
Comparison of diffusion-weighted whole-body magnetic resonance imaging and abdominal ultrasonography versus contrast-enhanced computed tomography in diagnosing acute focal bacterial nephritis: a retrospective cohort study
Introduction
Acute focal bacterial nephritis (AFBN), first described in 1979, is characterized by focal non-liquefying necrosis in the cortical region of the kidney (1). AFBN is considered a complex form of acute pyelonephritis or an intermediate stage between acute pyelonephritis and renal abscesses (2). Although AFBN is more commonly reported in infants and children, it can also occur in adults, and attention should be paid to this possibility. Since AFBN does not have specific characteristic symptoms, it is often difficult to diagnose. If diagnosis and treatment are not performed in a timely manner, renal abscess formation or renal scarring, which may require invasive procedures, may occur. Therefore, it is important to recognize that AFBN can also occur in adults and to ensure accurate diagnosis and appropriate treatment (3,4).
Contrast-enhanced computed tomography (CT) is the gold standard for diagnosing AFBN (5); however, its use is contraindicated for patients with contrast media allergies or chronic renal failure, presenting radiation risks and rendering it unsuitable for pregnant women. Conversely, diffusion-weighted whole body imaging with background body signal suppression (DWIBS) can help identify high signal areas indicative of acute inflammation, facilitating AFBN diagnosis (6). DWIBS involves the use of DWI technology to visualize the random motion of water molecules (Brownian motion), enhancing image diagnosis (7). By utilizing techniques such as multi-signal averaging, fat suppression, and high diffusion weighting, DWIBS can capture images without restricting patient respiration. This method provides strong contrast between cancerous and normal surrounding tissues, making it effective for cancer detection and staging with a sensitivity comparable with that of fluorodeoxyglucose-positron emission tomography (8). Additionally, DWIBS can help in detecting decreased diffusion motion associated with interstitial edema or necrosis, enabling the identification of acute inflammation (9). Moreover, DWIBS can be used to assess disease activity (9), and the lack of high signal intensity may be attributed to lower AFBN activity. As DWIBS does not use contrast media, it avoids the risks associated with contrast allergies and renal dysfunction and is safe for use during pregnancy. The exclusivity of utilizing only DWIBS, which can be executed within 166 s without incorporating other MRI modalities such as T1/T2 imaging, is also a significant advantage.
Despite these advantages, the diagnostic accuracy of DWIBS for AFBN remains under-evaluated. Abdominal ultrasonography is beneficial due to its non-exposure nature and user-friendly application, and it can differentiate between AFBN and acute pyelonephritis (APN) (10); however, its diagnostic accuracy is reported to be relatively low at 22.69% (5). Therefore, this study aimed to compare the diagnostic accuracy of DWIBS and abdominal ultrasonography against that of contrast-enhanced CT, the gold standard, among patients with AFBN undergoing inpatient treatment. We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1861/rc).
Methods
Study design
This was a retrospective cohort study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Naha City Hospital (approval No. 2023a61) and individual consent for this retrospective analysis was waived. The study details were published on Naha City Hospital website. Since the opt-out method was used, written informed consent was not required.
Patients
We enrolled patients aged ≥18 years hospitalized for AFBN under the Diagnosis Procedure Combination (DPC) system from January 1, 2013, to December 31, 2022.
The DPC system, a comprehensive payment model for acute inpatient care based on the diagnosis group classification, was initiated by the Japanese government in 2003 (11) and now registers over 7 million patients annually (12). Using the DPC system, we examined all previously hospitalized patients. In the DPC system, the diagnosis for AFBN is recorded as focal segmental glomerulosclerosis based on ICD-10 (13), and we focused our investigation on patients with this listed as their primary diagnosis. We excluded the following cases: (I) DPC diagnosis of AFBN, but radiologist interpretation indicated a renal abscess (3 cases) and (II) MRI was performed, but DWIBS was not available (2 cases).
Diagnosis with each modality
The diagnostic criteria for AFBN for each imaging modality are as follows. The choice of test and its timing are determined by the attending physician, as no standardized protocol is in place.
Abdominal ultrasonography
All images were obtained using a 4.5-MHz Vivid 7 convex probe (GE HealthCare Technologies, Inc., Chicago, IL, USA). Radiology technicians performed the ultrasonographic examinations. Positive findings were defined as hypoechoic areas in the renal cortex, and decreased blood flow in these areas was detected using Doppler imaging (3).
