
Value of multi-modal ultrasound in evaluation of the accessory renal artery
Introduction
The accessory renal artery (ARA) is a functional terminal branch of the kidney, characterized by a more tortuous and slender anatomical structure compared to the typical renal artery. The ARA represents the most prevalent and clinically significant variant of the renal artery. It has been reported that the incidence rates of ARA range from 4% to 61.5% (1,2). Moreover, several studies have underscored the significance of the evaluation of the presence of the ARA in renovascular hypertension related to isolated ARA stenosis and a range of renal vascular-related surgical procedures (3-6). Consequently, the precise assessment of the ARA utilizing non-invasive, safe, and convenient imaging modalities is crucial for the effective intervention of renal vascular-related conditions.
Digital subtraction angiography (DSA) is considered the preferred method for diagnosing the ARA due to its high accuracy, but its invasiveness and radiation dosage may limit its clinical applicability (7). Meanwhile, computed tomography angiography (CTA) has emerged as a non-invasive alternative for evaluating the ARA (8-12). However, CTA carries a risk of radiation exposure and requires exogenous contrast agents that are potentially nephrotoxic. Magnetic resonance angiography has the benefits of no ionizing radiation and high repeatability. However, the use of gadolinium-based contrast agent in contrast-enhanced magnetic resonance angiography poses a risk of nephrogenic systemic fibrosis (13). With technological innovations, the use of non-contrast-enhanced magnetic resonance angiography for assessing renal vessels is also under investigation (14). Nevertheless, the identification of novel non-invasive, secure, and effective techniques for assessing the ARA holds significant importance. Conventional ultrasound is the preferred modality for screening the ARA, typically employing two-dimensional grayscale ultrasound, color Doppler flow imaging (CDFI), and pulsed wave Doppler modes. Nonetheless, the ARA’s small diameter and deep positioning often pose challenges in achieving clear visualization, leading to a reduced detection rate due to interference from intestinal gas (15). The utilization of microbubble contrast agents in renal artery contrast-enhanced ultrasound (CEUS) amplifies the backscattered echoes, facilitating the visualization of blood flow within microvessels (16). Additionally, CEUS is particularly advantageous for individuals with allergies to iodinated contrast media or renal insufficiency (17). In recent years, our research team has found that the integration of modified coronal section imaging with renal artery conventional ultrasound and CEUS examinations enables the dynamic and precise visualization of the complete trajectory of the renal artery, along with the ability to assess the position, quantity, and diameter of renal arteries (18,19). Multi-modal ultrasound, which combines conventional ultrasound with CEUS, could be a potential examination method for evaluating the ARA.
This study aimed to evaluate the diagnostic accuracy of multi-modal ultrasound in detecting ARA, in comparison to CTA, and to analyze the factors contributing to misdiagnosis and missed diagnosis. We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1117/rc).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Beijing Hospital (No. 2018BJYYEC-043-02). The study was registered in the China Clinical Trial Registration Center (No. ChiCTR1800016252). As this study was retrospective, the requirement of informed consent was waived. Data collection (clinical and imaging information) was conducted on patients who underwent renal artery conventional ultrasound examination and CEUS examinations between August 2019 and November 2023 in Beijing Hospital. The inclusion criteria were as follows: (I) patients who underwent renal artery multi-modal examination and CTA examination; (II) patients who underwent renal artery multi-modal ultrasound examination prior to CTA examination; and (III) patients who were diagnosed with the ARA based on CTA examination. Additionally, the exclusion criteria were as follows: (I) patients with incomplete clinical data; (II) patients with poor quality ultrasound images. Ultimately, a total of 73 patients (144 kidneys) were included in the study, with two patients having undergone nephrectomy for a single kidney. The selection process for the study patients based on the inclusion and exclusion criteria is displayed in Figure 1.
