
The semiquantitative assessment of metacarpal head cartilage damage in rheumatoid arthritis via ultrahigh frequency ultrasound
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune inflammatory disease characterized by proliferative synovitis. It is found in approximately 0.5–1.0% of the overall population and frequently affects multiple joints throughout the body. Articular cartilage damage is an early stage of joint damage and is difficult to repair as the disease progresses, exerting a severely negative impact on the quality of life of patients with RA. In early RA, the vulnerable areas include the metacarpophalangeal (MCP) joint, proximal interphalangeal (PIP) joint, and the wrist (1). The cartilage of the metacarpal head (MH) is superficial and easily visible (2-4). Damage to cartilage can be detected at an early stage by monitoring the hyaline cartilage of MH, which can then be used to evaluate the functional condition of the whole joint.
At present, conventional radiography can only indirectly measure joint deterioration through the joint space and has insufficient sensitivity for revealing cartilage lesions (3,5-7). Magnetic resonance imaging (MRI), although capable of directly visualizing hyaline cartilage, is time-consuming, expensive, lacks mobility, and requires a specific coil (8-10). High frequency ultrasound (HFUS) has been applied in the evaluation of early articular cartilage damage due to its lack of radiation, low cost, and dynamic observation (11-13). However, the range in frequencies of the probes can lead to differences in ultrasound evaluation results (14), and ultrasound machines and probes vary in quality, with higher-frequency probes being more suited for shallow structures and providing increased spatial resolution.
Ultrahigh frequency ultrasound (UHFUS), a recent innovation with a probe frequency of ≥30 MHz and a spatial resolution of 30 µm, is able to observe and measure finer structures (15,16). The assessment of MCP cartilage via UHFUS mainly includes quantitative and semiquantitative approaches. Quantitative assessment involves the measurement of cartilage thickness, but the thinning of cartilage can also occur in healthy individuals, and these measurements are affected by the frequency of the probes. Semiquantitative assessment methods include the three-grade scoring method established by the European Society of Rheumatology in the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) (2) and the five-grade scoring proposed by Disler et al. (17). MH cartilage damage appears to be prevalent in the second and third fingers on the semiquantitative assessment of MCP 2–5 cartilage in patients with RA (3), but the specific portions of cartilage damaged in each of these sections has not been examined. Moreover, few studies have compared the efficacy of the two scoring methods in assessing RA cartilage.
Therefore, the aim of this study was to investigate the vulnerable portion of MH cartilage damage in patients with RA and evaluate the differences between two UHFUS-based semiquantitative scoring systems for assessing MH cartilage in patients with RA. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1539/rc).
Methods
Study participants
A total of 110 patients with RA were enrolled in this cross-sectional study from April 2023 to October 2023 in the West China Hospital of Sichuan University. Patients were included if they were diagnosed by clinicians or sonographers as having RA according to the 2010 American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) criteria, were 18–70 years of age, and demonstrated good compliance during the examination. Patients with any other diseases, trauma, or surgical history potentially associated with hand joints, including combined metabolic and infections joint diseases, were excluded. Data were also acquired in 110 healthy controls (HCs) who were enrolled as part of the methodological protocol for the sonographer and included hospital staff and their friends or relatives; HCs were required to be aged 18–70 years and have no history of related articular cartilage disorder. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the principles of Good Clinical Practice. The study was approved by the Biomedical Ethics Review Committee of West China Hospital of Sichuan University {No. 2023 review [1886]}. Written informed consent was obtained from all patients and healthy controls (HCs).
Ultrasound image acquisition
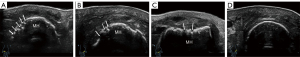
A Resona R9 ultrasound diagnostic instrument with an L30-8U ultrahigh-frequency linear probe (Mindray, Shenzhen, China) was applied to preset the musculoskeletal conditions, with the depth set to 1.0–1.5 cm and the focus adjusted to the level of the target MH cartilage for scanning. When participants had both their hands clenched into fists (MCP maximum flexion position), the transducer was lightly placed on the sound-guiding pad with a size of 140 mm × 110 mm × 7 mm (Beijing Deji Huizhong Medical Instrument Co., Ltd., Beijing, China), and the sound beam was positioned perpendicular to the surface of cartilage. The dorsal cartilage of the MH 1–5 of both hands was dynamically swept one by one from the radial to ulnar sides at transverse sections and from the proximal to distal at longitudinal sections (Figure 1). All the patients with RA and the HCs were scanned by one sonographer with more than 3 years of experience in musculoskeletal sonography. Additionally, 20 patients with RA were randomly selected and recalled 1 day after the examination to be reexamined with the same ultrasound instrumentation, method of examination, and sonographer, in addition to one other sonographer.