Contrast-enhanced CT
From January 1, 2013, to March 31, 2017, an Aquilion CX from Canon (Tokyo, Japan), was used, and from April 1, 2017, to December 31, 2022, an EVO Revolution CT from GE Healthcare was used. The voltage ranged from 100 to 120 kV and was automatically adjusted according to the body size of the patient. The section thickness was 5 mm, and a contrast agent was administered at a dose of 500–600 mL/kg, with a maximum of 135 mL. Imaging was performed in the corticomedullary and parenchymal phases. Positive findings were defined as wedge-shaped areas with poor contrast enhancement (14), with confirmation occurring during the parenchymal phase.
DWIBS
All images were obtained using a 1.5-Tesla Ingenia Ambition from Philips (Amsterdam, Netherlands). The sequence parameters were as follows: flip angle, 90°; b value, 1,000 s/mm2; repetition time, 5,000–6,000 ms; echo time, 120 ms; matrix size, 112×256; field of view, 460 mm × 460 mm; and section thickness, 5 mm. The respiratory motion was not controlled, and the average acquisition time was 166 s per section, focusing solely on the abdomen. Positive findings were defined as wedge-shaped, high-density areas of renal parenchyma.
Image interpretation
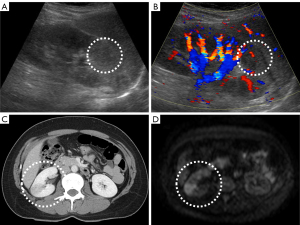
Both contrast-enhanced CT and DWIBS images were interpreted by two radiology specialists, each certified as a radiology specialist, with 34 and 30 years of experience. When one radiologist reviewed an image, the other would not review the same image. Therefore, we were unable to assess the interobserver variability between the two radiologists. Ultrasonography was performed by a radiology technician certified by the Japan Society of Ultrasonics in Medicine. An internal medicine physician overseeing the patient determined whether the ultrasonography findings were suggestive of AFBN. The radiologists were not involved in the interpretation of abdominal ultrasound images. Figure 1 illustrates the typical imaging findings for each modality.
Assessment parameters
We evaluated age, sex, period from symptom onset to hospitalization, period from the start of treatment to fever resolution, number of days from hospitalization to imaging studies, presence of urinary tract abnormalities, white blood cell (WBC) counts from blood and urine tests during hospitalization, C-reactive protein (CRP) levels, presence of cloudy urine, positive rate of blood cultures, positive rate of urine cultures, and duration of intravenous and oral antibiotic administration.
The period from symptom onset to hospitalization was defined as the time from the appearance of symptoms, such as fever, pain during urination, residual urine sensation, abdominal pain, and nausea, to hospitalization. After hospitalization, fever resolution was defined as a body temperature of 37.0 ℃ or lower sustained for over 24 h without the use of antipyretic medications. We recorded the number of days from hospitalization to the imaging tests from day 0 (day of hospitalization) to the day the tests were performed. Urinary tract abnormalities included urinary dysfunction, benign prostatic hyperplasia, renal stones, or ureteral stones. All patients submitted blood and urine cultures on admission. The first day an antibiotic was administered at a daily dose was considered day 1. All patients received intravenous antibiotics during hospitalization and were transitioned to oral antibiotics upon discharge.
We examined the proportion of patients who underwent abdominal ultrasonography, contrast-enhanced CT, and DWIBS, as well as the percentage of patients who exhibited AFBN findings (diagnostic rate). We focused on patients with AFBN findings confirmed by contrast-enhanced CT, in whom either abdominal ultrasonography or DWIBS, or both, was performed. We then evaluated the diagnostic accuracy of abdominal ultrasonography and DWIBS for detecting AFBN (accuracy rate).
Statistical analysis
Descriptive statistics were performed to analyze clinical profiles and treatment characteristics, bacterial distribution in blood and urinary cultures, and imaging modalities to aid in the diagnosis and their diagnostic accuracy. Diagnostic rates of ultrasonography and DWIBS were compared using the Chi-square test. Data analysis was performed using JMP (SAS Institute Inc., Tokyo, Japan).