Instruments
The CTA examination utilized the Toshiba 640-Slice Spiral CT Scanner (Toshiba, Tokyo, Japan) in conjunction with iodinated contrast media. Ultrasound contrast imaging: This study utilized three ultrasound instruments with specific ultrasound contrast imaging parameter settings. The instruments included the Samsung RS80A Ultrasound Diagnostic Instrument (Samsung, Seoul, Korea) with a CA1-7 convex array probe, operating at a frequency range of 2–5 MHz and machine parameters set to a mechanical index (MI) of 0.079 and gain of 56 dB. A Canon Aplio i800 Ultrasound Diagnostic Instrument (Canon, Tochigi, Japan) was also used with an i8CX convex array probe, operating at a frequency range of 1–8 MHz and machine parameters set to an MI of 0.07 and gain of 65 dB. Lastly, the GE LOGIQ E8 Color Doppler Ultrasound Instrument (GE Healthcare, Chicago, IL, USA), equipped with a C1 Probe and a frequency range of 3.5–5 MHz, was utilized for ultrasound imaging. The machine parameters in ultrasound contrast imaging mode included a MI of 0.12 and a gain of 30 dB. The ultrasound contrast agent employed was SonoVue (Bracco, Italy).
Ultrasound examination methods
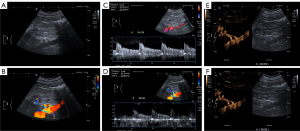
Patients were instructed to fast for a period of 8–12 hours prior to undergoing conventional ultrasound and CEUS examinations. The examinations were conducted with patients positioned in the supine, left lateral decubitus (at an angle of 45–60 degrees), and right lateral decubitus (at an angle of 60–90 degrees) positions. During the renal artery conventional ultrasound examination, particular attention was given to identifying the presence of multiple arteries originating from the abdominal aorta using two-dimensional grayscale ultrasound, CDFI, and pulsed wave Doppler modes. In the CEUS mode, 0.8–1 mL of SonoVue was administered via a three-way stopcock in the antecubital vein, followed by a flush with 5 mL of 0.9% saline solution. The ultrasound diagnostic instrument’s integrated timer initiated the recording of dynamic images at the onset of the flush and 30 seconds of dynamic imaging was recorded. Patients were directed to maintain a calm breathing pattern and stable position throughout the examination. The ultrasound physician reassessed the presence of multiple renal arteries arising from the abdominal aorta by comparing the results of conventional ultrasound examination and presented a comprehensive visualization of the renal artery’s trajectory from the abdominal aorta to the kidneys using the CEUS mode. Figure 2 depicts a representative example for the ARA multi-modal ultrasound examinations.
Diagnostic criteria for the ARA
In cases where a single kidney possesses multiple renal arteries providing blood supply, the artery with the greatest diameter is designated as the main renal artery (MRA), with the remaining arteries classified as ARAs, excluding early branches of the renal artery (20). Conversely, in instances where a single kidney is supplied by only one renal artery, that artery is also designated as the MRA (21). Multi-modal ultrasound examinations were independently evaluated by two proficient physicians, with any discrepancy resolved by a senior physician for final adjudication.
Statistical methods
Data analysis was conducted using the software SPSS 27.0 (IBM Corp., Armonk, NY, USA). Counting data were presented as either the number or percentage of cases, whereas measurement data were reported as mean ± standard deviation if they followed a normal distribution, or as median and interquartile range (IQR; 25th–75th percentile) if they did not. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of multi-modal ultrasound in diagnosing ARA were compared with CTA results. The diagnostic efficiency of multi-modal ultrasound was analyzed by receiver operating characteristic (ROC). The kappa consistency test was utilized to assess agreement between multi-modal ultrasound and CTA. Chi-squared or Fisher’s exact test and t-test or Mann-Whitney U test was used to compare the parameters between detected and missed ARAs. A P value <0.05 was considered statistically significant.
Results
Basic characteristics
The study comprised 73 patients, with a total of 144 kidneys (due to two patients having undergone unilateral nephrectomy), consisting of 46 males and 27 females. Patient ages ranged from 18 to 85 years, with body mass index (BMI) values ranging from 17.4 to 36.7 kg/m2. Most patients (89.0%) had a history of hypertension. The distribution of ARAs among patients showed a predominance of single ARAs, followed by double ARAs, with triple ARAs being infrequent. Unilateral ARAs were more prevalent than bilateral ARAs in this patient cohort. Table 1 describes the demographics and clinical parameters for the 73 patients enrolled.
