
An adult with polysplenia syndrome and sick sinus syndrome on cardiac magnetic resonance imaging: a case description and literature analysis
Introduction
Polysplenia syndrome (PPS) is a rare congenital disorder, often presenting with concurrent variations in multiple visceral organs, and it is believed to play a significant role in the normal development and lateralization of these organs (1). Individuals with mild variants can develop normally and are often discovered incidentally in adulthood (2,3); they often do not need special interventions. Here, we present a case of an adult PPS patient with concomitant sick sinus syndrome (SSS). The cardiovascular anomalies present in this patient may be associated with SSS. We employed cardiac magnetic resonance (CMR) imaging to conduct a comprehensive assessment of the patient’s cardiovascular anomalies and cardiac function.
Case presentation
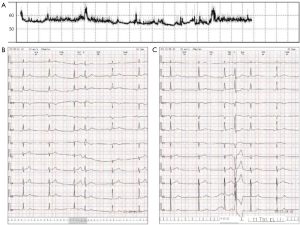
A 54-year-old female patient was admitted to the hospital due to recurrent episodes of dizziness lasting for 10 years, which had worsened over the past 2 months. Her previous diagnosis from a community hospital included sinus bradycardia and situs inversus of the abdominal organs. The patient had no history of hypertension, diabetes, coronary artery disease, or cerebral infarction and did not smoke, drink alcohol, or have a coffee addiction. After hospital admission, the patient underwent a 24-hour ambulatory electrocardiogram monitoring, which revealed bradycardia (<50 bpm) throughout the day (Figure 1A), occasional atrial premature beats with intraventricular conduction delay (Figure 1B), occasional ventricular premature beats (Figure 1C), and intermittent incomplete right bundle branch block. Based on these findings, the diagnosis of SSS was made. Following a comprehensive evaluation, it was decided to proceed with CMR examination initially. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
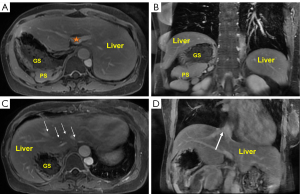
Within the abdominal cavity, in the transverse plane, the spleen and the gastric cavity were observed to be located on the left side. The portal vein entered the liver at the first hepatic portal, directly in front of the spine (Figure 2A). In the coronal plane, A parent spleen with multiple accessory spherules was seen in the coronal plane (Figure 2B). The volumes of the left and right liver lobes were approximately equal. All branches of the hepatic veins converged to form the inferior vena cava (IVC) (Figure 2C). Additionally, the infrahepatic IVC was absent (Figure 2D). In summary, we considered the patient to have partial abdominal inversion, a horizontal liver, and multiple spleens, accompanied by absence of infrahepatic IVC. The diagnosis was PPS.
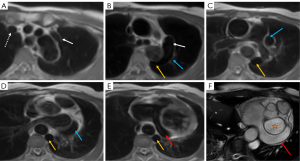
In the thoracic cavity, the persistent left superior vena cava (PLSVC) could be observed. The patient’s right superior vena cava (SVC) was normal and the bilateral SVC did not converge in front of the aortic arch but instead descended separately (Figure 3A). This condition is known as PLSVC (4). The hemiazygos vein, as a continuation of the left IVC, joined the PLSVC at the level of the tracheal bifurcation (Figure 3B), subsequently drained into the coronary sinus (CS), passed beneath the left atrium, and ultimately entered the right atrium, The patient’s CS was observed to be significantly dilated (Figure 3C-3F). The coronal enhanced image clearly showed the left IVC passing through the diaphragm into the thoracic cavity and continuing as the hemiazygos vein. Although this was a CMR, it was still possible to see that the left IVC originated from the infrahepatic region (Figure 4).
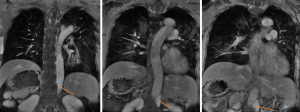
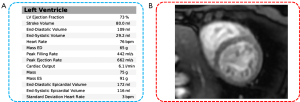
The functional magnetic resonance imaging (MRI) revealed that the patient’s cardiac function, as assessed by short-axis cardiac cine, was 73%. Additionally, no myocardial ischemia was detected during resting myocardial perfusion (Figure 5). Consequently, heart failure and coronary artery disease were not considered in the diagnostic evaluation.
Following a multidisciplinary discussion, considering the patient’s history of cardiac arrest and dynamic electrocardiogram findings, it was recommended to implant a permanent cardiac pacemaker. After obtaining consent from the patient and their family, we performed a dual-chamber permanent pacemaker implantation procedure. The patient’s CMR revealed no anomalies in the right SVC. Therefore, the ventricular leads were inserted via the right axillary vein and advanced into the right atrium and right ventricle through the right SVC respectively.
Follow-up
The patient underwent follow-up examinations with electrocardiogram and echocardiogram at 1 month, 3 months, and 1 year postoperatively. Ejection fraction was maintained above 60% in all echocardiographic assessments. As of the last follow-up on 9 December 2024, there was no recurrence of dizziness symptom, and the electrocardiogram showed normal findings (Figure 6).
