
Artificial intelligence-assisted enhancement of prostate volume metrics in the diagnosis of clinically significant prostate cancer: a retrospective analysis
Introduction
Prostate cancer is the most common solid malignancy in men and ranks among the top three causes of cancer-related death (1). Recent insights have identified Gleason pattern 3 and Gleason pattern 4 tumors as separate entities, which has been confirmed by distinct genomic signatures (2,3) and markedly different survival outcomes, as evidenced in extensive long-term follow-up studies (4,5). The integration of any percentage of Gleason pattern 4 into the final grade can reclassify a group of radical prostatectomy (RP) tumors, which is not the case for biopsy specimens (6). Importantly, the superior clinical outcomes in patients with prostate cancer with a tumor volume ≤0.5 cm3, a Gleason score (GS) 3+3=6, and absence of Gleason pattern 4 or 5 [clinically insignificant prostate cancer (insPC)] have been corroborated across numerous RP studies, which have reported a 10-year biochemical recurrence-free survival rate ranging from 87% to 100% (7-10). Consequently, there is a critical need for a reliable test to identify patients with clinically significant prostate cancer (csPC). However, biopsies have limited specificity and diagnostic accuracy in select patients suitable for active clinical surveillance in patients with GS 6 prostate cancer (11-13).
Previous studies have demonstrated that prostate serum antigen density (PSAD) and peripheral zone (PZ) PSAD and transition zone (TZ) PSAD can be used to minimize unnecessary biopsies and refine risk stratification (14,15). However, the precision of PSAD calculations is contingent on accurate prostate volume (PV) measurements. Magnetic resonance imaging (MRI), with its superior soft-tissue contrast resolution, offers advantages over transrectal ultrasonography (TRUS) (16,17). The emergence of automatic segmentation technology and the extensive application of artificial intelligence (AI) in PV measurement have made the measurement methods more convenient and consistent, thereby enabling the acquisition of the actual PV, not just relying on estimations. AI-based computer-aided diagnosis (CAD) systems have shown efficacy in prostate segmentation (18), yet challenges persist, particularly in delineating subarea boundaries due to variable MRI protocols and physiological differences among prostates (19). Thus, further developments are needed to account for the clinical diversity.
To the best of knowledge, the value of clinical factors and AI-assisted PV metrics in risk stratification of patients with biopsy-confirmed prostate cancer with GS 6 has not been extensively investigated. In this study, we developed an AI algorithm that demonstrated robust acquisition of AI-assisted PV metrics across varied magnetic resonance (MR) scanner types. We applied these metrics to differentiate between csPC and insPC within a biopsy-confirmed GS 6 cohort. The aim was to evaluate the integration of AI-derived PV measurements with clinical factors in cancer risk stratification, which could substantially improve the precision of prostate cancer diagnosis. We present this article in accordance with the TRIPOD + AI reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1015/rc).
Methods
Patient selection and ethical considerations
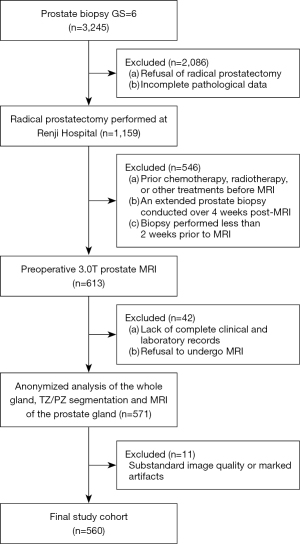
This retrospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and received approval from the Ethics Committee of Renji Hospital (No. KY2018-212-s). The institutional ethics committee waived the requirement for written informed consent due to the retrospective nature of the analysis. This study consisted of two stages. For the segmentation of prostate gland, we adopted previously published multicenter data (18). This study recruited a patient cohort from Renji Hospital between January 2017 and December 2021. The inclusion criteria were as follows: (I) initial diagnosis confirmed via ultrasound-guided transrectal 12-core system prostate biopsy with a GS of 6; (II) subsequent RP performed at Renji Hospital with comprehensive histopathological data; and (III) availability of complete clinical, imaging, and laboratory records. Meanwhile, the exclusion criteria were as follows: (I) prior chemotherapy, radiotherapy, or other treatments before MRI; (II) an extended prostate biopsy conducted over 4 weeks after MRI; (III) substandard image quality or marked artifacts; (IV) biopsies performed less than 2 weeks prior to MRI; (V) loss to follow-up or refusal of RP; and (VI) incomplete pathological data. The final cohort consisted of 560 patients (Figure 1).
