
A novel localization technique for margin distance assessment of deep-seated small pulmonary nodules in thoracoscopic wedge resection: a retrospective study
Introduction
In recent years, low-dose thoracic computed tomography (CT) has been widely utilized for lung cancer screening in China, leading to a significant increase in the identification of small pulmonary nodules (SPNs). Video-assisted thoracoscopic surgical (VATS) resection is recommended for the treatment of SPNs that are highly suspected of being early-stage lung cancer, contributing to both accurate diagnosis and curative intent (1,2). However, some SPNs, especially deep-seated ones, are not detectable during surgery by palpation or visualization. Although several localization methods have been developed with clinical success for superficial nodules (3,4), locating the position of deep-seated nodules and accurately obtaining sufficient surgical margin distance remain challenging. Failure to locate the nodules or an inadequate surgical margin distance may result in extended excision, even lobectomy, or a worse prognosis (5).
Conventional locating methods, such as CT-guided percutaneous localization with dye injection, hookwire, or microcoil implantation, cannot meet the needs for deep-seated nodules. These methods do not assist the surgeon in judging the depth of the nodules and surgical margin distance during thoracoscopic wedge resection. Therefore, it is crucial to find a reliable method for locating deep-seated lung nodules and obtaining sufficient resection margin distance. A few localization methods have been attempted for deep-seated nodules, such as combining multispot dye marks and a deeply-placed microcoil (6,7), CT-guided dual localization with microcoil and patent blue vital dye (8) or indocyanine green (9), and radiofrequency identification marker (10). However, each method has its limitations, such as the need for special equipment (virtual assisted lung mapping, C-arm portable fluoroscopic system or fluorescence thoracoscope, radiofrequency identification marking system, etc.), a complicated procedure, and increased radiation exposure for the patient and surgeon, leading to difficulties in widespread use.
Recently, a novel SPN localization device, characterized by a 4-hook anchor and a scaled suture, has been reported with good success, safety, and feasibility (11,12). The localization procedure with this device is similar to that of conventional CT-guided percutaneous lung puncture localization and does not require special equipment. However, these studies did not demonstrate the best indications for the device, localization techniques, and how to guarantee sufficient surgical margin distance. In our institute, we began utilizing this novel device for SPN localization from 2022, and the localization success rate was 95.6% (13). We preliminarily found that sufficient surgical margin distance could be obtained with this localization device through improving the localization procedure (13). However, it is still unknown whether the device and method are suitable for deep-seated nodules. Therefore, in this study, we utilized this device to explore whether it is suitable for deep-seated SPN localization and obtaining sufficient surgical margin distance. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1571/rc).
Methods
Patients and inclusion criteria
This retrospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and received approval from the Ethics Committee of The Third Affiliated Hospital of Sun Yat-sen University (No. II2023-257-02). Written informed consent for publication was provided by each participant. From 1 November 2021 to 31 October 2023, data from 73 patients recently diagnosed with a deep-seated SPN by CT, who underwent preoperative CT-guided 4-hook needle localization followed by VATS wedge resection, were collected via the electronic medical record system. Since 2021, we have initiated the use of a 4-hook needle for the localization of SPNs. The selection criteria for peripheral SPN wedge resection in this study included: (I) SPN with a maximum transverse diameter of ≥8 but ≤20 mm, and with a solid component less than 5 mm; (II) CT images showing enlargement or density increase of the nodule during follow-up; (III) CT demonstrating that all lung metastasis lesions can be wedge resected when the primary tumor is well controlled; (IV) the depth of SPN is ≥20 mm; and (V) secondary ipsilateral nodules <8 mm in size were synchronously localized and resected if suspected to be malignant. The parameters of SPNs were examined in the lung window settings [window level: −500 Hounsfield units (HU), window width: 1,500 HU]. In this study, a deep-seated nodule was defined as a nodule with the distance between the nearest visceral pleural surface and the nodular inner margin of ≥20 mm (14). Of the 203 patients with 235 SPN localizations, 73 patients met the criteria of having a deep-seated nodule and were enrolled in this study. A flow algorithm of patient selection is shown in Figure 1.