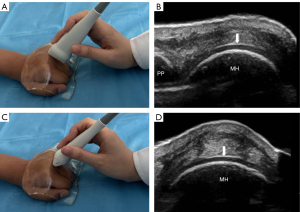
Ultrasound image interpretation
The degree of cartilage damage was assessed in both the transverse and longitudinal sections with the three-grade scoring and the five-grade scoring system (Figures 2,3), with the scoring results being recorded simultaneously. All pathological results were documented with at least two perpendicular scans. The semiquantitative scoring ratings greater than grade 0 were considered to indicate impairment. The most clearly displayed cartilage was divided into three parts, and the damage site was observed and recorded, with the transverse section being divided into the middle, radial, and ulnar sides and the longitudinal section being divided into middle, distal, and proximal sides. Homogeneous thinning was recorded as being widespread (Figures 4,5).
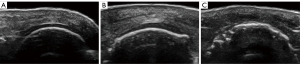
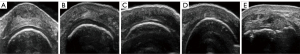
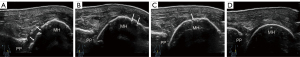
Statistical analysis
Statistical analysis was performed with SPSS 26.0 software (IBM Corp., Armonk, NY, USA). Count data were expressed as the frequency and rate, and the comparison of count data was performed using the χ2 test. Consistency was assessed using the unweighted Cohen kappa coefficient, with κ<0.4 considered as poor, 0.4–0.6 as moderate, 0.61–0.8 as good, and >0.8 as excellent. The Cochran Q test was applied to compare multiple groups, and two-by-two comparisons were completed using the test level α' (adjusted with the Bonferroni’s method: α' = α/N, where N is the number of two-by-two comparisons, and α is 0.05). Spearman correlation analysis was applied, with |r| ≥0.8 considered as highly correlated, 0.5≤ |r| <0.8 as moderately correlated, 0.3≤ |r| <0.5 as weakly correlated, and |r| <0.3 as very weakly correlated. Two-tailed P values <0.05 indicated statistical significance.
Results
Demographics of participants
The demographic characteristics of the participants are presented in Table 1. There were 110 HCs and 110 patients with RA who participated in this study. Regarding gender, age, and height, there were no statistically significant differences between HCs and patients with RA. However, there were statistically significant differences between the two groups in body weight and body mass index (BMI) (P<0.05), with patients with RA having a lower body weight and BMI than HCs.
Table 1
| Characteristic | RA patients | HCs | P value |
|---|---|---|---|
| Gender (male/female) | 18/92 | 19/91 | 0.857 |
| Age (years) | 51.00 (43.00–58.00) | 49.50 (37.00–56.00) | 0.068 |
| Height (m) | 1.58 (1.55–1.62) | 1.60 (1.55–1.75) | 0.050 |
| Weight (kg) | 55.00 (51.00–60.00) | 57.80 (52.00–63.30) | 0.018 |
| BMI (kg/m2) | 22.05 (20.50–23.40) | 23.20 (21.20–25.80) | 0.003 |
| Duration of disease (months) | 24.00 (10.00–84.00) | – | – |
| CRP (mg/L) | 5.08 (2.22–18.30) | – | – |
| Anti-CCP (<8.0/8.0–500.0/>500.0 U/mL) | 17/50/43 | – | – |
| RF (<20.0/>20.0 IU/mL) | 18/92 | – | – |
| DAS28 | 3.57 (2.22–5.31) | – | – |
Data are presented as mean (range) or number. RA, rheumatoid arthritis; HCs, healthy controls; BMI, body mass index; CRP, C-reactive protein; Anti-CCP, anti-cyclic citrullinated peptide antibody; RF, rheumatoid factor; DAS28, Disease Activity Score 28.
