
MRI signal simulation of liver DDVD (diffusion derived ‘vessel density’) with multiple compartments diffusion model
Our exploration of diffusion-weighted imaging (DWI) in liver fibrosis evaluation revealed that diffusion-derived ‘vessel density’ (DDVD) can reflect microvascular perfusion. Liver blood vessels including sub-pixel microvessels show high signal when there is no motion probing gradient (b=0 s/mm2) and low signal when even very low b-values (such as b=1, b=2 s/mm2) are applied (1,2). Thus, the signal difference between images when the motion probing gradient is ‘off’ and ‘on’ reflects the extent of tissue vessel density in physiological sense, and we term this as DDVD. DDVD is derived from Eq. [1]:
where and refer to the number of pixels in the selected region-of-interest (ROI) on b=0 and b=2 s/mm2 DWI, respectively. refers to the measured sum signal intensity within the ROI when b=0, and refers to the measured sum signal intensity within the ROI when b=2 s/mm2, thus equates to the mean signal intensity within the ROI. and can also be approximated by other low b-values (such as b=10 s/mm2) DWI. If we consider a pixel to be an individual ROI, DDVD pixelwise map can be constructed pixel-by-pixel with this same principle (3).
The analysis of DDVD requires only two b-values (with one being b=0 s/mm2 and the other being non-zero low b-value), with a significantly shorter scanning time than contrast enhanced computed tomography/magnetic resonance imaging (CT/MRI) while without the need of a contrast agent injection. DDVD measure based on this simple principle is useful as a straightforward imaging biomarker in diverse clinical scenarios. DDVD is a useful parameter for distinguishing livers with and without fibrosis, and livers with more severe fibrosis tend to have even lower DDVD measurements than those with milder liver fibrosis (1,2). He et al. (4) and Li et al. (5) reported that placenta DDVD as a perfusion biomarker allows excellent separation of normal and early pre-eclampsia pregnancies. Lu et al. (6) reported that placenta regional DDVD is significantly higher in pregnant women with placenta accreta spectrum disorders than women with normal placenta, and especially higher in patients with placenta percreta. Hu et al. (7) described that liver hemangiomas can be mostly differentiated from liver mass-forming lesions [hepatocellular carcinomas (HCCs) and focal nodular hyperplasia] solely based on DDVD map. Chen et al. (8) described a proof-of-concept study that a combination of DDVD map and high b-value DWI identifies the exitance and the size of penumbras. As absolute magnetic resonance (MR) signal intensity is influenced by various factors, including B0/B1 spatial inhomogeneity, coil loading, receiver gain, etc., we use the ratio of a lesion to its adjacent native tissue (such as the ratio of HCC’s DDVD to liver DDVD) to minimize these scaling factors (5,9,10).
In this letter, applying the multiple compartments diffusion MRI model, we conduct signal simulation for DDVDs of liver parenchyma and liver simple cyst.
Multiple compartments diffusion model
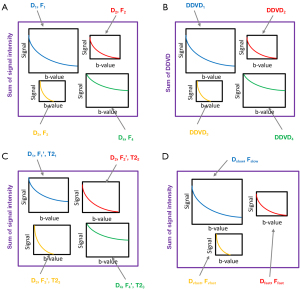
In DWI, each imaged tissue block can be considered to have multiple compartments with different levels of diffusion (Figure 1A). According to the Stejskal-Tanner Eq. [2], each compartment’s signal intensity exhibits mono-exponential decay with the b-value:
Where is the motion probing factor, is the signal intensity of the compartment when no motion probing gradient is applied, and is the diffusion coefficient of the compartment. The signal intensity of the entire tissue block can be considered as the sum of the signal intensities of all compartments:
is the signal intensity of each compartment, is the signal intensity of the entire tissue block when the b-value is 0, and is the fraction of each compartment. In this letter, symbol . The following equation must be satisfied for :
DDVD can also be considered as the sum of the differences in signal intensity for each compartment (Figure 1B).
represents the value of each compartment. Therefore, the discussion of the for an entire tissue block can be separated into the discussion of the for each compartment. This allows us to determine the contribution of each compartment to the value under different b-values and to observe the trend of change as the b-value changes.