Interventional equipment and localization procedure
A 64-slice CT scanner (AquilionTSX-101A, TOSHIBA, Tokyo, Japan) with a thin slice protocol (1.25 mm thickness) was utilized. All patients underwent CT-guided percutaneous localization performed by an experienced interventional radiologist. The novel localization device, known as the 4-hook needle (Sheng Jie Kang Biological Technology Co., Ltd., Ningbo, China), was characterized by its 4-hook anchor and a tri-colored suture with a scale (Figure 2). The 4-hook anchor, made of nickel titanium memory alloy, could securely fix the lung tissue and the nodule. The tri-colored suture with a scale was 9 cm in length, with each colored suture being 3 cm respectively, divided into 6 sections on average with 5 mm for each section. When pulling the lung tissue during wedge resection, the 4-hook anchor could prevent nodule shifting, and the tri-colored suture could estimate the depth of the SPN.

Details of the CT-guided localization procedure have been described in previous studies (11-13). Briefly, first, the patient selected an appropriate position according to the planned puncture path and then underwent a CT scan. Second, after infiltration anesthesia with lidocaine, the needle was advanced to less than 10 mm beside the nodule (Figure 3A), which was confirmed by a CT scan. Third, we removed the card clasp and pushed the pusher, releasing the 4-hook anchor near the expected position (Figure 3B). Fourth, the pusher was reinserted into the coaxial needle to release the tri-colored suture in the chest wall. Finally, the patient underwent a final CT scan to confirm the site of the device and any complications. Unlike other studies, we required the puncture path to be as perpendicular to the pleura as possible, with the anchor either lateral or behind the nodule within 10 mm, rather than through or in front of the nodule. Patients typically underwent the operation the day after the localization.
Surgical procedure
After evaluation, most patients were anesthetized with a laryngeal mask, whereas some patients were anesthetized with a double-lumen endotracheal tube (such as those with the possibility of lobectomy due to deep nodule location or pleural adhesions). Depending on the surgeon’s preference, either single or double port VATS was used. An incision of about 3 cm was made in the 4th or 5th intercostal space of the anterior axillary line. For the 2-port method, an observation port of about 1 cm was made in the 7th intercostal space of the midaxillary line. All patients initially underwent successful thoracoscopic lung wedge resection.
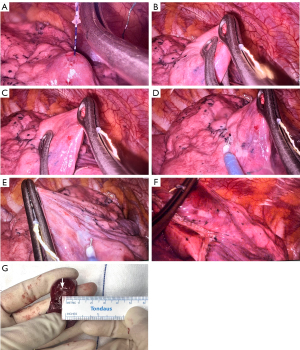
Upon entering the thoracic cavity, the patient’s breathing was stopped and the respiratory circuit was opened (anesthesia via a laryngeal mask) or single lung ventilation was initiated (anesthetized with a double-lumen endotracheal tube). The lung tissue was compressed by oval forceps to completely collapse the lung, making it easy to find the tri-colored suture on the surface of the pleura. Based on the 3-dimensional (3D) relationship between the pleural puncture point, hook, and nodule, the location of the nodule was determined and then the scale suture near the pleura was gently pulled to evaluate the depth of the nodule (Figure 4A). Oval forceps with a diameter of about 1 cm were used to squeeze the residual air in the lung and measure the expected resection margin (Figure 4B,4C), which was marked bilaterally by an electrotome (Figure 4D,4E). Then, the nodule and anchor were resected with staplers below the marked line (Figure 4F). The resection margin distance was measured with a ruler from the border of the nodule to the nearest staple line (Figure 4G). The resection margin was determined by the reading of the scale suture, the 3D relationship between the anchor and the nodule, and the diameter of the nodule. The resection margin distance was expected to be at least equal to the maximum diameter of the nodule.
The specimen was transected along the maximum diameter of the nodule and perpendicular to the resection line in its natural state, and the resection margin distance was measured with a ruler. The resection margin distance is defined as the distance from the inner margin of the nodule to the resection line, including the staple line. The resection margin distance was recorded by the surgeon in the surgical record. All cases underwent rapid frozen pathology examination during the operation. Complete resection is sufficient if the disease is benign or metastatic, regardless of the resection margin distance. The resection margin distance is required to be more than 5 mm if the disease is carcinoma in situ or malignant. If the resection margin distance is less than 5 mm for invasive adenocarcinoma (IAC), an expanded wedge resection is needed. If an extended wedge resection is not possible, segmental or lobectomy is performed to obtain sufficient margins.