Consistency analysis of MH cartilage on UHFUS
The results of the inter- and intraobserver reliability analyses of the two grade scoring systems for both the transverse and longitudinal sections are presented in Table 2. There was excellent intraobserver consistency for both sections under the three-grade scoring system and for the transverse section under the five-grade scoring system. The interobserver agreement was good for the transverse section under both scoring systems but was moderate for the longitudinal section.
Table 2
| Consistency | Section | Kappa (95% CI) | P value | |
|---|---|---|---|---|
| The three-grade of scoring | The five-grade of scoring | |||
| Intra-observer consistency | Transverse section | 0.884 (0.780–0.963) | 0.812 (0.697–0.908) | <0.001 |
| Longitudinal section | 0.896 (0.773–0.977) | 0.624 (0.451–0.760) | <0.001 | |
| Inter-observer consistency | Transverse section | 0.721 (0.593–0.844) | 0.665 (0.559–0.759) | <0.001 |
| Longitudinal section | 0.485 (0.335–0.616) | 0.470 (0.342–0.591) | <0.001 | |
CI, confidence interval.
Comparison of the semiquantitative scoring methods
Patients with RA had greater cartilage damage than did HCs. The cartilage in about 24.18% of the 1,100 MHs among patients with RA was assessed as being degraded in the transverse section under the five-grade grading system. The transverse section exhibited greater incidence of cartilage damage than did longitudinal section (P<0.001) (Tables 3,4). The number of graded cases is shown in Table 5.
Table 3
| Method | Section | RA patients | HCs | P value |
|---|---|---|---|---|
| The three-grade scoring | Transverse section | 200 (18.18) | 38 (3.45) | <0.001 |
| Longitudinal section | 145 (13.18) | 20 (1.82) | <0.001 | |
| The five-grade scoring | Transverse section | 266 (24.18) | 49 (4.45) | <0.001 |
| Longitudinal section | 187 (17.00) | 31 (2.82) | <0.001 |
Data are presented as n (%). RA, rheumatoid arthritis; HCs, healthy controls.
Table 4
| Participants | Section | The three-grade scoring | The five-grade scoring | P value |
|---|---|---|---|---|
| RA patients | Transverse section | 200 (18.18) | 266 (24.18) | <0.001 |
| Longitudinal section | 145 (13.18) | 187 (17.00) | <0.001 | |
| HCs | Transverse section | 38 (3.45) | 49 (4.45) | <0.001 |
| Longitudinal section | 20 (1.82) | 31 (2.82) | <0.001 |
Data are presented as n (%). RA, rheumatoid arthritis; HCs, healthy controls.
Table 5
| Grade | Transverse section | longitudinal section | |||
|---|---|---|---|---|---|
| The three-grade of scoring | The five-grade of scoring | The three-grade of scoring | The five-grade of scoring | ||
| Grade 0 | 900 (81.82) | 834 (75.82) | 955 (86.82) | 913 (83.00) | |
| Grade 1 | 110 (10.00) | 66 (6.00) | 70 (6.36) | 42 (3.82) | |
| Grade 2 | 90 (8.18) | 125 (11.36) | 75 (6.82) | 90 (8.18) | |
| Grade 3 | 31 (2.82) | 19 (1.73) | |||
| Grade 4 | 44 (4.00) | 36 (3.27) | |||
Data are presented as n (%).
Sites of cartilage damage in patients with RA
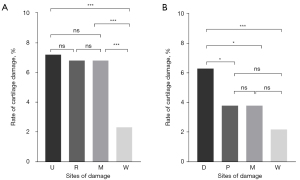
In the comparison of MH cartilage damage in patients with RA under the five-grade scoring system, the ulnar side of the transverse section and the distal side of the longitudinal section had the highest rate of cartilage damage (P<0.001), with the cartilage injury rate of the distal side being 6.30% and that on the ulnar side being 7.20% (Figure 6).
Correlation of semiquantitative scores with clinical and laboratory indicators
As can be seen in Table S1, cartilage damage was weakly positively correlated with age, course of disease, body weight, BMI, C-reactive protein (CRP), and Disease Activity Score 28 (DAS28) using CPR (r=0.068–0.192).