We have been using the first b-value being zero in previous studies, thus equals:
In the following discussion, we will continue to use this formula to explore the corresponding changes in the DDVD value when the second b-value () changes.
T2 effects between different tissue components
Even when each type of tissue block is considered being composed of only a single diffusion compartment, related to the ‘T2 shine-through’ effect, the differences in T2 relaxation times among different tissue components result in variations in . This means that the brightness of each tissue component on the DWI at b-value =0 differs, giving signal dependence on the b-value, according to (11-14):
Here, is a scalar independent of T2 and diffusion, is the echo time, is the T2 relaxation time of the tissue, and is the diffusion coefficient of the tissue as a whole. Therefore, the signal intensity is not solely determined by diffusion but is also influenced by the T2 values of the tissue components.
T2 effect between different T2 values of different compartments of a tissue block
With a tissue block having multiple diffusion compartments, and the T2 effect introduces another influence. If we consider that each component has its own T2 relaxation time, with a corresponding , the expression for signal intensity should be (Figure 1C):
Here, and are the original parameters without considering the T2 effect. After re-normalization, we obtain the following equation:
After simplification, we can further obtain:
The measured Fi, Di, and S0are always and already modulated by T2 effect, and the measured values will change following the adjustment of the time of echo (TE) parameter. This type of T2 effect will affect the measured Fi(i.e., the measured proportions of different diffusion compartments), while this T2 effect will not affect the measure of Di.
Tri-exponential diffusion model
It has been suggested that liver’s DWI signal attenuation follows a tri-exponential model, which was found to better describe the intravoxel incoherent motion (IVIM) signal decay in the liver when the b-value is within the 0–800 s/mm2 range (15-19). The tri-exponential IVIM model comprises three compartments: a slow diffusion compartment, a fast perfusion-related compartment, and a very fast perfusion-related compartment, and thus the signal intensity decay can be represented as (Figure 1D):
where denotes the slow diffusion coefficient, and represent the fast and very fast perfusion-related pseudo-diffusion coefficients, respectively. , , and are the fractions of each compartment. The measured values of the diffusion coefficients in literature vary substantially (17).
Numerical simulation of the liver DDVD
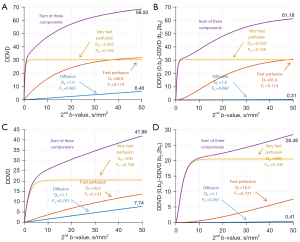
Considering the tri-exponential diffusion model, we attempt to simulate the numerical results of liver DDVD by varying the b2value. Due to the significant differences between the diffusion coefficients and based on our earlier research (20), we gradually increase b2from 0 to 10 s/mm2 and then to 50 s/mm2 to observe the numerical changes in liver DDVD.
In order to numerically simulate the liver’s DDVD, specific parameter values are required. In the literature, the measurements of , , , and are relatively stable, while the measurements of and show substantial variations (Table 1) (15-19). To show that our numerical simulation analysis is robust across variations of the reported parameter values, we use two sets of parameter measurements described by Riexinger et al. (18,19). As shown in Table 1, the first set has higher measurements for and , while the second set has lower measurements for and . The final simulation results of the DDVD curve indicate that the pattern of changes in DDVD values still follow our prediction even considering the substantial variations in tri-exponential diffusion model parameter values.
Table 1
| Source | B0 | TE | Ds | Fs | Df | Ff | Dvf | Fvf |
|---|---|---|---|---|---|---|---|---|
| Test 1 | 3.0T | 100 ms | 1.0 | 0.697 | 65.9 | 0.174 | 2,333 | 0.159 |
| Test 2 | 3.0T | 45 ms | 1.1 | 0.761 | 16.0 | 0.131 | 500 | 0.108 |
Test 1 had higher Ff and Fvf measures, and much higher Df and Dvf measures. Test 1 used a much longer TE. For acquired signal, a longer TE has the same effect as shorter T2, which have been noted to promote the ‘apparent’ values of perfusion related parameters relative to the ‘apparent’ values measured with a shorter TE (21,22). Ds, Df, Dvf unit in ×10−3 mm2/s. B0, field strength of the main magnetic field; TE, time of echo.