Results
Patient characteristics
We included 123 patients with a mean age of 51±20.4 years, of whom 107 (87.0%) were women. Among these patients, 72 (60.5%, n=119; with four missing values due to lack of symptom onset dates in the medical records) were hospitalized within 3 days of symptom onset. The period from the start of treatment to fever resolution was within 3 days for 44 patients (36.4%, n=121) and within 4–7 days for 68 patients (56.2%, n=121). Eighteen patients (14.6%; 8 men, 10 women) had urinary tract abnormalities as an underlying condition.
Laboratory and microbiological findings
At the initial visit, the WBC count was 12,597±4,483/µL, and the CRP level was 13.3±7.93 mg/dL. Cloudy urine was observed in 78 of the 119 patients (65.5%). The positive rates for blood and urine cultures were 28.7% (35/122) and 79.5% (97/122), respectively. Blood cultures helped identify Escherichia coli [n=30, including one extended-spectrum beta-lactamase (ESBL) producer], Klebsiella oxytoca (n=1), Klebsiella pneumoniae (n=1), Enterococcus faecalis (n=2), and Citrobacter diversus (n=1). Urine cultures yielded Escherichia coli (n=75, including one ESBL producer), Klebsiella pneumoniae (n=8), Enterococcus faecalis (n=3), Streptococcus agalactiae (n=4), Streptococcus anginosus (n=3), Pseudomonas aeruginosa (n=2), and Proteus mirabilis (n=1) (Table 1).
Table 1
| Bacterial species | Blood culture (n) | Urinary culture (n) |
|---|---|---|
| Escherichia coli [including ESBL] | 30 [1] | 75 [1] |
| Klebsiella oxytoca | 1 | – |
| Klebsiella pneumoniae | 1 | 8 |
| Enterococcus faecalis | 2 | 3 |
| Citrobacter diversus | 1 | – |
| Streptococcus agalactiae | – | 4 |
| Streptococcus anginosus | – | 3 |
| Pseudomonas aeruginosa | – | 2 |
| Proteus mirabilis | – | 1 |
ESBL, extended-spectrum beta-lactamase.
Treatment details
The selection, duration, and method of administration of antibiotics were left to the attending physician’s discretion, and no protocol was established. The duration of intravenous antibiotic administration was 13.6±5.82 days and that of oral administration was 6.99±7.22 days, totaling 20.4±9.56 days (Table 2). Patients were hospitalized during the period of intravenous antibiotic administration and were discharged when treatment was transitioned to oral antibiotics. Table 3 lists the names and frequencies of intravenously administered antibiotics. Antibiotics were modified up to two times, with the names and frequencies of the alternatives also listed. The primary reason for changing antibiotics was de-escalation; however, in some cases, escalation was performed based on culture results, or a switch to another class of antibiotics was required due to the emergence of an allergic reaction. Table 4 presents the names and frequencies of orally administered antibiotics.
Table 2
| Variable | Value |
|---|---|
| Patient characteristics | |
| Age (years) | 51±20.4 |
| Female sex | 87.0% (n=107/123) |
| Time to hospitalization ≤3 days | 60.5% (n=72/119) |
| Resolution of fever ≤3 days | 36.4% (n=44/121) |
| Resolution of fever 4–7 days | 56.2% (n=68/121) |
| Patients with urinary tract abnormalities | |
| Total patients with abnormalities | 14.6% (n=18/123) |
| Women | 8 |
| Men | 10 |
| Laboratory and microbiological findings | |
| White blood cell count (μL) | 12,597±4,483 |
| C-reactive protein (mg/dL) | 13.3±7.93 |
| Cloudy urine | 65.5% (n=78/119) |
| Positive blood culture | 28.7% (n=35/122) |
| Positive urine culture | 79.5% (n=97/122) |
| Treatment details | |
| Intravenous antibiotic duration (days) | 13.6±5.82 |
| Oral antibiotic duration (days) | 6.99±7.22 |
| Total antibiotic duration (days) | 20.4±9.56 |
Data are presented as mean ± standard deviation or n, unless otherwise indicated.