Histopathological evaluation
All specimens were examined by pathologists’ post-operation. The size of the nodule and the resection margin distance were measured macroscopically. The resection margin tissue was examined histologically. According to the 2015 World Health Organization (WHO) Classification of Lung Tumors, if lung adenocarcinoma was diagnosed, it was categorized into the following subtypes: adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and IAC. AIS was defined as small (≤3 cm) solitary adenocarcinomas without invasion, and MIA was defined as adenocarcinomas that exhibited a lepidic growth pattern with ≤5 mm invasion. IAC was defined as adenocarcinomas with ≥5 mm invasion, which could be further classified into the following subtypes: lepidic, acinar, papillary, micropapillary, solid adenocarcinoma, and others. The subtype and the percentage were recorded. These data were documented in the final pathological reports.
Main observation indicators
The indicators of the maximum diameter of the nodule, depth of the nodule, depth of the localization device, and the distance between the claw and the nodule were collected in the picture archiving and communication system. The depth of the nodule was defined as the distance between the nearest visceral pleural surface and the inner margin of the nodule. The duration of localization, success rate of localization, localization-related complications, operation time, margin distance, and margin-to-tumor ratio [margin distance (mm)/maximum tumor diameter (mm)] were recorded. Successful localization was defined as the distance between the claw and the nodule being ≤10 mm. All the indicators were determined by 2 reviewers and recorded after they reached a consensus.
Statistical analysis
Continuous variables were presented as a number, median, and range, whereas categorical variables were presented as a number and percentage. All descriptive statistics were conducted using SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline statistics
From 1 November 2021 to 31 October 2023, 73 patients (27 males and 46 females; median age 50.2 years; age range, 16–80 years) were selected according to the inclusion and exclusion criteria. All patients had a single deep-seated pulmonary nodule. The patient demographics and nodule characteristics are summarized in Table 1. Of the 73 nodules, the most common were pure ground-glass opacity (GGO) (37, 50.7%), followed by part-solid nodules (23, 31.5%) and solid nodules (13, 17.8%). The nodules were most commonly found in the right upper lobe, followed by the left upper lobe (37, 50.7% and 16, 21.9%, respectively). The median diameter and depth of the nodules were 10.7 mm (range, 5–23 mm) and 25.3 mm (range, 20–49 mm), respectively, according to the CT image in the lung window. Regarding the pathologic diagnosis, 53 of the 73 lesions (72.5%) were malignant (42 MIA, 6 IAC, and 5 metastatic cancers), 11 (15.1%) were benign, whereas 9 (12.4%) were precursor glandular lesions.
Table 1
| Variables | Value (N=73) |
|---|---|
| Gender | |
| Male | 27 (37.0) |
| Female | 46 (63.0) |
| Age (years) | 50.2 [16–80] |
| Smoker | |
| Yes | 17 (23.3) |
| No | 56 (76.7) |
| Nodule type | |
| Pure GGO | 37 (50.7) |
| Part-solid | 23 (31.5) |
| Solid | 13 (17.8) |
| Location | |
| Left upper lobe | 16 (21.9) |
| Left lower lobe | 5 (6.8) |
| Right upper lobe | 37 (50.7) |
| Right middle lobe | 5 (6.8) |
| Right lower lobe | 10 (13.7) |
| Size (diameter, mm) | 10.7 [5–23] |
| Nodule depth* (mm) | 25.3 [20–49] |
| ≥20 and <30 | 61 (83.6) |
| ≥30 | 12 (16.4) |
| Pathological diagnosis | |
| AAH | 1 (1.4) |
| AIS | 8 (11.0) |
| MIA | 42 (57.5) |
| IAC | 6 (8.2) |
| Metastatic tumor | 5 (6.8) |
| Others** | 11 (15.1) |
| Stage*** | |
| Benign | 12 (16.4) |
| 0 | 8 (11.0) |
| IA1 | 48 (65.8) |
| IV (metastatic cancer) | 5 (6.8) |
Values are presented as median [range] or n (%). N, the number of patients. *, nodule depth was defined as the distance between the nearest visceral pleural surface and the nodular inner margin. **, including granuloma, inflammation, benign tumor, and lymph node. ***, stage was determined by the eighth edition classification of lung cancer. GGO, ground-glass opacity; AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IAC, invasive adenocarcinoma.