Liver DDVD numerical simulation when the b2 is less than 10 s/mm2
For the diffusion and fast perfusion compartments, because of the much lower diffusion coefficient relative to that of the very fast compartment, the value at exponential term is small enough to take one order Taylor expansion and their can be approximated as:
Eq. [14] indicates that the DDVD values of these two compartments will increase linearly with b2. Regardless the significant variation in the measured values of (ranging from 16×10−3 to 65.9×10−3 mm2/s) (15-19), the Eq. [14] remains valid. Additionally, since is very small, the contribution of diffusion to DDVD is minimal and can be neglected.
For the very fast perfusion compartment, regardless of the numerical system used, the amount of related DDVD growth will gradually become close to zero with b2 changes to 10 s/mm2. In such a case, will approach a fixed value:
Therefore, when b2 is less than 10 s/mm2, liver DDVD can be written as:
The second term has a limited impact on the value and can be considered a correction term. When the very fast perfusion compartment has nearly completely plateaued, the above equation can be simplified to:
At this point, the measured fully reflects the perfusion information with minimal influence from diffusion. DDVD is predominantly affected by Fvf, Ffand Df. More importantly, the most unstable measurement, , does not contribute to the value of (Figure 2).

Liver DDVD numerical simulation when the b2 is between 10 and 50 s/mm2
For the diffusion compartment, the linear approximation remains valid, and its related DDVD variation follows Eq. [14]. The influence of this compartment on the DDVD value has gradually increased and can no longer be ignored but still remains a linear pattern (note that: this also works for the diffusion of other organs as Ds has a limited dynamic range and no matter which organ or structure, the change in corresponding to diffusion can always be approximated as a straight line).
For the very fast compartment which has plateaued and its influence on the DDVD remains unchanged, its related DDVD contribution follows Eq. [15]. Considering the sum of all sub-compartments, the liver DDVD can be represented as:
According to the numerical simulation of two sets of data (i.e., lower Dvf measure and high Dvf, Figure 3), following the increase of b2 from 0 to 50 s/mm2, liver (summed) DDVD had an initial phase of rapid increase, followed by a slow increase. Prior to the inflection point, the DDVD signal change is dominated by the very fast compartment; after the inflection point, the DDVD signal change is dominated by the fast compartment. When the values of and have a greater difference, the difference of the DDVD growth slopes before and after the inflection point is also greater. On the other hand, if the values of and have a smaller difference, the DDVD growth curve around the inflection point will be smoother.

An attempt to offset the impact of diffusion on DDVD value
According to the simulation results shown in Figure 4. When b2is relatively large, the impact of diffusion on DDVD measure can no longer be ignored. Since we intend to use DDVD to measure perfusion related information, following the parameters shown in Eq. [18], we proposed to use the following equation to mitigate the impact of diffusion on DDVD (3):
For the diffusion compartment, as the relationship between b2and DDVD always follow an appropriately linear pattern, thus Eq. [19] is approximated by:
For the signal growth of very fast perfusion, when is relatively ‘high’ (such as 30 s/mm2), it can be assumed that:
Considering the sum of very fast compartment, fast compartment, and slow (diffusion) compartment, Eq. [19] can be re-written as:
The simulation results for Eq. [19] shown in Figure 4 demonstrate that, after the application of Eq. [19], the diffusion compartment is well eliminated.
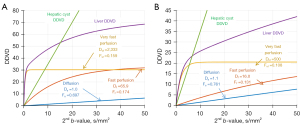
Numerical simulation in the case of liver simple cyst
It’s possible that a liver simple cyst is comprised of a single compartment. Given the lack of perfusion, cyst DWI signal follows a mono-exponential decay due to its relatively non-restricted diffusion. In the meantime, the corresponding diffusion coefficient should be less than 2.5×10−3 mm2/s (23,24).
In addition to its diffusion property, liver simple cyst has a markedly longer T2 relaxation time compared with that of the liver. Thus, cysts consistently display a higher signal on DWI images, irrespective of the b-value. Liver cyst DDVD can be represented as:
The intercept of Y-axis (Y-axis is DDVD and X-axis is b2) will always be zero in an idealized measurement. Though the coefficient is very high, the relationship between and DDVDcyst will follow a linear line, the slope of the line is determined by (S0,cyst × Ds,cyst), where T2 contributes to S0,cyst(Figure 5).
