
Assessment of common carotid artery wall stiffness using shear wave elastography: a promising technique for evaluating active and inactive Behçet’s disease
Introduction
Behçet’s disease (BD) is a systemic variable vasculitis of unknown etiology that mainly involves the skin and mucosa, but can also involve the eyes, joints, arteries, veins, nervous system, and gastrointestinal system (1). BD is a subtype of vasculitis that can affect arteries and veins of various sizes (2). The pathogenesis of vasculitis in BD is not completely clear at present. Vascular endothelial cell functional disorders play an important role in the development of vascular lesions in patients with BD (3). Research has shown that endothelial dysfunction in BD patients can cause an increase in vascular endothelial growth factor, soluble thrombomodulin, endothelium-derived von Willebrand factor, and E selectin (4-7). These changes can increase vascular wall fibrosis and smooth muscle cell proliferation, leading to an increase in vascular stiffness (8).
Pulse wave velocity (PWV) is a relatively reliable predictor of cardiovascular disease morbidity and mortality (9). However, there is still controversy as to whether these results indicate an increase in BD patients. Shear wave elastography (SWE) is a promising imaging modality that has been applied to vascular diseases in recent years (10-12). Measuring arterial stiffness by SWE can assess the endothelial function of BD (13). To date, no study has sought to compare the ability of SWE and PWV to evaluate arterial stiffness in BD. Nor has any study sought to compare the ability of SWE to evaluate arterial stiffness in active and inactive BD.
In this study, we aimed to investigate arterial stiffness as evaluated by SWE in active and inactive BD. We also aimed to compare the diagnostic efficacy of SWE and PWV. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1448/rc).
Methods
Patient population
Patients with BD who were admitted to the hospital (Peking University People’s Hospital) from October 2021 to October 2022 were selected for inclusion in the study. To be eligible for inclusion in the study, the patients had to meet the diagnostic criteria for BD as per the International Criteria for BD (14). Patients were excluded from the study if they met any of the following exclusion criteria include: (I) had an incomplete medical history; (II) were aged <20 or >45 years; (III) had abnormal liver, kidney, or thyroid function; (IV) were pregnant, anemic or a chronic smoker; (V) had a mixed chronic disease, such as ischemic cardiovascular or cerebrovascular disease, hypertension, hyperlipidemia, hyperglycemia, or another immune-related disease; (VI) had a tortuous carotid artery or an inner carotid vein >4 cm in thickness; (VII) had a mixed malignant tumor; (VIII) had a body mass index (BMI) <18 or >24 kg/m2; and/or (IX) had systolic blood pressure >120 or <100 mmHg, diastolic blood pressure >80 or <60 mmHg. In total, 39 patients met the criteria. Of these 39 patients, 19 were classified as active stage and 20 were classified as inactive stage according to their BD current activity form scores (15). In addition, 22 matched healthy adults were included in the study as the control group.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Review Committee of Peking University People’s Hospital (No. 2021PHB139-001). All patients gave their written informed consent.
PWV examination
A SuperSonic Imagine Aixplorer instrument (SuperSonic Imagine, France) equipped with a linear transducer (SL10-2) was used. Vascular PWV conditions were selected. The subjects were instructed to lie in the supine position and fully expose their neck area. The subjects lay flat without a pillow and their necks were relaxed. After stabilizing breathing, the common carotid artery (CCA) was first cross-sectionally scanned. A longitudinal section scan was then performed approximately 2 cm from the carotid bifurcation. The transducer was stably placed approximately 1–2 cm below the carotid bifurcation. The system then performed the image processing and analysis, and automatically calculated the PWV values. The measured data were found to be the most reliable when the corresponding standard deviations of the PWV values were 10–20%. Each subject was examined three times to obtain the mean values. The same procedure was performed on the left side of the neck. All the study subjects were independently examined by two operators.