Localization procedure and surgery outcomes
Table 2 summarizes the data on the localization procedure and surgery outcomes. The success rate of localization was 93.2% (68/73) in this study, with 5 patients experiencing failure. Successful localization was defined as the claw being less than 10 mm next to the nodule. The median times for localization and surgery were 23 minutes (range, 12–47 min) and 85.6 minutes (range, 35–300 min), respectively. Most patients (71, 97.3%) underwent surgery the day after localization; 2 patients underwent surgery on the same day. The average hospitalization time was 6.1 days (range, 3–13 days), and no deaths occurred.
Table 2
| Variables | Values (N=73) |
|---|---|
| Time of localization (min) | 23 [12–47] |
| Time of surgery (min) | 85.6 [35–300] |
| Time between localization and surgery | |
| One day before surgery | 71 (97.3) |
| The day of surgery | 2 (2.7) |
| Time of hospitalization (days) | 6.1 [3–13] |
| The depth of the anchor claw* (mm) | 22.5 [9–65] |
| Location of the anchor claw (mm) | |
| In nodules | 4 (5.5) |
| Around nodules | |
| >1 and ≤10 | 64 (87.7) |
| >10 | 4 (5.5) |
| Misplacement | 1 (1.4) |
| Distance between claw and nodule (mm) | |
| Successful localization | 68 (93.2) |
| Failed localization | 5 (6.8) |
| Localization related complications | 25 (34.2) |
| Parenchymal hemorrhage | 17 (23.3) |
| Small | 17 |
| Large | 0 |
| Pneumothorax | 8 (11.0) |
| Small | 8 |
| Large | 0 |
| Hemothorax | 0 |
| Hemoptysis | 0 |
| Surgical procedure | |
| Wedge resection | 71 (97.3) |
| Extended wedge resection | 0 |
| Segmentectomy | 0 |
| Lobectomy | 2 (2.7) |
| Retrieve of device after resection | 73 (100.0) |
Data are showed as median [range], n (%) or n. N, the number of patients. *, the depth of the anchor claw was defined as the distance between the nearest visceral pleural surface and inner margin of the anchor claw.
The median depth of the anchor claw was 22.5 mm (range, 9–65 mm), and the median distance between the claw and the nodule was 4.6 mm (range, 0–25 mm). The distance between the claw and the nodule was less than 10 mm in most cases (64, 87.7%), more than 10 mm in 4 cases, and within the nodule for 4 cases, with displacement occurring in 1 case.
Of the 5 failed cases, 1 failed due to the anchor being misplaced in the oblique fissure, while the puncture route passed through the dorsal segment to locate the nodule in the posterior segment. A total of 2 cases failed due to the occlusion of the bone structure, and the puncture path could not be properly perpendicular to the visceral pleura. The other 2 cases failed due to respiratory movement while the nodules were located in the lower lobe near the diaphragm. All 5 failed cases were successfully resected according to the 3D space of the nodule, claw, and tri-colored suture in the visceral pleural surfactant.
The common complications of localization included focal pulmonary parenchymal hemorrhage (17, 23.3%) and pneumothorax (8, 11%). There were no major complications such as hemoptysis or hemothorax, and no chest tube drainage was required after localization.
Wedge resection was successfully performed for all SPNs on the first attempt, and all patients received a complete resection, which was quickly confirmed by the surgeon and pathologists. A single case, a nodule in the anterior segment of the right upper lung with a 30 mm depth, with a resection margin distance of 4 mm and pathology of MIA, was subsequently converted to lobectomy. Another case was converted to lobectomy due to the solid nodule with a 9 mm diameter and frozen pathology indicating at least MIA.
Resection margin distance
The results of the wedge resection margin distance are summarized in Table 3. The median resection margin distance was 14.4 mm (range, 4–29 mm). There were 8 cases with a resection margin distance of less than 10 mm, including 1 case with 4 mm, 6 cases with 8 mm, and 1 case with 9 mm. Additionally, there were 52 cases with a resection margin distance between 10 to 19 mm, and 13 cases with a distance greater than 20 mm. The median resection margin distance in nodules with depth ≥30 mm was 12.5 mm (range, 4–22 mm), whereas that was 15.3 mm (range, 8–29 mm) in nodules with depth <30 mm. A single case with a resection margin distance of 4 mm was the nodule in the anterior segment of the right upper lung with 30 mm depth.