Acknowledgments
None.
Footnote
Funding: This study received funding from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2693/coif). Y.X.J.W. serves as the Editor-in-Chief of Quantitative Imaging in Medicine and Surgery. Y.X.J.W. is the founder of Yingran Medicals Ltd., which develops medical image-based diagnostics software. B.H.X. contributed to the development of Yingran Medicals Ltd. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wáng YXJ. Living tissue intravoxel incoherent motion (IVIM) diffusion MR analysis without b=0 image: an example for liver fibrosis evaluation. Quant Imaging Med Surg 2019;9:127-33. [Crossref] [PubMed]
- Xiao BH, Huang H, Wang LF, Qiu SW, Guo SW, Wáng YXJ. Diffusion MRI Derived per Area Vessel Density as a Surrogate Biomarker for Detecting Viral Hepatitis B-Induced Liver Fibrosis: A Proof-of-Concept Study. SLAS Technol 2020;25:474-83. [Crossref] [PubMed]
- Yao DQ, Zheng CJ, Deng YY, Lu BL, Lu T, Hu GW, Li XM, Xiao BH, Ma FZ, Sabarudin A, King AD, Wáng YXJ. Potential diverse applications of diffusion-derived vessel density (DDVD) pixel-by-pixel mapping. Quant Imaging Med Surg 2024;14:2136-45. [Crossref] [PubMed]
- He J, Chen C, Xu L, Xiao B, Chen Z, Wen T, Wáng YXJ, Liu P. Diffusion-Derived Vessel Density Computed From a Simplified Intravoxel Incoherent Motion Imaging Protocol in Pregnancies Complicated by Early Preeclampsia: A Novel Biomarker of Placental Dysfunction. Hypertension 2023;80:1658-67. [Crossref] [PubMed]
- Li CY, Chen L, Ma FZ, Chen JQ, Zhan YF, Wáng YXJ. High performance of the diffusion magnetic resonance imaging biomarker diffusion-derived ‘vessel density’ (DDVD) for separating placentas associated with pre-eclampsia from placentas in normal pregnancy. Quant Imaging Med Surg 2025;15:1-14. [Crossref]
- Lu T, Wang L, Li M, Wang Y, Chen M, Xiao BH, Wáng YXJ. Diffusion-derived vessel density (DDVD) computed from a simple diffusion MRI protocol as a biomarker of placental blood circulation in patients with placenta accreta spectrum disorders: A proof-of-concept study. Magn Reson Imaging 2024;109:180-6. [Crossref] [PubMed]
- Hu GW, Li CY, Zhang G, Zheng CJ, Ma FZ, Quan XY, Chen W, Sabarudin A, Zhu MSY, Li XM, Wáng YXJ. Diagnosis of liver hemangioma using magnetic resonance diffusion-derived vessel density (DDVD) pixelwise map: a preliminary descriptive study. Quant Imaging Med Surg 2024;14:8064-82. [Crossref] [PubMed]
- Chen JQ, Li CY, Wang W, Yao DQ, Jiang RF, Wáng YXJ. Diffusion-derived vessel density (DDVD) for penumbra delineation in brain acute ischemic stroke: initial proof-of-concept results using single NEX DWI. Quant Imaging Med Surg 2024;14:9533-42. [Crossref] [PubMed]
- Li XM, Yao DQ, Quan XY, Li M, Chen W, Wáng YXJ. Perfusion of hepatocellular carcinomas measured by diffusion-derived vessel density biomarker: Higher hepatocellular carcinoma perfusion than earlier intravoxel incoherent motion reports. NMR Biomed 2024;37:e5125. [Crossref] [PubMed]
- Lu BL, Yao DQ, Wáng YXJ, Zhang ZW, Wen ZQ, Xiao BH, Yu SP. Higher perfusion of rectum carcinoma relative to tumor-free rectal wall: quantification by a new imaging biomarker diffusion-derived vessel density (DDVD). Quant Imaging Med Surg 2024;14:3264-74. [Crossref] [PubMed]
- Stejskal EO, Tanner JE. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time Dependent Field Gradient. J Chem Phys 1965;42:288-92. [Crossref]
- Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986;161:401-7. [Crossref] [PubMed]
- Le Bihan D, Turner R, Moonen CT, Pekar J. Imaging of diffusion and microcirculation with gradient sensitization: design, strategy, and significance. J Magn Reson Imaging 1991;1:7-28. [Crossref] [PubMed]
- Jerome NP, d'Arcy JA, Feiweier T, Koh DM, Leach MO, Collins DJ, Orton MR. Extended T2-IVIM model for correction of TE dependence of pseudo-diffusion volume fraction in clinical diffusion-weighted magnetic resonance imaging. Phys Med Biol 2016;61:N667-80.