Table 3
| Antibiotic | n (%) |
|---|---|
| Initial antibiotic administered intravenously (n=123) | |
| Cefmetazole | 70 (56.9) |
| Cefotiam | 18 (14.6) |
| Ceftriaxone | 11 (8.9) |
| Cefotaxime | 6 (4.9) |
| Meropenem | 5 (4.1) |
| Ampicillin/sulbactam | 3 (2.4) |
| Ciprofloxacin | 3 (2.4) |
| Ampicillin | 2 (1.6) |
| Aztreonam | 1 (0.8) |
| Ceftazidime | 1 (0.8) |
| Panipenem/betamipron | 1 (0.8) |
| Vancomycin | 1 (0.8) |
| Cefmetazole + ampicillin | 1 (0.8) |
| First change in antibiotic regimen (n=69) | |
| Ampicillin | 26 (37.7) |
| Cefotiam | 20 (29.0) |
| Meropenem | 6 (8.7) |
| Cefmetazole | 4 (5.8) |
| Cefazolin | 3 (4.3) |
| Ceftriaxone | 3 (4.3) |
| Ampicillin/sulbactam | 2 (2.9) |
| Ceftazidime | 2 (2.9) |
| Clindamycin | 1 (1.4) |
| Minocycline | 1 (1.4) |
| Ciprofloxacin | 1 (1.4) |
| Second change in antibiotic regimen (n=10) | |
| Ampicillin | 4 (40.0) |
| Ciprofloxacin | 2 (20.0) |
| Cefotiam | 2 (20.0) |
| Ceftriaxone | 1 (10.0) |
| Panipenem/betamipron | 1 (10.0) |
Of the 123 patients with acute focal bacterial nephritis, antibiotics were changed in 69 patients. Among these 69 patients, antibiotics were further changed again in 10 patients.
Table 4
| Antibiotic | n (%) |
|---|---|
| Levofloxacin | 52 (64.2) |
| Amoxicillin | 16 (19.8) |
| Ampicillin/clavulanic acid | 3 (3.7) |
| Cefaclor | 3 (3.7) |
| Minocycline | 3 (3.7) |
| Cefotiam | 2 (2.5) |
| Ampicillin | 1 (1.2) |
| Sulfamethoxazole/trimethoprim | 1 (1.2) |
Imaging studies
We performed abdominal ultrasonography in 74.0% (91/123) of the patients, with 31.9% (29/91) showing characteristic findings of AFBN (diagnostic rate 31.9%). The average time to perform this imaging was 5.93±4.72 days after hospitalization. We conducted DWIBS in 11.4% (14/123) of the patients, with 85.7% (12/14) showing characteristic findings of AFBN (diagnostic rate 85.7%). The average time to perform DWIBS was 5.69±3.15 days after hospitalization. Although the timing of abdominal ultrasonography and DWIBS was similar, the diagnostic rate of DWIBS was considerably higher. We employed contrast-enhanced CT in 90.2% (111/123) of the patients, achieving a diagnostic rate of 100% (111/111), and the average time to perform this imaging was 4.17±3.17 days after hospitalization. Two patients were unable to undergo contrast-enhanced CT due to contrast media allergies. DWIBS had significantly higher diagnostic rates than abdominal ultrasonography (Fisher’s Exact Test, odds ratio: 0.08, P=0.002) (Table 5). Among the 111 patients with AFBN findings confirmed using contrast-enhanced CT, the accuracy rate of AFBN detection was 26.6% (21/79) using abdominal ultrasonography and 75.0% (6/8) using DWIBS, indicating that DWIBS had a significantly higher accuracy rate than that of abdominal ultrasonography [Chi-square test, χ2(1) =7.96, P=0.0048] (Table 6). We investigated two cases where AFBN findings were observed on contrast-enhanced CT but not on DWIBS. In one case (39-year-old woman), the interval from symptom onset to hospitalization was 8 days, and DWIBS was performed 11 days after hospitalization. DWIBS helps detect acute inflammation with high signal intensity; however, the duration of this persistence remains unclear. These findings suggest that AFBN may persist longer on contrast-enhanced CT than on DWIBS. Once the acute inflammatory phase has passed, the high signal intensity on DWIBS tends to disappear (9,15); thus, this observation suggests a potential reduction in inflammation. In the second case, a 38-year-old woman with DWIBS was hospitalized on day 3 of the acute inflammatory phase; however, no AFBN findings were observed. In this case, the CRP level was relatively low (2.97 mg/dL). DWIBS can be used to assess disease activity (9,15), and the lack of high signal intensity may be attributed to lower AFBN activity.