Discussion
High-frequency ultrasound can be used to assess articular cartilage (2,8,18-22), and previous studies in this area have focused on weight-bearing joints such as the hip and knee joints. Related research has been conducted on small joints such as the MCP and the PIP joints (1,14-16,23-26), because the joints of the hand are superficially located, are thus easy to visualize with ultrasound, and correlate well with the degree of damage to other joints. Semiquantitative assessment is a subjective evaluation of cartilage damage, and standardized training in MCP cartilage scanning and measurement methods has demonstrated good consistency in semiquantitative assessment, but probe frequency and sound beam orientation may affect the results (27,28). Evaluation of cartilage damage by UHFUS has not been extensively researched, and there are no comparative studies regarding site of cartilage damage or the differences between different semiquantitative scores.
In this study, the transverse and longitudinal sections of the MH 1–5 dorsal cartilage were examined through the three- and five- grade scoring systems with a 30-MHz probe. The results at the transverse and longitudinal sections assessed on the three-grade scoring system had intraobserver agreement as did those of the transverse section scored in the five-grade scoring systems. However, there was only moderate-to-good interobserver consistency for different sections under both grade scoring systems. Filippucci et al. (26) assessed the dorsal cartilage of the MCP in patients with RA on two occasions using the five-grade scoring system with 6- to 18-MHz and 22-MHz probes, respectively. The 6- to 18-MHz probe showed moderate interobserver agreement while the 22-MHz probe showed good intraobserver and interobserver agreement. Meanwhile, Mandl et al. (2) used 8- to 18-MHz and 10- to 22-MHz probes to assess dorsal cartilage damage to the MCP joints in longitudinal section in patients with RA and HCs using the three-grade scoring system, reporting good-to-excellent inter- and intraobserver agreement. Our results are basically consistent with those in these previous studies. These findings may be explained by the high frequency of probe, which could provide enhanced resolution and ultrasound image quality. However, Ogura et al. (24) reported excellent intraobserver agreement and moderate interobserver agreement using a 7- to 14-MHz probe to scan the longitudinal section of the dorsal cartilage of the MCP 2–5 and PIP joints in patients with RA and HCs. This suggests that probe frequency affects the assessment results, with patient position, examiner experience, and other factors also exerting an influence.
The MH cartilage is equally damaged in HCs regardless of age and gender; therefore, there is a need to investigate whether there is a difference in MH cartilage damage between patients with RA and HCs. In this study, we found that the incidence of cartilage damage was significantly higher in patients with RA than in HCs (P<0.05), which is consistent with previous studies (24,25), and can be explained by the fact that RA is an autoimmune disease with a high degree of joint destruction.
In this study, we evaluated three-grade scoring and the five-grade scoring systems. The results revealed that the detection rate of cartilage damage was higher at the transverse section than that at the longitudinal section for both scoring methods, which may be related to the more complete display at the transverse section. Furthermore, the rate for cartilage damage was higher for the five-grade scoring system than for the three-grade scoring system. Among the two scoring methods, the three-grade system method is simpler, while the five-grade scoring method is more detailed and provides more assessment information. Chondral edema is one of the manifestations of early articular cartilage damage (29) and can cause the blurring of cartilage-synovial membrane interface and loss of cartilage edge sharpness. The five-grade scoring system incorporates this type of damage and presents a more detailed delineation of the degree of cartilage damage, resulting in a higher rate of positive detection (3,26). However, the cartilage-synovial membrane interface may lead to differences in assessment between examiners, and the use of UHFUS may be able to overcome this problem by providing high spatial resolution. The portions of MH susceptible to cartilage damage have not been extensively investigated. Some studies (30,31) have reported that mechanical stresses on the articular surfaces can harm the articular tissues. In our study, we found that the ulnar side on the transverse section and the distal side on the longitudinal section had the highest rate of cartilage damage, which may be attributed to the high mobility of the ulnar and distal portions and the wide contact surface area resulting in abnormal or greater mechanical stresses.
We further identified that age, course of disease, body weight, and inflammatory degree as factors associated with cartilage damage, which is consistent with previous studies (3,24,25,32-37). This finding further confirms that controlling body weight and inflammation can help reduce cartilage damage.