Table 3
| Variables | Values (N=73) |
|---|---|
| Margin distance (mm) | 14.4 [4–29] |
| <10 | 8 (11.0) |
| ≥10 and <20 | 52 (71.2) |
| ≥20 | 13 (17.8) |
| Margin distance/tumor size ratio | |
| <1 | 11 (15.1) |
| ≥1 | 62 (84.9) |
Values are presented as median [range] or n (%).
The median resection margin-to-tumor diameter ratio in the first resected specimen was 1.42 (range, 0.4–2.5), with 62 cases (84.9%) having a margin distance to tumor size ratio of ≥1 and 11 cases (15.1%) having a ratio of <1. A total of 2 cases with a margin distance to tumor size ratio of <1 were subsequently converted to lobectomy.
Discussion
The preoperative or intraoperative identification of deep-seated SPNs and the acquisition of a sufficient resection margin distance pose challenges during wedge resection via VATS. In this study, we utilized a novel device in CT-guided percutaneous localization for deep-seated SPNs prior to wedge resection via VATS. The results showed that this localization technique is simple, accurate, and efficient. This study provides a simple and precise technique for obtaining an adequate resection margin distance for deep-seated SPNs during wedge resection by VATS.
Preoperative localization enables surgeons to rapidly find the target lesion, perform accurate resections, and shorten the operation time for SPN wedge resection. However, most localization procedures provide accurate pleural surface localization and facilitate subsequent resection using staplers for superficial lesions, but not for deep-seated SPNs. This is because when lung collapse and surface tissue grasping occur during surgery, these methods do not provide an accurate depth of the lesion. Thus, the surgical margin distance cannot be assessed accurately, leading to insufficient surgical margin distance or positive margins during wedge resection, especially for deep-seated SPNs. Therefore, the localization for deep-seated SPNs requires not only precise location of the pleural surface but also an accurate evaluation of the nodule depth during wedge resection.
Several preoperative and intraoperative localization methods have been developed for deep-seated SPNs, including a virtual-assisted lung mapping system (6,7), multispot dye marks, combining microcoil and patent blue vital dye (8), or indocyanine green (9), and a radiofrequency identification marker (10). Each method has its disadvantages, such as additional radiation exposure, the need for special equipment (such as fluoroscopy, a radiofrequency system, and a hybrid operating room), and a complicated procedure, leading to difficulties in widespread use. Therefore, a simple and easy method that can be applied in most hospitals is urgently needed.
Recently, Fan et al. first reported a novel device, with a 4-hook and scaled suture, for SPN localization and wedge resection with good success, safety, feasibility, and tolerability (11). This device does not require any special equipment or technique. The localization procedure with this novel device is similar to conventional CT-guided percutaneous lung puncture localization and has been widely used in China (12,15-17). However, there is still a lack of experience in using this device for deep-seated nodules, and no details about the means of determining the resection margin accurately have been published in the literature (11,12,15-18). Given the design of the 4-hook and scale suture system, we speculated that it could not only accurately locate the nodules but also achieve precise wedge resection and sufficient surgical margin distance in thoracoscopic surgery. The target lesion and surrounding lung tissue can be fixed by the 4-hook system, thus preventing the nodule from shifting when grasping the surface tissue. The resection margin distance can be reliably determined when lung collapse occurs during surgery by the scale suture, which is different from the distance measured on preoperative CT images when the lung is expanded. In our previous study, we modified the localization point to be as close as possible beside or behind the nodule and improved the method for evaluating the depth of the nodule. We preliminarily found that it is possible to accurately locate and obtain sufficient surgical margins distance, and this technique showed some advantages by the subgroup analysis of deep-seated nodules in a small sample (13). Therefore, in this study, we expanded the sample size to focus on the localization for deep-seated SPNs with this novel device.