SWE examination
The transducer was placed lightly on the surface of the cervical skin. The instrument was switched to SWE mode and the color measure range was set to 0–100 kPa. The frame was placed in the CCA, 5 cm from the bulb below the sternocleidomastoid muscle. The subjects were instructed to hold their breath for 3 seconds. The image was frozen after the color was uniformly distributed. The sternocleidomastoid muscle was used as a control to ensure that its stiffness of the sternocleidomastoid muscle fell within the stable normal range of 9.9±4.1 kPa (16). The region of interest (ROI) was selected. A ROI was established in every SWE frame. Q-BOX tracing was used to defined an area of 0.2±0.01 cm2 in the carotid anterior wall to obtain the best measurement results. The system automatically generated the mean Young’s modulus. Each ROI was measured three times to obtain the average value. All the study subjects were independently examined by two operators.
Statistical analysis
The statistical analysis was performed using SPSS 26.0 (IBM Corp., Armonk, NY, USA). The normally distributed data (SWE) are presented as the mean ± standard deviation, and were analyzed by one-way analysis of variance followed by the Bonferroni test. The non-normally distributed data (PWV) are expressed as the median and interquartile range (IQR) and were analyzed using a non-parametric Kruskal-Wallis H test. A P value <0.05 was considered statistically significant. Moreover, the intra-observer agreements in measuring PWV and SWE were assessed using the intraclass correlation coefficient (ICC). An ICC >0.80 indicated excellent agreement. A receiver operating characteristic (ROC) curve analysis was performed to determine the performance of PWV and SWE in distinguishing between the inactive and active stage patients. The areas under the curve (AUC) were compared between the two methods using the DeLong method.
Results
The results of the comparisons between the control, inactive, and active stage groups in terms of age, sex, blood pressure and BMI are shown in Table 1. There were no statistically significant differences among the three groups in terms of sex, age, blood pressure, and BMI.
Table 1
| Characteristics | Inactive stage (n=20) | Active stage (n=19) | Control (n=22) |
|---|---|---|---|
| Sex (male/female) | 11/9 | 11/8 | 13/9 |
| Age (years) | 32.70±6.27 | 30.79±5.73 | 30.77±7.53 |
| BMI (kg/m2) | 20.48±1.24 | 20.26±0.99 | 20.98±1.63 |
| Systolic blood pressure (mmHg) | 116.15±4.17 | 117.47±3.50 | 115.5±4.36 |
| Diastolic blood pressure (mmHg) | 75.80±3.38 | 77.05±2.46 | 75.73±2.98 |
Data are presented as n or mean ± standard deviation. BMI, body mass index.
All data on the SWE and PWV values for the inactive stage group, active stage group, and control group are presented in Table 2 and Figure 1. The BD (inactive and active) patients had higher SWE and PWV values of the CCA wall than the control subjects (P<0.05) (Figures 2,3). The elastic modulus values of the CCA wall were higher in the in active stage group (44.42±5.23 kPa) than the inactive stage group (35.17±4.60 kPa) (P<0.05) (Figure 2). There were no statistically significant differences in the PWV values of CCA wall between the active group [5.96 m/s (IQR, 5.35–6.62 m/s)] and inactive group [5.42 m/s (IQR, 4.63–6.30 m/s)] (P>0.05) (Figure 3). In terms of the intra-observer variability for SWE and PWV, the ICC values were 0.812 [95% confidence interval (CI): 0.658–0.897] and 0.824 (95% CI: 0.702–0.911), respectively.
Table 2
| Group | SWE (kPa) | PWV (m/s) |
|---|---|---|
| Inactive stage | ||
| Left | 36.11±4.22 | 5.75 (4.76–7.61) |
| Right | 34.24±4.87 | 5.62 (4.80–6.79) |
| Active stage | ||
| Left | 44.07±5.37 | 6.06 (5.36–6.55) |
| Right | 44.76±5.21 | 5.83 (5.20–6.93) |
| Control | ||
| Left | 29.59±5.52 | 4.91 (4.50–5.58) |
| Right | 30.00±5.39 | 4.87 (4.67–5.24) |
Data are presented as mean ± SD or median (IQR). SWE, shear wave elastography; PWV, pulse wave velocity; SD, standard deviation; IQR, interquartile range.