Table 1
| Parameter | Values |
|---|---|
| Age (years) | 69 [59, 76] |
| BMI (kg/m2) | 25.0±4.1 |
| Serum creatinine (μmol/L) | 104.0 [72.5, 141.0] |
| Uric acid (μmol/L) | 358.3±120.2 |
| History of hypertension | 65 (89.0) |
| History of renal dysfunction | 28 (38.4) |
| The number of the ARA | |
| 1 | 63 (86.3) |
| 2 | 8 (11.0) |
| 3 | 2 (2.7) |
| Kidney with the ARA | |
| Unilateral | 65 (89.0) |
| Bilateral | 8 (11.0) |
| Location of the ARA | |
| Left | 36 (42.3) |
| Right | 49 (57.7) |
Data are presented as median [IQR, 25th–75th percentile], mean ± SD or n (%). ARA, accessory renal artery; BMI, body mass index; IQR, interquartile range; SD, standard deviation.
The diagnostic performances of the multi-modal ultrasound
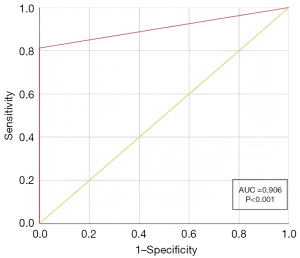
The results of the CTA and multi-modal ultrasound on 144 kidneys revealed that a total of 85 ARAs were detected by CTA, whereas multi-modal ultrasound detected 70 ARAs. However, there were 15 ARAs missed by multi-modal ultrasound, with each missed ARA corresponding to a kidney. Interestingly, when CTA did not detect any ARAs in a kidney, multi-modal ultrasound also did not find any ARA. Table 2 provides a detailed overview of the diagnostic outcomes of CTA and multi-modal ultrasound in detecting ARAs. Utilizing CTA as the reference standard, the sensitivity, specificity, PPV, NPV, and accuracy of multi-modal ultrasound for detecting ARAs were calculated as 82.4%, 100%, 100%, 81.0%, and 90.9%, respectively. The area under the curve (AUC) of the ROC for multi-modal ultrasound in detecting ARAs was 0.906 (P<0.001). Furthermore, the consistency analysis, measured by the kappa value, yielded a result of 0.806 (P<0.01). The ROC curve of multi-modal ultrasound is shown in Figure 3.
Table 2
| Multi-modal ultrasound | CTA | |||
|---|---|---|---|---|
| 0 | 1 | 2 | Total | |
| 0 | 64 | 15 | 0 | 79 |
| 1 | 0 | 60 | 0 | 60 |
| 2 | 0 | 0 | 5 | 5 |
| Total | 64 | 75 | 5 | 144 |
ARA, accessory renal artery; CTA, computed tomography angiography.
Comparisons between the group with detected ARAs and missed ARAs during multi-modal ultrasound
Multi-modal ultrasound identified 65 ARAs while failing to detect 15 ARAs. The group of patients for whom ARA was missed by multi-modal ultrasound but detected by CTA displayed older age and higher BMI compared to the group that was detected. The disparities in age and BMI between the two groups were found to be statistically significant (P<0.05). Conversely, there were no statistically significant variances in renal length, renal width, renal parenchymal thickness, or the diameter of the MRA between the two groups (P>0.05). Table 3 provides a detailed overview of the clinical and imaging characteristics of both groups.
Table 3
| Parameter | Detected ARA | Missed ARA | P value |
|---|---|---|---|
| Age (years) | 67 [58, 76] | 73 [70, 80] | <0.05 |
| BMI (kg/m2) | 23.7 [21.1, 26.3] | 27.1 [25.6, 30.4] | <0.01 |
| Renal length (cm) | 10.3±1.1 | 10.2±1.9 | >0.05 |
| Renal width (cm) | 5.0±0.8 | 5.1±0.8 | >0.05 |
| Renal parenchymal thickness (cm) | 1.4±0.3 | 1.3±0.5 | >0.05 |
| Diameter of the MRA (mm) | 5.0±0.7 | 5.1±0.7 | >0.05 |
| Sex | >0.05 | ||
| Male | 40 (61.5) | 10 (66.7) | |
| Female | 25 (38.5) | 5 (33.3) | |
| Number of the ARA | >0.05 | ||
| 1 | 60 (92.3) | 15 (100.0) | |
| >1 | 5 (7.7) | 0 (0.0) | |
| Location of the ARA | >0.05 | ||
| Left | 28 (43.1) | 6 (40.0) | |
| Right | 37 (56.9) | 9 (60.0) |
Counting data were presented as number (percentage) of cases, while measurement data were reported as mean ± standard deviation or median and interquartile range (IQR, 25th–75th percentile). ARA, accessory renal artery; BMI, body mass index; IQR, interquartile range; MRA, main renal artery.
