
The first case of primary extragastrointestinal stromal tumor of the kidney: a case description
Introduction
Gastrointestinal stromal tumors (GISTs) are the predominant type of mesenchymal tumors found in the gastrointestinal system. These tumors originate from interstitial cells of Cajal (ICCs), which serve as pacemaker cells for peristalsis (1). GISTs can occur throughout the digestive system but occur most frequently in the stomach (45–65%), followed by the small intestine (15–25%), and less frequently in the colon, rectum (5–10%), and esophagus (5–10%) (2). Although the majority of GISTs are confined to the gastrointestinal tract, a minority arise outside this region and are classified as extragastrointestinal stromal tumors (EGISTs). EGISTs are rare, with an incidence of less than 1.5% and account for 6% of all GISTs and only just 1% of all gastrointestinal malignancies (3). In this report, we present the first documented case of primary EGIST originating from the kidney that was confirmed by pathology. We describe the imaging characteristics of this disease on abdominal computed tomography (CT) and magnetic resonance imaging (MRI). Although renal EGISTs have not been previously reported on, they should nonetheless be considered in the differential diagnosis of renal tumors to aid radiologists and clinicians in early and accurate diagnosis.
Case presentation
A 58-year-old female patient was admitted to hospital after a routine examination revealed a mass in her left kidney. She was asymptomatic, with no signs of urinary urgency, frequency, dysuria, hematuria, fever, nausea, vomiting, flank plain, or abdominal pain. Physical examination and laboratory tests revealed no abnormalities.
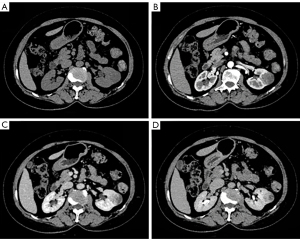
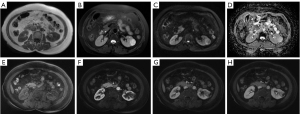
The patient underwent abdominal ultrasound, which showed a hypoechoic mass in the left kidney. The abdominal contrast-enhanced CT scan indicated that the mass of the left kidney was round and slightly hypodense, with the mass measuring approximately 3.0 cm × 2.7 cm in diameter. There was mild inhomogeneous enhancement during the arterial phase and progressive enhancement in the venous and delayed phases (Figure 1). The MRI enhanced scan indicated hypointensity on T1-weighted imaging (T1WI), inhomogeneous hyperintensity on T2-weighted imaging (T2WI), hyperintensity on diffusion-weighted imaging (DWI), and on the apparent diffusion coefficient (ADC). Moreover, there was mild inhomogeneous enhancement during the arterial phase, progressive enhancement observed in the venous and delayed phases, and significant ring enhancement of the lesion envelope (Figure 2). Based on these imaging findings, potential chromophobe renal cell carcinoma (RCC) was initially considered.
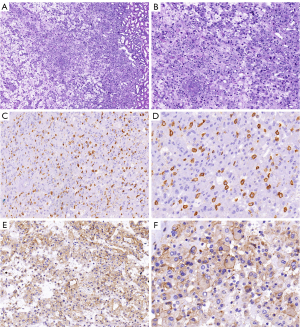
Six days after admission, the patient underwent a retroperitoneoscopic partial nephrectomy under general anesthesia. During the procedure, a round mass measuring approximately 3.5 cm × 3.0 cm was observed protruding from the kidney’s surface. Pathological examination revealed eosinophilic tumor cells arranged predominantly in sheets with focal edema. There was no evidence of intravascular tumor thrombi or perineural invasion. The relevant immunohistochemical results were as follows: cluster of differentiation 117 (CD117/c-Kit) (+), discovered on gastrointestinal stromal tumor-1 (DOG-1) (+), Ki-67 (+1%), Vimentin (+), Desmin (−), cytokeratin 7 (CK7) (−), carbonic anhydrase IX (CAIX) (−), and CD10 (−). The combination of the histological and immunohistochemical results suggested that the tumor was an EGIST (Figure 3). Genetic testing confirmed positive c-Kit and platelet-derived growth factor receptor α (PDGFRα) mutations. No additional treatment was administered postoperatively. Clinical and imaging assessments at 6-month follow-up revealed no signs of tumor recurrence.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee (s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
We report the first rare case of a primary EGIST of the kidney. EGISTs are primarily found in the mesentery, omentum, and retroperitoneum. Notably, recent literature has also documented occurrences in other organs, including the liver, pancreas, bladder, and prostate gland (4-7). Until now, there have been no clinical case reports of primary renal EGISTs in the medical literature.
The clinical symptoms of primary renal EGISTs likely depend on tumor size, location, and growth rate. Smaller tumors (<5 cm) are usually asymptomatic and detected incidentally through imaging or palpation (8). Given the absence of previous reports, the imaging characteristics of renal EGISTs have not been comprehensively summarized. However, by reviewing cases of retroperitoneal, hepatic, pancreatic, and prostatic EGISTs from the literature, we identified the common imaging characteristics: EGISTs are typically large, irregular in shape, and have clear tumor boundaries. These tumors are prone to cystic degeneration and necrosis. On enhanced scans, the solid part of the mass shows moderate, inhomogeneous progressive enhancement. The solid portion of the tumor is rich in blood supply, with numerous thickened and tortuous vessels at the edge and center of the lesion (5-7).