Discussion
In adult PPS patients, the most commonly associated cardiovascular anomaly is the PLSVC, accounting for approximately 65% of cases. Typically, patients with this variant do not exhibit significant associated symptoms and the anomaly is often discovered incidentally. In this case, the infrarenal portion of the IVC returned to the thoracic cavity via the hemiazygos vein, where it converged with PLSVC to enter the CS, ultimately returning to the right atrium. It is precisely this anomaly that leads to a significant increase in blood flow in the CS. It was confirmed in a previous animal study that hypertension in rabbit CS can lead to an increase in the content of hemosiderin in the surrounding myocardial cells (5). The iron overload can impair the function of L-type calcium channels in myocardial cells (6). Due to the presence of embryonic pacemaker cells around the opening of the CS, this chronic hypertension may lead to arrhythmias (7).
Furthermore, several studies have also noted the concurrent occurrence of CS dilation and arrhythmias (3,4,8). Zhang et al. categorized PLSVC patients into four groups. Among them, only patients with PLSVC who had CS drainage in groups I and II experienced arrhythmias, whereas those with normal CS in groups III and IV all showed sinus rhythm. This suggests a potential association between high CS pressure and arrhythmias (8). Lin et al. reported a patient with cardiovascular anomalies identical to those in this case, and that patient had a history of pacemaker implantation and was hospitalized due to atrial fibrillation (4). In contrast, the case reported by Ladak et al. did not exhibit a hemiazygos continuation of the IVC or CS dilation, and thus presented with no significant symptoms (3). We speculate that the variant of a persistent left SVC with a hemiazygos continuation of the IVC converging through the CS may be associated with arrhythmias such as SSS.
In patients with PPS, compared to the currently most commonly used contrast-enhanced computed tomography (CT) imaging, CMR provides multi-parametric imaging capabilities, allowing for the acquisition of comprehensive data such as myocardial perfusion and tissue viability, which are crucial for a thorough evaluation of cardiac pathology. Furthermore, MRI’s ability to generate images in any plane affords a panoramic view of the heart’s anatomy and function, enhancing diagnostic precision and enabling a more holistic assessment of cardiac structures. In the case of a patient with a PLSVC combined with an occluded right SVC, the ventricular lead should be introduced through the PLSVC and the CS to the right atrium (9). Fortunately, in this patient, CMR clearly showed that there was no anomaly in the right SVC.
In summary, this case serves as a cautionary example that PPS in adults is not always asymptomatic, even when the cardiovascular anomalies appear to be mild. The comprehensive one-stop assessment based on CMR multi-sequence imaging is instrumental in identifying cardiovascular anomalies in patients, evaluating myocardial perfusion and cardiac function, and aiding in the exclusion of other potential cardiac diagnoses; the presence of dilated CS should alert clinicians to the risk of concomitant arrhythmias.
Acknowledgments
None.
Footnote
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2294/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nelson LW, Bugenhagen SM, Lubner MG, Bhalla S, Pickhardt PJ. Spectrum of Heterotopic and Ectopic Splenic Conditions. Radiographics 2024;44:e240004. [Crossref] [PubMed]
- Lin Z, Rahman A, Quintero S. A Case of Pancreatic Ductal Adenocarcinoma in an Elderly Adult With Heterotaxy Syndrome. Cureus 2024;16:e63664. [Crossref] [PubMed]
- Ladak R, Magnuson W. Polysplenia with situs inversus totalis, azygos continuation of the inferior vena cava, and duplication of the superior vena cava in a healthy adult: A case report. Radiol Case Rep 2024;19:4184-9. [Crossref] [PubMed]
- Lin SX, Sun JZ. Persistent Left Superior Vena Cava with Hemiazygos Continuation of Left Inferior Vena Cava. Radiology 2024;310:e232050. [Crossref] [PubMed]
- Akşit E, Büyük B, Oğuz S. Histopathological changes in myocardial tissue due to coronary venous hypertension. Arch Med Sci 2023;19:1714-20. [PubMed]
- Siri-Angkul N, Xie LH, Chattipakorn SC, Chattipakorn N. Cellular Electrophysiology of Iron-Overloaded Cardiomyocytes. Front Physiol 2018;9:1615. [Crossref] [PubMed]
- Morgan DR, Hanratty CG, Dixon LJ, Trimble M, O'Keeffe DB. Anomalies of cardiac venous drainage associated with abnormalities of cardiac conduction system. Europace 2002;4:281-7. [Crossref] [PubMed]
- Zhang L, Ling G, Gang Y, Yang Z, Lu Z, Gan X, Liang H, Zeng Y, Zhang X. Classification and quantification of double superior vena cava evaluated by computed tomography imaging. Quant Imaging Med Surg 2022;12:1405-14. [Crossref] [PubMed]
- Archontakis S, Sanidas E, Sideris K, Arsenos P, Gatzoulis K, Sideris S. Optimal technique for right ventricular lead implantation in isolated persistent left superior vena cava. Europace 2022;24:11. [Crossref] [PubMed]