According to the JCOG 0804 study (19), a minimum sublobar resection margin distance of 5 mm is sufficient for local control and recurrence-free survival for GGO dominant peripheral lung tumors 20 mm or less in size. In this study, resection margin distances of more than 5 mm were obtained in 72 (72/73, 98.6%) patients, with only 1 case having a resection margin distance of 4 mm. The median resection margin distance was 14.4 mm. The lesion of the case with a 4 mm resection margin distance was located in the anterior segment of the right upper lobe with a size of 10 mm and a depth of 30 mm. The patient was converted to lobectomy due to the difficulty of extended excision after initial wedge resection. Meanwhile, there were 62 (84.9%) cases with a margin distance to tumor size ratio of ≥1, and 11 (15.1%) cases with a ratio of <1. The results are satisfactory and consistent with our previous research (13). In general, it showed that sufficient surgical margins can be obtained by this localization device and measurement method for deep-seated nodules.
Accurate evaluation of the depth of the nodule is key to ensuring complete resection and an adequate resection margin. However, the depth of the nodule and the resection distance are usually evaluated based on the surgeon’s personal experience during the operation, which lacks accuracy and repeatability. Therefore, we standardized the evaluation of nodule depth and the procedure of wedge resection. The detailed steps of localization are as follows (Figure 4). First, the puncture path was made as perpendicular to the pleura as possible, and the hook was placed as near to the lesion as possible. Second, after evaluating the depth of the claw by reading the scaled suture, the suture and some visceral pleura were gently pulled with an oval or curved forceps. Third, the expected resection margin was measured using oval forceps with a diameter of 10 mm or a ruler. Fourth, the bilateral resection line was marked with an electrotome. Lastly, wedge resection was performed using staplers under the markers. The depth of the nodule and resection margin distance were evaluated by reading the scaled suture and the 3D relationship between the claw and the nodule. In this study, all deep-seated SPNs were completely resected on the first attempt.
Although this method presents certain advantages in localizing and performing wedge resection for deep-seated SPN, it also has some drawbacks. It remains incapable of overcoming the disadvantages associated with CT-guided percutaneous localization, such as the inability to locate paramediastinal nodules, scapular obstruction, pain, radiation exposure for patients, and so forth. In addition, it is necessary to carefully select patients on whom to use this method to locate nodules with depth ≥30 mm for wedge resection. For nodules with depth ≥30 mm, except for some locations that are easy to perform wedge resection, such as the apex of the lung, a wedge resection should be carefully selected, and segmentectomy should be considered instead to ensure an adequate resection margin distance. Therefore, it is necessary to carefully select patients for the application of this approach.
There are limitations in this study, including its retrospective, single-center design, small sample size, and lack of a control group (i.e., superficial dye marking combined with deeply-placed microcoil), which makes it difficult to differentiate the advantage of this novel device and method for localization of deep-seated SPNs. Further investigations are necessary to clarify the applicability and advantage of this method for wedge resection of deep-seated SPNs with different characteristics and locations.
Conclusions
Our preliminary experience suggests that preoperative CT-guided 4-hook needle with scaled suture localization is an efficient, simple, and precise strategy for the wedge resection of deep-seated SPNs via VATS, ensuring a sufficient resection margin distance. Although the benefits of this novel localization technique should be confirmed in further studies, we believe that this localization technique can enhance surgical accuracy and avoid unnecessary trauma to the patient, making it worthy of further promotion.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1571/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1571/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and received approval from the Ethics Committee of The Third Affiliated Hospital of Sun Yat-sen University (No. II2023-257-02). Written informed consent for publication was provided by each participant.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Krochmal R, Arias S, Yarmus L, Feller-Kopman D, Lee H. Diagnosis and management of pulmonary nodules. Expert Rev Respir Med 2014;8:677-91. [Crossref] [PubMed]