MRI protocol and prostate segmentation technique
Imaging was performed on four 3.0-T MRI scanners, including Ingenia (Philips Healthcare, Best, the Netherlands), Signa (GE HealthCare, Chicago, IL, USA), Skyra (Siemens Healthineers, Erlangen, Germany), and uMR560 (United Imaging, Shanghai, China). Axial T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and apparent diffusion coefficient (ADC) maps formed the basis of our imaging analysis (the MR protocol is summarized in Table 1). Interpretations were conducted by a senior radiologist (G.W.) with more than 12 years of experience in MRI of the prostate. Prostate contour delineation was executed via ITK-SNAP software (version 3.6.0), as illustrated in Figure 2.
Table 1
| Parameter | Ingenia | Signa | Skyra | uMR560 |
|---|---|---|---|---|
| Platform | Philips Healthcare | GE HealthCare | Siemens Healthineers | United Imaging |
| Axial T2WI | ||||
| TR/TE (ms) | 3,371/95 | 3,320/72 | 4,650/105 | 4,737/69 |
| Section thickness (mm) | 3.0 | 3.5 | 3.0 | 3.0 |
| Intersection gap (mm) | 0.3 | 0.3 | 0.3 | 0.3 |
| FOV (mm) | 220×220 | 240×240 | 230×230 | 230×220 |
| Matrix | 368×326 | 320×288 | 384×384 | 288×270 |
| Axial DWI | ||||
| TR/TE (ms) | 4,284/54 | 6,000/73 | 3,960/57 | 5,600/91 |
| Section thickness (mm) | 3.0 | 3.5 | 3.0 | 3.0 |
| Intersection gap (mm) | 0.3 | 0.3 | 0.3 | 0.3 |
| FOV (mm) | 200×280 | 350×350 | 280×280 | 230×220 |
| Matrix | 80×134 | 128×128 | 185×185 | 144×75 |
| b value (s/mm2) | 0/1,500 | 0/1,500 | 0/1500 | 0/1,500 |
FOV, field of view; TR, repetition time; TE, echo time; T2WI, T2-weighted imaging; DWI, diffusion-weighted imaging.

We employed a convolutional neural network (CNN) for segmentation of the prostate gland and its zonal divisions based on PyTorch framework (https://pytorch.org/). Python 3.5. T2-weighted sequences facilitated prostate gland delineation via the modified virtual net (Vnet) architecture with a supervised approach (18,19).
For prostate gland segmentation with the CNN algorithm, the main architecture of the Vnet consists of one input block, four downsampling blocks, four upsampling blocks, and one output block. The input block contains one convolution layer with 16 filters, and each filter has a 3×3×3 filter shape. Each downsampling block consists of one residual block and one convolution layer with a 2×2×2 filter shape and a stride of 2. The residual block has three convolution layers and one residual connection, and each convolution layer has 32×N filters, where N denotes the current downsampling block. Each filter has 3×3×3 filter shape, and after each convolution batch, normalization is conducted, and the leaky rectified linear unit (ReLU) is used as the activation function. Each upsampling block consists of one residual block and one deconvolution layer with a 2×2×2 filter shape and a stride of 2. The residual block has three convolution layers and one residual connection, and each convolution layer has 128/N filters, where N denotes the current upsampling block. Each filter has a 3×3×3 filter shape, and after each convolution batch, normalization is conducted, and leaky ReLU is used as the activation function. The output block contains one convolution layer and one softmax layer, and the convolution layer has 2 filters with a 1×1×1 filter shape (Figure 3A).