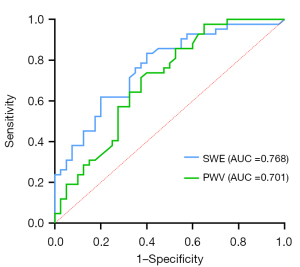
The AUC values for SWE and PWV in distinguishing between the inactive stage and control groups were 0.768 (95% CI: 0.666–0.869) and 0.701 (95% CI: 0.5877–0.8152), respectively (Figure 4). There was no significant difference in the AUC values for SWE and PWV (P=0.145). The AUC values for SWE and PWV in distinguishing between the active stage and control groups were 0.978 (95% CI: 0.953–1.000) and 0.776 (95% CI: 0.675–0.877), respectively (Figure 5). The AUC value for SWE was significantly greater than that for PWV (P<0.01).

Discussion
BD is associated with multiple pathological pathways and vasculitis accompanied by vascular pathological changes, as shown by vascular endothelial dysfunction (17), vascular integrity deterioration, erythrocyte extravasation, fibrinoid necrosis of the vessel walls, and thrombosis (6,18). Inflammatory cell infiltration, composed mainly of neutrophils, can be observed in the blood vessels and surrounding tissues (19). The prolonged presence of endothelial cells in an inflammatory environment result in vascular fibrosis and hyperplasia of smooth muscle cells, leading to increased arterial stiffness (20). Artery elasticity is an important indicator of pathological changes in arterial walls and reflects the compliance of arterial walls (21). Research has reported a correlation between increased arterial stiffness and the evolvement of atherosclerosis (22).
PWV was thought to be a reliable method for assessing artery wall stiffness, and can be used to predict cardiovascular disease morbidity and mortality (9). PWV is defined as the velocity of movement of the arterial pulse along the vessel wall, and it is negatively correlated with arterial dilation or relative arterial compliance (23). Contrary to the results reported by Kürüm et al. (24), many studies have reported that PWV is increased in patients with BD (3,25,26). Accordingly, the present study sought to compare changes in the wall stiffness of the CCA between active and inactive stage BD patients. We found that PWV was increased in both the active and inactive stage groups compared to the control group; however, no statistically significant difference in PWV was found between the active and inactive stage groups. This may be due to the relatively young age of the patients in the active and inactive stage groups in this study.
SWE is an imaging modality based on the principle of ultrasound that can be used to quantitatively measure tissue hardness. SWE uses ultrafast ultrasonic scanning probes to generate supersonic shear sources and image based on the propagation information of transient plane shear waves. It can estimate the shear modulus of tissues by imaging the propagation of shear waves. The propagation speed of the shear waves depends on the density and elasticity of tissues, which exhibit different characteristics in different human tissues and pathological states (12). It was thought that SWE, which is a widely available non-invasive diagnostic tool, could be a promising tool for detecting vascular endothelial dysfunction (11). Research has also reported good agreement between Young’s modulus values acquired by SWE and those acquired by mechanical testing (27). Alis et al. (13) reported that SWE may be a promising modality for assessing endothelial dysfunction in BD by assessing arterial stiffness. However, no previous research study has analyzed the arterial stiffness of active and inactive stage BD patients by SWE.
In this study, we investigated arterial stiffness in patients with inactive and active stage BD, and control subjects. The results showed that the Young’s modulus values were higher in the active than inactive stage patients, indicating an increase in the vascular wall stiffness of active stage BD patients. Our results are similar to those previously reported by Yilmaz et al. (25), who used PWV. Increased arterial stiffness may be attributed to endothelial dysfunction and acute or chronic inflammatory processes associated with BD. This result may be explained by more prominent inflammatory changes in the vascular wall in the active stage. We also found that the stiffness of the CCA detected by SWE was increased in the active stage patients compared with the inactive stage patients. The AUC values for SWE and PWV in distinguishing between the active stage and control groups were 0.978 and 0.776, respectively. These results showed that SWE had better sensitivity than PWV in active stage BD patients.
Many studies have reported promising results on the application of SWE to arterial wall stiffness assessment (10,11,28). However, only a few studies have measured artery wall stiffness in BD. The limited research may be due to the relatively thin wall of the carotid artery and the susceptibility of the measured elastic modulus to various factors. Alis et al. (13) reported that the wall stiffness of the CCA was significantly higher in BD patients than controls. However, the evaluation value of SWE for distinguishing active and inactive stage BD was unclear. Further, to date, no comparative study of SWE and PWV in the evaluation of arterial stiffness in active and inactive stage BD patients had been conducted. This appears to have been the first study to examine the use of SWE in assessing the stiffness of the CCA in active and inactive stage BD patients, and to compare the SWE and PWV methods.
