brett A, Segal NA, Miller M, Rosen D, Kumagai Y. Study TPX-100-5: intra-articular TPX-100 significantly delays pathological bone shape change and stabilizes cartilage in moderate to severe bilateral knee OA. Arthritis Res Ther 2021;23:242. [Crossref] [PubMed]
- Bacon K, LaValley MP, Jafarzadeh SR, Felson D. Does cartilage loss cause pain in osteoarthritis and if so, how much? Ann Rheum Dis 2020;79:1105-10. [Crossref] [PubMed]
- Hunter DJ, Deveza LA, Collins JE, Losina E, Katz JN, Nevitt MC, Lynch JA, Roemer FW, Guermazi A, Bowes MA, Dam EB, Eckstein F, Kwoh CK, Hoffmann S, Kraus VB. Multivariable Modeling of Biomarker Data From the Phase I Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Care Res (Hoboken) 2022;74:1142-53. [Crossref] [PubMed]
- Sharma K, Eckstein F, Maschek S, Roth M, Hunter DJ, Wirth W. Association of quantitative measures of medial meniscal extrusion with structural and symptomatic knee osteoarthritis progression - Data from the OAI FNIH biomarker study. Osteoarthritis Cartilage 2023;31:1396-404. [Crossref] [PubMed]
- Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage 2008;16:1433-41. [Crossref] [PubMed]
- Roemer FW, Guermazi A, Collins JE, Losina E, Nevitt MC, Lynch JA, Katz JN, Kwoh CK, Kraus VB, Hunter DJ. Semi-quantitative MRI biomarkers of knee osteoarthritis progression in the FNIH biomarkers consortium cohort - Methodologic aspects and definition of change. BMC Musculoskelet Disord 2016;17:466. [PubMed]
- Fong FJY, Ong BWL, Lee YHD. Medial Meniscal Extrusion in Patients With Medial Meniscus Root Tears: A Systematic Review and Meta-analysis. Orthop J Sports Med 2023;11:23259671231151698. [Crossref] [PubMed]
- Teichtahl AJ, Cicuttini FM, Abram F, Wang Y, Pelletier JP, Dodin P, Martel-Pelletier J. Meniscal extrusion and bone marrow lesions are associated with incident and progressive knee osteoarthritis. Osteoarthritis Cartilage 2017;25:1076-83. [PubMed]
- Lee DH, Lee BS, Kim JM, Yang KS, Cha EJ, Park JH, Bin SI. Predictors of degenerative medial meniscus extrusion: radial component and knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2011;19:222-9. [PubMed]
- Zhang F, Bierma-Zeinstra SM, Oei EHG, Turkiewicz A, Englund M, Runhaar J. The association between meniscal body extrusion and the development/enlargement of bone marrow lesions on knee MRI in overweight and obese women. Osteoarthr Cartil Open 2020;1:100015. [PubMed]
- Khokhar K, Conaghan PG. Bone in osteoarthritis: imaging and interventions. Curr Opin Rheumatol 2022;34:73-8. [PubMed]
- Ghouri A, Muzumdar S, Barr AJ, Robinson E, Murdoch C, Kingsbury SR, Conaghan PG. The relationship between meniscal pathologies, cartilage loss, joint replacement and pain in knee osteoarthritis: a systematic review. Osteoarthritis Cartilage 2022;30:1287-327. [PubMed]
- Gale DR, Chaisson CE, Totterman SM, Schwartz RK, Gale ME, Felson D. Meniscal subluxation: association with osteoarthritis and joint space narrowing. Osteoarthritis Cartilage 1999;7:526-32. [PubMed]
- Ishii Y, Ishikawa M, Nakashima Y, Hashizume T, Okamoto S, Kamei G, Okada K, Takagi K, Takahashi M, Adachi N. Unique patterns of medial meniscus extrusion during walking and its association with limb kinematics in patients with knee osteoarthritis. Sci Rep 2023;13:12513. [PubMed]
- Berthiaume MJ, Raynauld JP, Martel-Pelletier J, Labonté F, Beaudoin G, Bloch DA, Choquette D, Haraoui B, Altman RD, Hochberg M, Meyer JM, Cline GA, Pelletier JP. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis 2005;64:556-63. [PubMed]
- Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, Beary JF, Cline GA, Meyer JM, Martel-Pelletier J. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther 2007;9:R74. [PubMed]
- Cox LG, van Donkelaar CC, van Rietbergen B, Emans PJ, Ito K. Alterations to the subchondral bone architecture during osteoarthritis: bone adaptation vs endochondral bone formation. Osteoarthritis Cartilage 2013;21:331-8. [PubMed]
- Sekiya I, Sasaki S, Miura Y, Aoki H, Katano H, Okanouchi N, Tomita M, Masumoto J, Koga H, Ozeki N. Medial Tibial Osteophyte Width Strongly Reflects Medial Meniscus Extrusion Distance and Medial Joint Space Width Moderately Reflects Cartilage Thickness in Knee Radiographs. J Magn Reson Imaging 2022;56:824-34. [PubMed]
- Brandt KD, Dieppe P, Radin EL. Etiopathogenesis of osteoarthritis. Rheum Dis Clin North Am 2008;34:531-59. [PubMed]
- Gajjar SM, Solanki KP, Shanmugasundaram S, Kambhampati SBS. Meniscal Extrusion: A Narrative Review. Orthop J Sports Med 2021;9:23259671211043797. [PubMed]
- Chiba D, Sasaki T, Ishibashi Y. Greater medial meniscus extrusion seen on ultrasonography indicates the risk of MRI-detected complete medial meniscus posterior root tear in a Japanese population with knee pain. Sci Rep 2022;12:4756. [Crossref] [PubMed]