Discussion
The findings indicate that multi-modal ultrasound possesses a high diagnostic value for the assessment of ARA. The combination of hemodynamic evaluation through conventional ultrasound and the dynamic visualization of renal arteries via CEUS appears to be mutually reinforcing, thereby enhancing the diagnostic accuracy for identifying ARA. The accurate evaluation of the ARA through non-invasive, safe, and convenient imaging modalities is essential for the effective management of renal vascular-related conditions. This necessity is particularly pronounced during various renal vascular-related surgical procedures, including aortic aneurysm reconstruction, renal transplantation, laparoscopic nephron-sparing surgery for renal tumors, and the treatment of hydronephrosis (6,22-25). Several studies have reported an increased incidence of acute kidney injury and long-term deterioration of renal function following the occlusion of the ARA (6,26,27). Furthermore, several studies have suggested that ARAs may contribute to elevated blood pressure in patients with essential hypertension and could potentially facilitate the progression of the condition (3-5,28-31). Consequently, precise evaluation of ARAs is crucial for ensuring that patients with refractory hypertension receive effective treatment.
CTA is presently considered the imaging modality of choice for evaluating ARAs, with research indicating a sensitivity approaching 100% in identifying renal vascular variations (6,32). Nonetheless, it is not the preferred imaging examination method due to its inherent limitations, such as radiation exposure and the potential risk of contrast-induced nephropathy. In this study, multi-modal ultrasound demonstrated superior diagnostic accuracy for identifying ARA, with a sensitivity of 82.4% and a specificity of 100%. Additionally, multi-modal ultrasound exhibited a high level of diagnostic consistency and effectiveness. The kappa value was 0.806 (P<0.01), and the AUC was 0.906 (P<0.001). Therefore, multi-modal ultrasound is an effective tool for the dynamic visualization of the origin and trunk of the renal artery. It also offers valuable diagnostic information through the analysis of specific renal artery spectral waveforms, facilitating the confirmation of ARA. Initially, a detailed examination was performed to identify any additional arteries originating from the abdominal aorta, utilizing two-dimensional grayscale ultrasound and CDFI modes. Secondly, the spectral waveform of the suspected ARA was identified utilizing pulsed wave Doppler mode. Subsequently, the existence of the ARA was confirmed under CEUS conditions. The CEUS examination selectively visualizes arterial vessels during the arterial phase, thereby minimizing potential interference from abdominal intestines and enabling rapid, precise, and clear dynamic visualization of the entire course of the ARA. This comprehensive imaging modality appears to significantly reduce the likelihood of false-negative results.
Nonetheless, 15 ARAs were not identified by multi-modal ultrasound in the present study. Statistically significant distinctions were noted between the detected and missed groups. The missed group exhibited higher BMI and older age compared to the detected group. There are two potential explanations for this phenomenon. Firstly, the narrower diameter of the ARA in comparison to the MRA, combined with the deeper positioning of renal arteries in obese individuals, may present challenges for ultrasound imaging to accurately depict the lumen of the ARA, thereby hindering detection and potentially leading to overlooked diagnoses. Secondly, advanced age is often associated with diminished digestive system function and decreased gastrointestinal motility, which can result in heightened abdominal gas production. Consequently, it is imperative for patients to adhere to an 8–12-hour fasting period and to evacuate their bowels prior to undergoing renal artery ultrasound examinations. Deep positioning of renal arteries individuals with severe obesity remains a challenge even when utilizing modified coronal section imaging techniques, resulting in suboptimal visualization of the ARA. The present study did not reveal any statistically significant variances between the two groups with respect to renal dimensions, parenchymal thickness, MRA internal diameter, ARA count, or ARA location. Interestingly, in this study, no kidney with two ARAs was missed by multi-modal ultrasound examination. There are two reasons accounting for this. Firstly, multi-modality ultrasound in evaluation of the renal artery is operator dependent, a kidney with more than one ARA could be easily observed, and operators might pay more attention to the presence of the ARA. Besides, a kidney with two ARAs in this study was so small that we did not find this situation.