Our research had several limitations. First, the sample size of our study was relatively small. Second, the treatment status of the patients and the intima-media thickness of the CCA, which might affect the stiffness of the CCA, were not taken into account. Third, our study mainly focused on patients with a short disease duration. Therefore, the patients in our study were relatively young. The correlation between artery stiffness and different BD duration times was not investigated in this study. Also, the correlation between artery stiffness and the severity score was not examined. These issues could be addressed in future research. Our results revealed differences in PWV and SWE between active and inactive stage BD; however, further research is needed from a physical perspective due to the influence of blood viscosity and other factors. Finally, only the wall stiffness of the CCA was evaluated in this study. The wall stiffness of other regional arteries in BD, such as the femoral and axillary arteries, should be examined in future studies.
Conclusions
By interpreting the stiffness of the CCA, SWE appears to be a promising modality for evaluating the active and inactive stages of BD. SWE was better able to evaluate the BD active stage than PWV.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1448/rc
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1448/coif). D.L. reports that this research was supported by the Peking University People’s Hospital Scientific Research Development Fund (No. RDJ2022-12), of which he is the person in charge. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Review Committee of Peking University People’s Hospital (No. 2021PHB139-001). All patients gave their written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1-11. [Crossref] [PubMed]
- Nair JR, Moots RJ. Behcet's disease. Clin Med (Lond) 2017;17:71-7. [Crossref] [PubMed]
- Chang HK, Kim SK, Lee SS, Rhee MY. Arterial stiffness in Behcet's disease: increased regional pulse wave velocity values. Ann Rheum Dis 2006;65:415-6. [Crossref] [PubMed]
- Kayikçioğlu M, Aksu K, Hasdemir C, Keser G, Turgan N, Kültürsay H, Doganavsargil E. Endothelial functions in Behçet's disease. Rheumatol Int 2006;26:304-8. [Crossref] [PubMed]
- Oztürk MA, Unverdi S, Oktar SO, Bukan N, Gülbahar O, Ureten K, Göker B, Haznedaroglu S, Sungur G, Ciftçi TU, Onat AM. Vascular endothelial growth factor and carotid intima-media thickness in patients with Behçet's disease. Clin Rheumatol 2008;27:961-6. [Crossref] [PubMed]
- Kiraz S, Ertenli I, Oztürk MA, Haznedaroğlu IC, Celik I, Calgüneri M. Pathological haemostasis and "prothrombotic state" in Behçet's disease. Thromb Res 2002;105:125-33. [Crossref] [PubMed]
- Haznedaroglu E, Karaaslan Y, Büyükaşik Y, Koşar A, Ozcebe O. Haznedaroglu bC, Kirazli E, Dündar SV. Selectin adhesion molecules in Behçet's disease. Ann Rheum Dis 2000;59:61-3. [Crossref] [PubMed]
- Patel RS, Al Mheid I, Morris AA, Ahmed Y, Kavtaradze N, Ali S, Dabhadkar K, Brigham K, Hooper WC, Alexander RW, Jones DP, Quyyumi AA. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis 2011;218:90-5. [Crossref] [PubMed]
- Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 2006;113:657-63. [Crossref] [PubMed]
- Couade M, Pernot M, Prada C, Messas E, Emmerich J, Bruneval P, Criton A, Fink M, Tanter M. Quantitative assessment of arterial wall biomechanical properties using shear wave imaging. Ultrasound Med Biol 2010;36:1662-76. [Crossref] [PubMed]
- Gülşen F, Samanci C, Memis Durmaz ES, Durmaz E, Tel C, Gencturk M, Ağırman A. Brachial Artery Wall Stiffness Assessment by Shear Wave Elastography: A Promising New Diagnostic Tool for Endothelial Dysfunction Detection. J Ultrasound Med 2018;37:1977-83. [Crossref] [PubMed]