Table 5
| Modality | Performance rate | Diagnostic rate |
|---|---|---|
| Abdominal ultrasonography | 74.0% (n=91/123) | 31.9% (n=29/91) |
| Contrast-enhanced CT | 90.2% (n=111/123) | 100.0% (n=111/111) |
| DWIBS | 11.4% (n=14/123) | 85.7% (n=12/14) |
CT, computed tomography; DWIBS, diffusion-weighted whole body magnetic resonance imaging with background body signal suppression.
Table 6
| Modality | Accuracy rate (%) |
|---|---|
| Abdominal ultrasonography | 26.6 (n=21/79) |
| DWIBS | 75.0 (n=6/8) |
DWIBS, diffusion-weighted whole body magnetic resonance imaging with background body signal suppression.
Discussion
In this study, we evaluated the accuracy of abdominal ultrasonography and DWIBS versus contrast-enhanced abdominal CT in patients with AFBN undergoing inpatient treatment. Abdominal ultrasonography exhibited an accuracy of 26.6% (21/79), whereas DWIBS demonstrated an accuracy of 75.0% (6/8), indicating that DWIBS was considerably more accurate than abdominal ultrasonography.
A noteworthy characteristic of these patients was the high positivity rate of urine and blood cultures. In a study by Sieger et al. (3), the positivity rate of urine cultures was 59%, while the positivity rate of blood cultures was 19%. In contrast, Jiao et al. (5) reported a urine and blood culture positivity rate of 38.24% and 6.3%, respectively. In our study, the positivity rates of blood and urine cultures were higher than those reported in previous reports. However, another report noted that the positivity rate of blood cultures in uncomplicated pyelonephritis is 25.2% (16), suggesting that our results are reasonable.
Although no established guidelines exist for the duration of antibiotic treatment for AFBN, prolonged antibiotic administration may be desirable, considering the high positivity rate of blood cultures. Cheng et al. recommend a 3-week course of antibiotic treatment for AFBN to prevent recurrence, noting that a 2-week regimen may be insufficient (17). Jiao et al. reported no recurrences with a 4-week antibiotic regimen (5). In our study, we administered an average of 20.4±9.56 days of antibiotic therapy, and no patients were re-admitted due to AFBN.
DWIBS demonstrated higher diagnostic and accuracy rates than that of abdominal ultrasonography. In our study, the correct response rate for DWIBS was 75.0%. Upon reviewing two cases of AFBN identified on contrast-enhanced CT but not on DWIBS, the inflammation had resolved in one case, leading to the absence of high signal intensity on DWIBS. In the other case, it is hypothesized that the inflammation activity was minimal and not detectable by DWIBS. Although DWIBS achieved a correct response rate of 75.0%, this method may have demonstrated greater sensitivity in detecting inflammation activity than that of contrast-enhanced CT.
In a study by Jiao et al. (5), abnormal findings were observed on abdominal ultrasonography in 22.69% of the patients diagnosed with AFBN using contrast-enhanced CT. These abnormal findings included renal enlargement (21.85%) and hypoechoic areas (0.84%). We did not include renal enlargement as a diagnostic criterion for AFBN, as it is not specific to AFBN and can also be observed in pyelonephritis (18,19). Further, including renal enlargement as a diagnostic criterion would have decreased the accuracy rate. In a small-scale study involving pediatric patients, the focal loss of corticomedullary differentiation proved to be useful in distinguishing AFBN from pyelonephritis on abdominal ultrasonography, rather than renal enlargement (10). Contrast-enhanced CT findings of wedge-shaped areas with poor contrast enhancement reflect localized blood flow reduction; hence, it is more appropriate to assess blood flow reduction using abdominal ultrasonography. A previous report indicates that hypoechoic areas changes in AFBN evolve into high-echoic areas as the disease progresses, ultimately disappearing. This suggests that echogenicity may differ depending on the stage of the disease (20). In our study, we evaluated hypoechoic areas but did not assess high-echoic areas, which may have resulted in the exclusion of some cases of AFBN at more advanced stages. The relatively low diagnostic rate of abdominal ultrasonography, despite the high quality of examinations by our radiology technicians, may be attributed to the difficulty in visualizing lesions based on the patient physique and the complexity of assessing blood flow using Doppler imaging.