In our case, the primary renal EGIST radiologically demonstrated hypointensity on T1WI, inhomogeneous hyperintensity on T2WI, and hyperintensity on DWI (b =1,000 s/mm2). There was mild heterogeneous enhancement in the arterial phase and progressive enhancement in the venous and delayed phases. These imaging features are consistent with those of EGISTs in other locations but also share similarities with those of conventional RCC, which complicates diagnosis. Nevertheless, there are some differences between primary renal EGISTs and RCC subtypes. Clear cell renal cell carcinoma (ccRCC) is typically hypervascular and often exhibits features such as hemorrhage, necrosis, and cystic changes. On corticomedullary-phase imaging, ccRCC demonstrates enhancement similar to that of the renal cortex, along with a rapid contrast washout pattern. Papillary renal cell carcinoma (PRCC) commonly originates at the cortico-medullary junction. Smaller lesions are usually homogeneous, while larger tumors frequently present with necrosis, cystic changes, hemorrhage, and calcifications. On dynamic contrast-enhanced imaging, PRCC shows mild to moderate enhancement with a pattern of gradual increase. Chromophobe renal cell carcinoma (ChRCC) is typically hypovascular and presents as a solitary, well-defined mass. On imaging, it often appears homogeneous, with occasional calcifications and central scars. On dynamic contrast-enhanced scans, ChRCC demonstrates mild-to-moderate enhancement, which is consistently lower than that of the renal cortex, and shows a gradual washout pattern. These features help distinguish primary renal EGIST from conventional RCC in clinical practice.
The histomorphological and immunohistochemical features of EGISTs resemble those of GISTs, with both tumors arising from Cajal or Cajal-like cells. These tumors typically exhibit expression of KIT tyrosine kinase and are commonly accompanied by activating mutations in c-Kit or PDGFRα (9). The diagnosis of EGISTs is primarily determined via histological and immunohistochemical criteria. Around 95% of GISTs express CD117, which is widely recognized as the gold standard for diagnosing these tumors (10). Moreover, DOG-1, a protein present in nearly all GISTs, serves as a valuable biomarker for this tumor type (11). The combination of CD117 and DOG-1 is clinically recommended to enhance diagnostic accuracy. CD117 and DOG-1 typically show dual-positive expression in most cases, with only a minority presenting as single positive or dual negative. Therefore, c-Kit or PDGFRα gene testing is now advocated to assist in diagnosis (1,12).
Regarding the origins of EGISTs, numerous studies have confirmed the presence of ICC in the extragastrointestinal tract, including the urinary bladder, gallbladder, uterus, omentum, and prostate (13). Independent studies have highlighted the crucial role of Cajal-like cells in regulating urinary kinetics, suggesting that these cells function similarly to the ICC found in the gastrointestinal tract (14,15). Furthermore, research has identified these interstitial cells in the renal pelvis and being associated with the c-Kit receptor (16,17). Therefore, it can be inferred that primary renal EGISTs likely originate from ICC-like cells.
Patients with EGISTs generally have a poorer prognosis compared to those with GISTs, with a lower 5-year overall survival rate postsurgery (18). Surgical resection is the preferred first-line treatment for both EGISTs and GISTs. The most significant genetic features of EGISTs and GISTs are activating mutations in the c-Kit or PDGFRα genes, which provide personalized treatment options for patients. Imatinib, a medication used to treat GISTs and EGISTs, works by selectively inhibiting protein tyrosine kinases. Neoadjuvant imatinib offers several potential benefits for tumors, including downsizing, reduced mitotic activity, and a lower risk of recurrence (19). For GISTs and EGISTs with a high risk of recurrence, adjuvant targeted therapy with imatinib after surgery improves prognosis (20).
EGISTs are exceedingly uncommon and are often identified incidentally. We have limited information on primary EGISTs of the kidney. Clinicians should consider the possibility of the primary renal EGISTs when they encounter atypical renal lesions that are difficult to diagnose through conventional clinical and radiological methods. Pathological examination and immunohistochemical analysis are crucial for diagnosing these tumors, with CD117 and DOG-1 positivity being specific biomarkers. Early and complete surgical resection is the preferred treatment approach for primary renal EGISTs.
Acknowledgments
None.