- Li Z, Du L, Wang F, Luo X. Assessment of the arterial stiffness in patients with acute ischemic stroke using longitudinal elasticity modulus measurements obtained with Shear Wave Elastography. Med Ultrason 2016;18:182-9. [Crossref] [PubMed]
- Alis D, Durmaz ESM, Civcik C, Tutuncu M, Saip S, Kocer N, Islak C, Kizilkilic O. Assessment of the common carotid artery wall stiffness by Shear Wave Elastography in Behcet's disease. Med Ultrason 2018;20:446-52. [Crossref] [PubMed]
- The International Criteria for Behçet's Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol 2014;28:338-47. [Crossref] [PubMed]
- Bhakta BB, Brennan P, James TE, Chamberlain MA, Noble BA, Silman AJ. Behçet's disease: evaluation of a new instrument to measure clinical activity. Rheumatology (Oxford) 1999;38:728-33. [Crossref] [PubMed]
- Herman J, Sedlackova Z, Vachutka J, Furst T, Salzman R, Vomacka J. Shear wave elastography parameters of normal soft tissues of the neck. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2017;161:320-5. [Crossref] [PubMed]
- Arica DA, Akşan B, Örem A, Altinkaynak BA, Yayli S, Sönmez M. High levels of endothelial progenitor cells and circulating endothelial cells in patients with Behçet's disease and their relationship to disease activity. An Bras Dermatol 2019;94:320-6. [Crossref] [PubMed]
- Bozkirli ED, Keşkek SÖ, Kozanoğlu I, Yücel AE. High levels of endothelial progenitor cells can be associated with thrombosis in patients with Behçet's disease. Clin Exp Rheumatol 2014;32:S49-53.
- Kim MK, Kwon HC, Song JJ, Park YB, Lee SW. Antineutrophil Cytoplasmic Antibody Positivity Is Associated with Vascular Involvement in Behçet's Disease. Yonsei Med J 2021;62:149-58. [Crossref] [PubMed]
- Protogerou AD, Nasothimiou EG, Sfikakis PP, Tzioufas AG. Non-invasive vascular biomarkers in patients with Behçet's disease: review of the data and future perspectives. Clin Exp Rheumatol 2017;35:100-7.
- Novo G, Di Miceli R, Novo S. Is local stiffness, as measured by radio frequency, more sensitive than intima-media thickness? Int Angiol 2013;32:575-80.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010;55:1318-27. [Crossref] [PubMed]
- Imura T, Yamamoto K, Kanamori K, Mikami T, Yasuda H. Non-invasive ultrasonic measurement of the elastic properties of the human abdominal aorta. Cardiovasc Res 1986;20:208-14. [Crossref] [PubMed]
- Kürüm T, Yildiz M, Soy M, Ozbay G, Alimgil L, Tüzün B. Arterial distensibility as determined by carotid-femoral pulse wave velocity in patients with Behçet's disease. Clin Rheumatol 2005;24:134-8. [Crossref] [PubMed]
- Yilmaz S, Celik G, Esmen SE. Assessment of arterial stiffness in patients with inactive and active Behçet's disease. Scand J Rheumatol 2014;43:63-9. [Crossref] [PubMed]
- Caldas CA, Borba EF, Bortolotto LA, Medeiros DM, Bonfa E, Gonçalves CR. Increased arterial stiffness assessed by pulse wave velocity in Behçet's disease and its association with the lipid profile. J Eur Acad Dermatol Venereol 2013;27:454-9. [Crossref] [PubMed]
- Maksuti E, Widman E, Larsson D, Urban MW, Larsson M, Bjällmark A. Arterial Stiffness Estimation by Shear Wave Elastography: Validation in Phantoms with Mechanical Testing. Ultrasound Med Biol 2016;42:308-21. [Crossref] [PubMed]
- Caenen A, Shcherbakova D, Verhegghe B, Papadacci C, Pernot M, Segers P, Swillens A. A versatile and experimentally validated finite element model to assess the accuracy of shear wave elastography in a bounded viscoelastic medium. IEEE Trans Ultrason Ferroelectr Freq Control 2015;62:439-50. [Crossref] [PubMed]