DWIBS is effective not only for the diagnosis of AFBN but also for morphological evaluations. Nephromegaly and presence of hydronephrosis can be identified. In our study, two patients could not undergo contrast-enhanced abdominal CT due to contrast media allergies. DWIBS is noninvasive, avoids radiation exposure, and can be performed even in patients with poor renal function or contrast media allergies.
In cases where CT is challenging, abdominal ultrasonography is commonly performed; however, we demonstrated the utility of DWIBS. To the best of our knowledge, this study is the first to evaluate the diagnostic accuracy of DWIBS for the diagnosis of AFBN versus that of abdominal ultrasonography and contrast-enhanced CT. Diagnosing AFBN using ultrasonography requires advanced skills and training, whereas DWIBS allows for easier diagnosis due to its shorter examination time and the high signal intensity visualization.
Limitations
First, the diagnosis of AFBN using abdominal ultrasonography was based on positive findings of hypoechoic areas in the renal cortex and decreased blood flow in those areas, excluding renal enlargement which improved the diagnostic accuracy (18,19). Second, the time interval between hospitalization and performance of each examination varied across cases. The timing of the examinations may have influenced the diagnostic and accuracy rates. DWIBS is believed to sensitively reflect inflammation, and its diagnostic and accuracy rates may decrease when inflammation is weak or beyond the acute inflammatory phase (9). However, overall, the timing of the abdominal ultrasonography and DWIBS examinations was almost the same; therefore, the impact of the timing of the examinations on the results of this comparison was considered minimal. Furthermore, we believe that the time interval between admission and DWIBS does not significantly impact diagnostic accuracy. This is because DWIBS has high sensitivity for identifying inflammation, and the time from symptom onset to imaging has a greater influence on the diagnosis. Third, this was a single-center retrospective study, which may have introduced bias. The sample sizes of abdominal ultrasonography (79 cases) and DWIBS (8 cases) differ remarkably, which warrants careful consideration when interpreting the results. However, despite this disparity, the statistical analysis demonstrated a significant difference between the two modalities. Increasing the number of cases may alter the diagnostic and correct response rates. Prospective trials with consistent examination schedules are required to gain deeper insight into the effectiveness of abdominal ultrasonography, CT, and DWIBS. In this study, we did not confirm whether the abnormal findings identified by each imaging modality disappeared after the diagnosis of AFBN. Establishing specific evaluation dates for each modality and conducting imaging re-evaluations not only before the diagnosis of AFBN but also after clinical improvement could enhance diagnostic accuracy, enable a more detailed assessment of the degree of inflammation, and provide deeper insights into the healing process.
Conclusions
DWIBS demonstrated higher diagnostic and correct response rates than those of abdominal ultrasonography for AFBN.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1861/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1861/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosenfield AT, Glickman MG, Taylor KJ, Crade M, Hodson J. Acute focal bacterial nephritis (acute lobar nephronia). Radiology 1979;132:553-61. [PubMed]
- Zaontz MR, Pahira JJ, Wolfman M, Gargurevich AJ, Zeman RK. Acute focal bacterial nephritis: a systematic approach to diagnosis and treatment. J Urol 1985;133:752-7. [PubMed]
- Sieger N, Kyriazis I, Schaudinn A, Kallidonis P, Neuhaus J, Liatsikos EN, Ganzer R, Stolzenburg JU. Acute focal bacterial nephritis is associated with invasive diagnostic procedures - a cohort of 138 cases extracted through a systematic review. BMC Infect Dis 2017;17:240. [PubMed]
- Cheng CH, Tsau YK, Chang CJ, Chang YC, Kuo CY, Tsai IJ, Hsu YH, Lin TY. Acute lobar nephronia is associated with a high incidence of renal scarring in childhood urinary tract infections. Pediatr Infect Dis J 2010;29:624-8. [PubMed]
- Jiao S, Yan Z, Zhang C, Li J, Zhu J. Clinical features of acute focal bacterial nephritis in adults. Sci Rep 2022;12:7292. [PubMed]
- Fujita Y, Kuwashima S, Nomura K, Kano Y, Yoshihara S. Diagnosis and Treatment for Acute Focal Bacterial Nephritis With Renal Abscess Based on Magnetic Resonance Imaging Evaluation. Pediatr Infect Dis J 2021;40:e278-80. [PubMed]
- Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med 2004;22:275-82. [PubMed]
- Ochiai R, Kobayashi H, Yoshida T, Kitagawa M, Ono K, Omagari J. Comparison of Body Diffusion Weighted Imaging Using Diffusion Weighted Whole Body Imaging with Background Body Signal Suppression (DWIBS) and 18FDG-PET for the Detection of Tumors. Nichidoku-iho 2005;50:86-98.