Our study involved several limitations that should be addressed. First, we did not compare the UHFUS-based quantitative and semiquantitative assessments. Second, we did not examine if there were any differences in the assessment results between UHFUS and HFUS. Finally, no comparison with other imaging examinations was performed.
Conclusions
In this study, the semiquantitative assessment of MH cartilage by UHFUS was found to be reliable and reproducible. The cartilage damage in patients with RA was greater than that in HCs, and the five-grade scoring system proved to be more accurate. Moreover, the ulnar side of the transverse section and the distal side of the longitudinal section were the common damage sites in patients with RA. In conclusion, the assessment of cartilage damage in transverse sections via a five-grade scoring system is reliable and valid. Further investigations should be conducted to ascertain the potential benefits of semiquantitative scoring methods for treatment.
Acknowledgments
The authors would like to thank Yujia Yang, Xi Xiang, Linyan Zhang and Zhenling Zhou (Department of Medical Ultrasound, West China Hospital of Sichuan University, Chengdu, Sichuan, China) for their contributions in providing cases.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1539/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1539/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the principles of Good Clinical Practice. The study was approved by the Biomedical Ethics Review Committee of West China Hospital of Sichuan University {No. 2023 review [1886]}. Written informed consent was obtained from all patients and healthy controls (HCs).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Luz KR, Pinheiro MM, Petterle GS, Dos Santos MF, Fernandes AR, Natour J, Furtado RN. A new musculoskeletal ultrasound scoring system (US10) of the hands and wrist joints for evaluation of early rheumatoid arthritis patients. Rev Bras Reumatol Engl Ed 2016;56:421-31. [Crossref] [PubMed]
- Mandl P, Studenic P, Filippucci E, Bachta A, Backhaus M, Bong D, et al. Development of semiquantitative ultrasound scoring system to assess cartilage in rheumatoid arthritis. Rheumatology (Oxford) 2019;58:1802-11. [Crossref] [PubMed]
- Hurnakova J, Filippucci E, Cipolletta E, Di Matteo A, Salaffi F, Carotti M, Draghessi A, Di Donato E, Di Carlo M, Lato V, Horvath R, Komarc M, Pavelka K, Grassi W. Prevalence and distribution of cartilage damage at the metacarpal head level in rheumatoid arthritis and osteoarthritis: an ultrasound study. Rheumatology (Oxford) 2019;58:1206-13. [Crossref] [PubMed]
- Mitra S, Samui PP, Samanta M, Mondal RK, Hazra A, Mandal K, Sabui TK. Ultrasound detected changes in joint cartilage thickness in juvenile idiopathic arthritis. Int J Rheum Dis 2019;22:1263-70. [Crossref] [PubMed]
- Gherghe AM, Ramiro S, Landewé R, Mihai C, van der Heijde D. Association of the different types of radiographic damage with physical function in patients with rheumatoid arthritis: analysis of the RAPID trials. RMD Open 2016;2:e000219. [Crossref] [PubMed]
- Landewé R, van der Heijde D. Joint space narrowing, cartilage and physical function: are we deceived by measurements and distributions? Ann Rheum Dis 2011;70:717-8. [Crossref] [PubMed]
- Navarro-Compán V, Landewé R, Provan SA, Ødegård S, Uhlig T, Kvien TK, Keszei AP, Ramiro S, van der Heijde D. Relationship between types of radiographic damage and disability in patients with rheumatoid arthritis in the EURIDISS cohort: a longitudinal study. Rheumatology (Oxford) 2015;54:83-90. [Crossref] [PubMed]
- Abrar DB, Schleich C, Frenken M, Vordenbäumen S, Richter J, Schneider M, Ostendorf B, Nebelung S, Sewerin P. DGEMRIC in the Assessment of Pre-Morphological Cartilage Degeneration in Rheumatic Disease: Rheumatoid Arthritis vs. Psoriatic Arthritis. Diagnostics (Basel) 2021.