In the last five years, the renal artery multi-modal ultrasound examination has been a research focus of our angiography group in the ultrasound department, and more than 1,000 patients has undergone this examination (19,33). Our findings suggested that the combined application of the conventional ultrasound and CEUS could improve the accuracy of grading diagnosis of stenosis degree. Accidentally, we found that multi-modal ultrasound also could enhance the detection rate and diagnostic accuracy of assessing the ARA. Furthermore, multi-modal ultrasound represents a non-invasive, safe, and reproducible imaging modality, particularly well-suited for elderly patients with renal impairment (17). However, few studies have recognized the value of multi-modal ultrasound in the evaluation of the ARA. When physicians recognize the value of multi-modal ultrasound in the evaluation of the ARA and receive specialized training in renal artery multi-modal ultrasound examinations, the detection rate of the ARA can be significantly enhanced.
It is also important to recognize the limitations of this study. Firstly, this study is retrospective in nature and involves a limited sample size, which may introduce selection and statistical biases. Secondly, the diagnostic accuracy of multi-modal ultrasound for evaluating the ARA was compared with that of CTA, rather than gold standard imaging modality (DSA). Thirdly, the accuracy of ARA evaluation is operator-dependent, underscoring the necessity for standardized procedures in renal artery ultrasound examinations. Future research should involve a large, multicenter sample to yield more robust and convincing conclusions.
Conclusions
In summary, multi-modal ultrasound exhibits certain detection rates and diagnostic accuracy for the evaluation of the ARA. The integrated use of two-dimensional grayscale ultrasound, CDFI, pulsed wave Doppler, and CEUS facilitates a comprehensive assessment of both the morphological and hemodynamic characteristics of the ARA. These modalities complement and corroborate each other, thereby enhancing the overall diagnostic capability. Consequently, multi-modal ultrasound emerges as a promising and valuable technique for the evaluation of the ARA.
Acknowledgments
The authors are grateful for the support of the angiography group at the Department of Sonography, Beijing Hospital.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1117/rc
Funding: This work was supported by grants from the National High-Level Hospital Clinical Research Funding (No. BJ-2023-096) and the National High-Level Hospital Clinical Research Funding (No. BJ-2022-198).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1117/coif). All authors report that this work was supported by grants from the National High-Level Hospital Clinical Research Funding (No. BJ-2023-096) and the National High-Level Hospital Clinical Research Funding (No. BJ-2022-198). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Hospital (No. 2018BJYYEC-043-02). Since this study was retrospective, the requirement of informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Johnson PB, Cawich SO, Shah SD, Aiken W, McGregor RG, Brown H, Gardner MT. Accessory renal arteries in a Caribbean population: a computed tomography based study. Springerplus 2013;2:443. [Crossref] [PubMed]
- Palmieri BJ, Petroianu A, Silva LC, Andrade LM, Alberti LR. Study of arterial pattern of 200 renal pedicle through angiotomography. Rev Col Bras Cir 2011;38:116-21. [Crossref] [PubMed]
- Satyapal KS, Haffejee AA, Singh B, Ramsaroop L, Robbs JV, Kalideen JM. Additional renal arteries: incidence and morphometry. Surg Radiol Anat 2001;23:33-8. [Crossref] [PubMed]
- MARSHALL AG. Aberrant renal arteries and hypertension. Lancet 1951;2:701-5. [Crossref] [PubMed]
- Shen J, Lyu L, Wu X, Ji J, Zeng C, Li S, Zhao Y, Xu J, Lin L, Lu C, Mao W, Wei T. Correlation between Renal Artery Anatomy and Hypertension: A Retrospective Analysis of 3000 Patients. Evid Based Complement Alternat Med 2021;2021:9957361. [Crossref] [PubMed]
- Tenorio ER, Kärkkäinen JM, Marcondes GB, Lima GBB, Mendes BC, DeMartino RR, Macedo TA, Oderich GS. Impact of intentional accessory renal artery coverage on renal outcomes after fenestrated-branched endovascular aortic repair. J Vasc Surg 2021;73:805-818.e2. [Crossref] [PubMed]
- Hänninen EL, Denecke T, Stelter L, Pech M, Podrabsky P, Pratschke J, Ricke J, Schindler R, Neuhaus P, Felix R, Tullius SG. Preoperative evaluation of living kidney donors using multirow detector computed tomography: comparison with digital subtraction angiography and intraoperative findings. Transpl Int 2005;18:1134-41. [Crossref] [PubMed]
- Türkvatan A, Ozdemir M, Cumhur T, Olçer T. Multidetector CT angiography of renal vasculature: normal anatomy and variants. Eur Radiol 2009;19:236-44. [Crossref] [PubMed]
- Urban BA, Ratner LE, Fishman EK. Three-dimensional volume-rendered CT angiography of the renal arteries and veins: normal anatomy, variants, and clinical applications. Radiographics 2001;21:373-86; questionnaire 549-55.