- Oguro E, Ohshima S, Kikuchi-Taura A, Murata A, Kuzuya K, Okita Y, Matsuoka H, Teshigawara S, Yoshimura M, Yoshida Y, Isoda K, Kudo-Tanaka E, Harada Y, Kaminou T, Saeki Y. Diffusion-weighted Whole-body Imaging with Background Body Signal Suppression (DWIBS) as a Novel Imaging Modality for Disease Activity Assessment in Takayasu's Arteritis. Intern Med 2019;58:1355-60. [PubMed]
- Hosokawa T, Tanami Y, Sato Y, Oguma E. Comparison of imaging findings between acute focal bacterial nephritis (acute lobar nephronia) and acute pyelonephritis: a preliminary evaluation of the sufficiency of ultrasound for the diagnosis of acute focal bacterial nephritis. Emerg Radiol 2020;27:405-12. [Crossref] [PubMed]
- Hayashida K, Murakami G, Matsuda S, Fushimi K. History and Profile of Diagnosis Procedure Combination (DPC): Development of a Real Data Collection System for Acute Inpatient Care in Japan. J Epidemiol 2021;31:1-11. [Crossref] [PubMed]
- Akaishi T, Tarasawa K, Fushimi K, Hamada H, Saito M, Kobayashi N, Kikuchi S, Tomita H, Ishii T, Fujimori K, Yaegashi N. Risk Factors Associated With Peripartum Suicide Attempts in Japan. JAMA Netw Open 2023;6:e2250661. [Crossref] [PubMed]
- World Health Organization. ICD-10: International Statistical Classification of Diseases and Related Health Problems. 10th revision, 2nd ed. 2005. Available online: https://apps.who.int/iris/handle/10665/43110
- Huang JJ, Sung JM, Chen KW, Ruaan MK, Shu GH, Chuang YC. Acute bacterial nephritis: a clinicoradiologic correlation based on computed tomography. Am J Med 1992;93:289-98. [Crossref] [PubMed]
- Tomizawa M, Shinozaki F, Tanaka S, Sunaoshi T, Kano D, Sugiyama E, Shite M, Haga R, Fukamizu Y, Fujita T, Kagayama S, Hasegawa R, Shirai Y, Motoyoshi Y, Sugiyama T, Yamamoto S, Ishige N. Diffusion-weighted whole-body magnetic resonance imaging with background body signal suppression/T2 image fusion for the diagnosis of acute cholecystitis. Exp Ther Med 2017;14:730-4. [Crossref] [PubMed]
- Velasco M, Martínez JA, Moreno-Martínez A, Horcajada JP, Ruiz J, Barranco M, Almela M, Vila J, Mensa J. Blood cultures for women with uncomplicated acute pyelonephritis: are they necessary? Clin Infect Dis 2003;37:1127-30. [Crossref] [PubMed]
- Cheng CH, Tsau YK, Lin TY. Effective duration of antimicrobial therapy for the treatment of acute lobar nephronia. Pediatrics 2006;117:e84-9. [Crossref] [PubMed]
- Morehouse HT, Weiner SN, Hoffman JC. Imaging in inflammatory disease of the kidney. AJR Am J Roentgenol 1984;143:135-41. [PubMed]
- Zulfiqar M, Ubilla CV, Nicola R, Menias CO. Imaging of Renal Infections and Inflammatory Disease. Radiol Clin North Am 2020;58:909-23. [Crossref] [PubMed]
- Rianthavorn P. Progression and resolution of acute focal bacterial nephritis. Iran J Kidney Dis 2011;5:271-4. [PubMed]