- Yang SM, Hsu HH, Chen JS. Recent advances in surgical management of early lung cancer. J Formos Med Assoc 2017;116:917-23. [Crossref] [PubMed]
- Thistlethwaite PA, Gower JR, Hernandez M, Zhang Y, Picel AC, Roberts AC. Needle localization of small pulmonary nodules: Lessons learned. J Thorac Cardiovasc Surg 2018;155:2140-7. [Crossref] [PubMed]
- Hsu PK, Chuang LC, Wu YC. Electromagnetic Navigation-Guided Preoperative Localization of Small Malignant Pulmonary Tumors. Ann Thorac Surg 2020;109:1566-73. [Crossref] [PubMed]
- Ajmani GS, Wang CH, Kim KW, Howington JA, Krantz SB. Surgical quality of wedge resection affects overall survival in patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg 2018;156:380-391.e2. [Crossref] [PubMed]
- Sato M, Nagayama K, Kobayashi M, Nakajima J. Virtual-Assisted Lung Mapping 2.0: Preoperative Bronchoscopic Three-Dimensional Lung Mapping. Ann Thorac Surg 2019;108:269-73. [Crossref] [PubMed]
- Sato M, Kobayashi M, Sakamoto J, Fukai R, Takizawa H, Shinohara S, Kojima F, Sakurada A, Nakajima J. The role of virtual-assisted lung mapping 2.0 combining microcoils and dye marks in deep lung resection. J Thorac Cardiovasc Surg 2022;164:243-251.e5. [Crossref] [PubMed]
- Lin CW, Ko HJ, Yang SM, Chen YC, Ko WC, Huang HC, Chen JS, Chang YC. Computed tomography-guided dual localization with microcoil and patent blue vital dye for deep-seated pulmonary nodules in thoracoscopic surgery. J Formos Med Assoc 2019;118:979-85. [Crossref] [PubMed]
- Chao YK, Leow OQY, Wen CT, Fang HY. Image-guided thoracoscopic lung resection using a dual-marker localization technique in a hybrid operating room. Surg Endosc 2019;33:3858-63. [Crossref] [PubMed]
- Yutaka Y, Sato T, Tanaka S, Miyahara S, Yoshizawa A, Morita S, Date H. Feasibility study of a novel wireless localization technique using radiofrequency identification markers for small and deeply located lung lesions. JTCVS Tech 2022;12:185-95. [Crossref] [PubMed]
- Fan L, Yang H, Yu L, Wang Z, Ye J, Zhao Y, Cai D, Zhao H, Yao F. Multicenter, prospective, observational study of a novel technique for preoperative pulmonary nodule localization. J Thorac Cardiovasc Surg 2020;160:532-539.e2. [Crossref] [PubMed]
- Chen ZM, Xu JY, Cai WQ, Liao FC, Huo SQ, Yang JW, Peng J. The 4-hook anchor coaxial needle with scaled suture is superior to the double spring coil for preoperative localization. J Thorac Dis 2021;13:4455-63. [Crossref] [PubMed]
- Wu W, Li X, Wu Y, Zhang K, Xu J, Zhang J, Chen H. A novel localization device for small pulmonary nodules in thoracoscopic wedge resection with adequate margin distance: a retrospective study. J Thorac Dis 2023;15:6515-24. [Crossref] [PubMed]
- Tsai TM, Chiang XH, Liao HC, Tsou KC, Lin MW, Chen KC, Hsu HH, Chen JS. Computed tomography-guided dye localization for deeply situated pulmonary nodules in thoracoscopic surgery. Ann Transl Med 2019;7:31. [Crossref] [PubMed]
- Huang YY, Wang T, Fu YF, Shi YB, Cao W, Hou JP. Comparison of the effectiveness of anchoring needles and coils in localizing multiple nodules in the lung. BMC Pulm Med 2022;22:393. [Crossref] [PubMed]
- Kong J, Guo J, Zhang H, Li Y, Wang G, Zhang Y. CT-guided localization techniques of small pulmonary nodules: a prospective non-randomized controlled study on pulmonary nodule localization needle and methylene blue staining with surgical glue. J Thorac Dis 2020;12:6826-35. [Crossref] [PubMed]
- Wang L, He J, Zhang L, Chen C, Chen B, Shen W. A novel preoperative image-guided localization for small pulmonary nodule resection using a claw-suture device. Sci Rep 2023;13:18950. [Crossref] [PubMed]
- Fan L, Ma W, Ma J, Yang L, Wang Z, Xu K, Jia Y, Sun B, Sieren JC, Yang H, Yao F. The improved success rate and reduced complications of a novel localization device vs. hookwire for thoracoscopic resection of small pulmonary nodules: a single-center, open-label, randomized clinical trial. Transl Lung Cancer Res 2022;11:1702-12. [Crossref] [PubMed]
- Suzuki K, Watanabe SI, Wakabayashi M, Saji H, Aokage K, Moriya Y, Yoshino I, Tsuboi M, Nakamura S, Nakamura K, Mitsudomi T, Asamura HWest Japan Oncology Group and Japan Clinical Oncology Group. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg 2022;163:289-301.e2. [Crossref] [PubMed]