- Tao XF, Zhu JQ, Wu YW, Tang GY, Shi YZ, Zhang L, Lin Y, Wang ZQ. Dual-energy computed tomography angiography for evaluating the renal vascular variants. Chin Med J (Engl) 2013;126:650-4.
- Gulas E, Wysiadecki G, Cecot T, Majos A, Stefańczyk L, Topol M, Polguj M. Accessory (multiple) renal arteries - Differences in frequency according to population, visualizing techniques and stage of morphological development. Vascular 2016;24:531-7. [Crossref] [PubMed]
- Zhao XY, Tian J, Ru YH, Sun B, Sun CF, Zhang AM, Shao YH. Application value of multislice spiral computed tomography angiography in the evaluation of renal artery variation in living donor kidney transplantation. Genet Mol Res 2015;14:314-22. [Crossref] [PubMed]
- Prince MR, Zhang H, Morris M, MacGregor JL, Grossman ME, Silberzweig J, DeLapaz RL, Lee HJ, Magro CM, Valeri AM. Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology 2008;248:807-16. [Crossref] [PubMed]
- Fu J, Lin Z, Zhang B, Song L, Qin N, Qiu J, Yang M, Zou Y. Magnetic Resonance Imaging in Atherosclerotic Renal Artery Stenosis: The Update and Future Directions from Interventional Perspective. Kidney Dis (Basel) 2024;10:23-31. [Crossref] [PubMed]
- Ma N, Wang SY, Sun YJ, Ren JH, Guo FJ. Diagnostic value of contrast-enhanced ultrasound for accessory renal artery among patients suspected of renal artery stenosis. Zhonghua Yi Xue Za Zhi 2019;99:838-40. [Crossref] [PubMed]
- Schneider A, Johnson L, Goodwin M, Schelleman A, Bellomo R. Bench-to-bedside review: contrast enhanced ultrasonography--a promising technique to assess renal perfusion in the ICU. Crit Care 2011;15:157. [Crossref] [PubMed]
- Mao M, Xia B, Chen W, Gao X, Yang J, Li S, et al. The Safety and Effectiveness of Intravenous Contrast-Enhanced Sonography in Chinese Children-A Single Center and Prospective Study in China. Front Pharmacol 2019;10:1447. [Crossref] [PubMed]
- Wang Y, Li Y, Wang S, Ma N, Ren J. Role of Contrast-Enhanced Ultrasound in the Evaluation of Patients With Suspected Renal Arterial Stenosis. Front Cardiovasc Med 2022;9:721201. [Crossref] [PubMed]
- Li Y, Wang Y, Liu ZS, Ma N, Zhang WD, Ren JH. Clinical Practice Report of Contrast-Enhanced Ultrasound in Renal Artery Disease. J Ultrasound Med 2024;43:117-25. [Crossref] [PubMed]
- Mihaylova E, Groudeva V, Nedevska M. Multidetector computed tomography angiography study of the renal arterial vasculature anatomy and its variations in a Bulgarian adult population. Surg Radiol Anat 2023;45:289-96. [Crossref] [PubMed]
- Qaseem SMD, Singhal A, Ghonge NP. Renal Volumetry-based Prediction of the Presence of Accessory Renal Artery: Computed Tomographic Angiography-based Study with Clinical Implications on Renal Doppler. J Med Ultrasound 2021;29:22-5. [Crossref] [PubMed]