- Cercueil JP, Petit JM, Nougaret S, Soyer P, Fohlen A, Pierredon-Foulongne MA, Schembri V, Delhom E, Schmidt S, Denys A, Aho S, Guiu B. Intravoxel incoherent motion diffusion-weighted imaging in the liver: comparison of mono-, bi- and tri-exponential modelling at 3.0-T. Eur Radiol 2015;25:1541-50. [Crossref] [PubMed]
- Chevallier O, Zhou N, Cercueil JP, He J, Loffroy R, Wáng YXJ. Comparison of tri-exponential decay versus bi-exponential decay and full fitting versus segmented fitting for modeling liver intravoxel incoherent motion diffusion MRI. NMR Biomed 2019;32:e4155. [Crossref] [PubMed]
- Chevallier O, Wáng YXJ, Guillen K, Pellegrinelli J, Cercueil JP, Loffroy R. Evidence of Tri-Exponential Decay for Liver Intravoxel Incoherent Motion MRI: A Review of Published Results and Limitations. Diagnostics (Basel) 2021;11:379. [Crossref] [PubMed]
- Riexinger AJ, Martin J, Rauh S, Wetscherek A, Pistel M, Kuder TA, Nagel AM, Uder M, Hensel B, Müller L, Laun FB. On the Field Strength Dependence of Bi- and Triexponential Intravoxel Incoherent Motion (IVIM) Parameters in the Liver. J Magn Reson Imaging 2019;50:1883-92. [Crossref] [PubMed]
- Riexinger A, Martin J, Wetscherek A, Kuder TA, Uder M, Hensel B, Laun FB. An optimized b-value distribution for triexponential intravoxel incoherent motion (IVIM) in the liver. Magn Reson Med 2021;85:2095-108. [Crossref] [PubMed]
- Ju ZG, Leng XM, Xiao BH, Sun MH, Huang H, Hu GW, Zhang G, Sun JH, Zhu MSY, Guglielmi G, Wáng YXJ. Influences of the second motion probing gradient b-value and T2 relaxation time on magnetic resonance diffusion-derived ‘vessel density’ (DDVD) calculation: the examples of liver, spleen, and liver simple cyst. Quant Imaging Med Surg 2025;15:74-87. [Crossref]
- Lemke A, Laun FB, Simon D, Stieltjes B, Schad LR. An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magn Reson Med 2010;64:1580-5. [Crossref] [PubMed]
- Ma FZ, Wáng YXJ T. (2) relaxation time elongation of hepatocellular carcinoma relative to native liver tissue leads to an underestimation of perfusion fraction measured by standard intravoxel incoherent motion magnetic resonance imaging. Quant Imaging Med Surg 2024;14:1316-22. [Crossref] [PubMed]
- Gatidis S, Schmidt H, Martirosian P, Schwenzer NF. Development of an MRI phantom for diffusion-weighted imaging with independent adjustment of apparent diffusion coefficient values and T2 relaxation times. Magn Reson Med 2014;72:459-63. [Crossref] [PubMed]
- Kalaitzakis G, Boursianis T, Gourzoulidis G, Gourtsoyianni S, Lymperopoulou G, Marias K, Karantanas A, Maris TG. Apparent diffusion coefficient measurements on a novel diffusion weighted MRI phantom utilizing EPI and HASTE sequences. Phys Med 2020;73:179-89. [Crossref] [PubMed]