- Saltzherr MS, Muradin GSR, Haugen IK, Selles RW, van Neck JW, Coert JH, Hazes JMW, Luime JJ. Cartilage evaluation in finger joints in healthy controls and early hand osteoarthritis patients using high-resolution MRI. Osteoarthritis Cartilage 2019;27:1148-51. [Crossref] [PubMed]
- Rubin DA. MRI and ultrasound of the hands and wrists in rheumatoid arthritis. I. Imaging findings. Skeletal Radiol 2019;48:677-95. [Crossref] [PubMed]
- Sakthiswary R, Rajalingam S, Hussein H, Sridharan R, Asrul AW. Cartilage oligomeric matrix protein (COMP) in rheumatoid arthritis and its correlation with sonographic knee cartilage thickness and disease activity. Clin Rheumatol 2017;36:2683-8. [Crossref] [PubMed]
- Roth J. Emergence of Musculoskeletal Ultrasound Use in Pediatric Rheumatology. Curr Rheumatol Rep 2020;22:14. [Crossref] [PubMed]
- Quílez Caballero E, Bueno Horcajadas ÁL, Cebada Chaparro E, De Iruarrizaga Gana M, López-Vidaur Franco I. Martel Villagrán J. Ultrasound (US) of the fingers: anatomy and pathology. Quant Imaging Med Surg 2024;14:8012-27. [Crossref] [PubMed]
- Andersen C, Griffin JF 4th, Jacobsen S, Østergaard S, Walters M, Mori Y, Lindegaard C. Validation of ultrasonography for measurement of cartilage thickness in the equine carpus. Vet Radiol Ultrasound 2022;63:478-89. [Crossref] [PubMed]
- Izzetti R, Vitali S, Aringhieri G, Nisi M, Oranges T, Dini V, Ferro F, Baldini C, Romanelli M, Caramella D, Gabriele M. Ultra-High Frequency Ultrasound, A Promising Diagnostic Technique: Review of the Literature and Single-Center Experience. Can Assoc Radiol J 2021;72:418-31. [Crossref] [PubMed]
- Fogante M, Carboni N, Argalia G. Clinical application of ultra-high frequency ultrasound: Discovering a new imaging frontier. J Clin Ultrasound 2022;50:817-25. [Crossref] [PubMed]
- Disler DG, Raymond E, May DA, Wayne JS, McCauley TR. Articular cartilage defects: in vitro evaluation of accuracy and interobserver reliability for detection and grading with US. Radiology 2000;215:846-51. [Crossref] [PubMed]
- Aletaha D, Funovits J, Smolen JS. Physical disability in rheumatoid arthritis is associated with cartilage damage rather than bone destruction. Ann Rheum Dis 2011;70:733-9. [Crossref] [PubMed]
- van der Heijde DM. Joint erosions and patients with early rheumatoid arthritis. Br J Rheumatol 1995;34:74-8.
- Möller B, Bonel H, Rotzetter M, Villiger PM, Ziswiler HR. Measuring finger joint cartilage by ultrasound as a promising alternative to conventional radiograph imaging. Arthritis Rheum 2009;61:435-41. [Crossref] [PubMed]
- Cipolletta E, Filippucci E, Di Matteo A, Tesei G, Cosatti MA, Di Carlo M, Grassi W. The Reliability of Ultrasound in the Assessment of Hyaline Cartilage in Rheumatoid Arthritis and Healthy Metacarpal Heads. Ultraschall Med 2022;43:e65-72. [Crossref] [PubMed]
- Iagnocco A, Conaghan PG, Aegerter P, Möller I, Bruyn GA, Chary-Valckenaere I, Filippucci E, Gandjbakhch F, Loeuille D, Naredo E, D'Agostino MA. The reliability of musculoskeletal ultrasound in the detection of cartilage abnormalities at the metacarpo-phalangeal joints. Osteoarthritis Cartilage 2012;20:1142-6. [Crossref] [PubMed]
- Onat ŞŞ, Ata AM, Serrano S, Shyu SG, Constantino J, Yalçın S, Açıkel C, Kara M, Özçakar L. Ultrasonographic measurements of the metacarpophalangeal and talar cartilage thicknesses: A reliability study in healthy subjects. J Back Musculoskelet Rehabil 2018;31:253-7. [Crossref] [PubMed]