- Sadeghi-Azandaryani M, Zimmermann H, Korten I, Klose A, Scheiermann P, Treitl M, Heyn J. Altered renal functions in patients with occlusion of an accessory renal artery after endovascular stenting of an infrarenal aneurysm. J Vasc Surg 2017;65:635-42. [Crossref] [PubMed]
- Carter JT, Freise CE, McTaggart RA, Mahanty HD, Kang SM, Chan SH, Feng S, Roberts JP, Posselt AM. Laparoscopic procurement of kidneys with multiple renal arteries is associated with increased ureteral complications in the recipient. Am J Transplant 2005;5:1312-8. [Crossref] [PubMed]
- Guan WH, Han Y, Zhang X, Chen DS, Gao ZW, Feng XS. Multiple renal arteries with renal cell carcinoma: preoperative evaluation using computed tomography angiography prior to laparoscopic nephrectomy. J Int Med Res 2013;41:1705-15. [Crossref] [PubMed]
- Zhang Q, Ji Y, He T, Wang J. Ultrasound-guided percutaneous renal biopsy-induced accessory renal artery bleeding in an amyloidosis patient. Diagn Pathol 2012;7:176. [Crossref] [PubMed]
- Chan HL, Papazoglou DD, Jungi S, Weiss S, Becker D, Kotelis D, Makaloski V. Fenestrated Physician-Modified Endografts for Preservation of Main and Accessory Renal Arteries in Juxtarenal Aortic Aneurysms. J Clin Med 2023;12:4708. [Crossref] [PubMed]
- Spanos K, Nana P, Brotis AG, Kouvelos G, Behrendt CA, Tsilimparis N, Kölbel T, Matsagkas M, Giannoukas A. Clinical effect of accessory renal artery coverage after endovascular repair of aneurysms in abdominal and thoracoabdominal aorta. J Vasc Surg 2021;74:2104-2113.e7. [Crossref] [PubMed]
- Shakeri AB, Tubbs RS, Shoja MM, Pezeshk P, Farahani RM, Khaki AA, Ezzati F, Seyednejad F. Bipolar supernumerary renal artery. Surg Radiol Anat 2007;29:89-92. [Crossref] [PubMed]
- Bakker J, Beek FJ, Beutler JJ, Hene RJ, de Kort GA, de Lange EE, Moons KG, Mali WP. Renal artery stenosis and accessory renal arteries: accuracy of detection and visualization with gadolinium-enhanced breath-hold MR angiography. Radiology 1998;207:497-504. [Crossref] [PubMed]
- Glodny B, Cromme S, Wörtler K, Winde G. A possible explanation for the frequent concomitance of arterial hypertension and multiple renal arteries. Med Hypotheses 2001;56:129-33. [Crossref] [PubMed]
- Wu F, Yuan X, Sun K, Zhang Y, Zhu L, Bai C, Cheng Y, Lu Y, Jiang Y, Song W. Effect of Accessory Renal Arteries on Essential Hypertension and Related Mechanisms. J Am Heart Assoc 2024;13:e030427. [Crossref] [PubMed]
- Kim JK, Park SY, Kim HJ, Kim CS, Ahn HJ, Ahn TY, Cho KS. Living donor kidneys: usefulness of multi-detector row CT for comprehensive evaluation. Radiology 2003;229:869-76. [Crossref] [PubMed]
- Ren JH, Ma N, Wang SY, Sun YJ, Zhang YW, Guo FJ, Li YJ, Li TH, Ai H, Zhang WD, Li P, Ma WH. Rationale and study design for one-stop assessment of renal artery stenosis and renal microvascular perfusion with contrast-enhanced ultrasound for patients with suspected renovascular hypertension. Chin Med J (Engl) 2019;132:63-8. [Crossref] [PubMed]