Footnote
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1678/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee (s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- von Mehren M, Joensuu H. Gastrointestinal Stromal Tumors. J Clin Oncol 2018;36:136-43. [Crossref] [PubMed]
- Machairas A, Karamitopoulou E, Tsapralis D, Karatzas T, Machairas N, Misiakos EP. Gastrointestinal stromal tumors (GISTs): an updated experience. Dig Dis Sci 2010;55:3315-27. [Crossref] [PubMed]
- Costa Almeida C, Caroço TV, Albano M, Carvalho L. Extragastrointestinal stromal tumour (EGIST) presented as a mesenteric and retroperitoneal mass. BMJ Case Rep 2019;12:e232481. [Crossref] [PubMed]
- Harouachi A, Harhar M, Mhand M, Atmani A, Elamrani A, Kharkhach A, Bouhout T, Serji B, Harroudi TE. Extra gastrointestinal stromal tumor EGIST in the recto-vesical pouch: A case report and literature review. Ann Med Surg (Lond) 2022;74:103283. [Crossref] [PubMed]
- Nguyen Thi My Xuan A, Le Thi Bich V, Le Van D, Bui Van P, Nguyen Tri T, Le Van P. A case of primary gastrointestinal stromal tumor of the liver. Radiol Case Rep 2023;18:4533-6. [Crossref] [PubMed]
- Etit D, Kar H, Ekinci N, Yenipazar AE, Çakalağaoğlu F. Extra-Gastrointestinal Stromal Tumor of Prostate. Balkan Med J 2017;34:168-71. [Crossref] [PubMed]
- Song T, Hong Q, Wu Y. Pancreatic Extragastrointestinal Stromal Tumor: A Case Report. Cureus 2024;16:e54514. [Crossref] [PubMed]
- Toutounji Z, Alahmad Z, Attar M, Sarminy M, Alsado WM, Mohammad M. Unusual location of gastrointestinal stromal tumor (GIST): A case report and literature review of greater omentum location. Int J Surg Case Rep 2024;119:109793. [Crossref] [PubMed]
- Li J, Ye Y, Wang J, Zhang B, Qin S, Shi Y, He Y, Liang X, Liu X, Zhou Y, Wu X, Zhang X, Wang M, Gao Z, Lin T, Cao H, Shen LChinese Society Of Clinical Oncology Csco Expert Committee On Gastrointestinal Stromal Tumor. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res 2017;29:281-93. [Crossref] [PubMed]
- Poveda A, García Del Muro X, López-Guerrero JA, Cubedo R, Martínez V, Romero I, Serrano C, Valverde C, Martín-Broto J. GEIS (Grupo Español de Investigación en Sarcomas/Spanish Group for Sarcoma Research). GEIS guidelines for gastrointestinal sarcomas (GIST). Cancer Treat Rev. 2017;55:107-19. [Crossref] [PubMed]
- Yi JH, Park BB, Kang JH, Hwang IG, Shin DB, Sym SJ, Ahn HK, Lee SI, Lim DH, Park KW, Won YW, Lim SH, Park SH. Retrospective analysis of extra-gastrointestinal stromal tumors. World J Gastroenterol 2015;21:1845-50. [Crossref] [PubMed]
- Joensuu H, Rutkowski P, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Braconi C, Bordoni A, Magnusson MK, Sufliarsky J, Federico M, Jonasson JG, Hostein I, Bringuier PP, Emile JF. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol 2015;33:634-42. [Crossref] [PubMed]
- Lin J, Liao W, Wang J, Li W, Tang X, Li H, Yi X, Lu X, Chen Z, Zhu B, Feng X, Diao D. Primary extra-gastrointestinal stromal tumor of retroperitoneum: Clinicopathologic characteristics and prognosis of six cases. Front Oncol 2023;13:1033598. [Crossref] [PubMed]
- Lang RJ, Hashitani H, Tonta MA, Parkington HC, Suzuki H. Spontaneous electrical and Ca2+ signals in typical and atypical smooth muscle cells and interstitial cell of Cajal-like cells of mouse renal pelvis. J Physiol 2007;583:1049-68. [Crossref] [PubMed]
- Wolnicki M, Aleksandrovych V, Gil K. Interstitial cells of Cajal and telocytes in the urinary system: facts and distribution. Folia Med Cracov 2016;56:81-9.
- Lang RJ, Klemm MF. Interstitial cell of Cajal-like cells in the upper urinary tract. J Cell Mol Med 2005;9:543-56. [Crossref] [PubMed]
- Juszczak K, Maciukiewicz P, Drewa T, Thor PJ. Cajal-like interstitial cells as a novel target in detrusor overactivity treatment: true or myth? Cent European J Urol 2014;66:413-7. [Crossref] [PubMed]
- Feng H, Hu W, Zheng C, Wang W, Zheng G, Feng X, Xiong W, Lin G, Zhou Y, Zhao Y, Li Y. Clinical Features of Extragastrointestinal Stromal Tumor Compared with Gastrointestinal Stromal Tumor: A Retrospective, Multicenter, Real-World Study. J Oncol 2021;2021:1460131. [Crossref] [PubMed]
- Wilkinson MJ, Fitzgerald JE, Strauss DC, Hayes AJ, Thomas JM, Messiou C, Fisher C, Benson C, Tekkis PP, Judson I. Surgical treatment of gastrointestinal stromal tumour of the rectum in the era of imatinib. Br J Surg 2015;102:965-71. [Crossref] [PubMed]
- Qian XH, Yan YC, Gao BQ, Wang WL. Prevalence, diagnosis, and treatment of primary hepatic gastrointestinal stromal tumors. World J Gastroenterol 2020;26:6195-206. [Crossref] [PubMed]