- Ogura T, Hirata A, Hayashi N, Imaizumi C, Ito H, Takenaka S, Inoue Y, Takakura Y, Mizushina K, Katagiri T, Kameda H. Finger Joint Cartilage Evaluated by Semiquantitative Ultrasound Score in Patients With Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2021;73:173-9. [Crossref] [PubMed]
- Cipolletta E, Mandl P, Di Matteo A, Mashadi Mirza R, Passarini G, Grassi W, Filippucci E. Sonographic assessment of cartilage damage at the metacarpal head in rheumatoid arthritis: qualitative versus quantitative methods. Rheumatology (Oxford) 2022;61:1018-25. [Crossref] [PubMed]
- Filippucci E, da Luz KR, Di Geso L, Salaffi F, Tardella M, Carotti M, Natour J, Grassi W. Interobserver reliability of ultrasonography in the assessment of cartilage damage in rheumatoid arthritis. Ann Rheum Dis 2010;69:1845-8. [Crossref] [PubMed]
- Mandl P, Supp G, Baksa G, Radner H, Studenic P, Gyebnar J, Kurucz R, Niedermayer D, Aletaha D, Balint PV, Smolen JS. Relationship between radiographic joint space narrowing, sonographic cartilage thickness and anatomy in rheumatoid arthritis and control joints. Ann Rheum Dis 2015;74:2022-7. [Crossref] [PubMed]
- Torp-Pedersen S, Bartels EM, Wilhjelm J, Bliddal H. Articular cartilage thickness measured with US is not as easy as it appears: a systematic review of measurement techniques and image interpretation. Ultraschall Med 2011;32:54-61. [Crossref] [PubMed]
- Calvo E, Palacios I, Delgado E, Sánchez-Pernaute O, Largo R, Egido J, Herrero-Beaumont G. Histopathological correlation of cartilage swelling detected by magnetic resonance imaging in early experimental osteoarthritis. Osteoarthritis Cartilage 2004;12:878-86. [Crossref] [PubMed]
- Visser AW, de Mutsert R, le Cessie S, den Heijer M, Rosendaal FR, Kloppenburg MNEO Study Group. The relative contribution of mechanical stress and systemic processes in different types of osteoarthritis: the NEO study. Ann Rheum Dis 2015;74:1842-7. [Crossref] [PubMed]
- Tateiwa D, Yoshikawa H, Kaito T. Cartilage and Bone Destruction in Arthritis: Pathogenesis and Treatment Strategy: A Literature Review. Cells 2019;8:818. [Crossref] [PubMed]
- Renner N, Kleyer A, Krönke G, Simon D, Söllner S, Rech J, Uder M, Janka R, Schett G, Welsch GH, Pachowsky ML. T2 Mapping as a New Method for Quantitative Assessment of Cartilage Damage in Rheumatoid Arthritis. J Rheumatol 2020;47:820-5. [Crossref] [PubMed]
- Pamukoff DN, Vakula MN, Holmes SC, Shumski EJ, Garcia SA. Body mass index moderates the association between gait kinetics, body composition, and femoral knee cartilage characteristics. J Orthop Res 2020;38:2685-95. [Crossref] [PubMed]
- Turesson C, Bergström U, Pikwer M, Nilsson JÅ, Jacobsson LT. A high body mass index is associated with reduced risk of rheumatoid arthritis in men, but not in women. Rheumatology (Oxford) 2016;55:307-14. [Crossref] [PubMed]
- Karlsson T, Hadizadeh F, Rask-Andersen M, Johansson Å, Ek WE. Body Mass Index and the Risk of Rheumatic Disease: Linear and Nonlinear Mendelian Randomization Analyses. Arthritis Rheumatol 2023;75:2027-35. [Crossref] [PubMed]
- de Hair MJ, Landewé RB, van de Sande MG, van Schaardenburg D, van Baarsen LG, Gerlag DM, Tak PP. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann Rheum Dis 2013;72:1654-8. [Crossref] [PubMed]
- Schulman E, Bartlett SJ, Schieir O, Andersen KM, Boire G, Pope JE, Hitchon C, Jamal S, Thorne JC, Tin D, Keystone EC, Haraoui B, Goodman SM, Bykerk VP. CATCH Investigators. Overweight, Obesity, and the Likelihood of Achieving Sustained Remission in Early Rheumatoid Arthritis: Results From a Multicenter Prospective Cohort Study. Arthritis Care Res (Hoboken) 2018;70:1185-91. [Crossref] [PubMed]