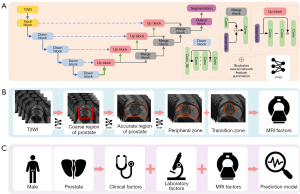
For the segmentation of TZ and PZ, the original model was based on the primary architecture, with the TZ and PZ labels serving as the input. In the coarse model, two cascaded Vnets were employed. The first Vnet was trained using the cropped coarse region as input, while the second Vnet employed the TZ and PZ labels for training. The fine model introduced an additional Vnet between the two Vnets of the coarse model, with the accurate prostate boundary being used as the input for network training (Figure 3B).
In designing the CNN architecture, we accounted for the fact that whole-gland segmentation assists in localizing the prostate region, and the gland segmentation result was also used in the subarea segmentation stage to remove most of the unrelated tissues, such as the pelvic bones and muscles. Additionally, accurately delineation of the prostate boundary in fine model helps minimize interference from periprostatic fat and vascular tissue, thereby improving the robustness of lesion detection results.
The ReLU activation function was selected to avoid the “dying Relu” problem. In standard ReLU, negative input values result in zero, which can cause neurons to stop learning. Leaky ReLU addresses this by allowing a small, nonzero gradient for negative inputs, thus enabling the network to continue updating its weights even when encountering negative input values. This feature is particularly beneficial in medical image segmentation, where certain features might not be captured if neurons are inactive. By using leaky ReLU, the model can better capture fine-grained details, improving segmentation performance in challenging areas of the prostate.
Evaluation metrics
Our method of segmentation was appraised via the Dice similarity coefficient (DSC), which can be used as a measure to compare two different regions and ground truth-overlapping regions. The DSC formula can be expressed as follows:
where N denotes the total number of pixels in the image, and pi and qi denote the pixel of manual segmentation and algorithm segmentation results, respectively. One minus the Dice coefficient was chosen as the loss function, so the loss tended toward zero. The learning rate was set as 0.0001, and the cascaded UNet models were trained for up to 100 iterations. The ground truth in our datasets were manually outlined by one expert with more than 12 years of experience.
Dataset allocation and risk stratification model building
For the risk stratification, factors including age, prostate serum antigen (PSA) levels, Prostate Imaging Reporting and Data System (PI-RADS), positivity in TZ or PZ biopsy, number of positive cores, and AI-assisted PV metrics were used to establish the model. The foundational model included clinical factors such as age, PSA levels, PI-RADS, positivity in TZ or PZ biopsy, and number of positive cores. Model 1 was based on the foundational model and further incorporated PV as an anatomical factor. Model 2 was based on model 1 with greater refinement of the anatomical factors of PZ volume and TZ volume (Figure 3C).
We employed computer-generated random assignments to allocate 60% of the dataset to the training set and the remaining 40% to the independent test set. In this study, we applied Pearson correlation coefficient (PCC) and principal component analysis (PCA) to integrate features in establishing the model. The efficiency of PCC and PCA in model building was assessed with total variance.
Biopsy methodology and pathological evaluation
Our standard protocol entailed ultrasound-guided transrectal biopsy (TRUS-biopsy), with 12 cores being extracted in accordance with international guidelines (20). Each core was independently processed and evaluated by blinded expert uropathologists, and RP specimens were assessed by uropathologists following established procedures (21). Patients were classified as having csPC or insPC based on RP specimen analyses. The csPC was defined as a single biopsy core with a GS of 3+4 or above [International Society of Urological Pathology (ISUP) grade group (GG) >1] (21).
Statistical analysis
Statistical evaluations were conducted using SPSS 26.0 (IBM Corp., Armonk, NY, USA) and R (R-3.4.4; https://www.r-project.org; The R Foundation for Statistical Computing). Continuous variables are represented as the median and interquartile range (IQR), while categorical variables are expressed as frequencies and percentages. The DSC was used to measure the overlap between the AI result and ground truth. Predictive models were constructed in Python (Python Software Foundation, Wilmington, DE, USA) to determine the occurrence of csPC and insPC. A foundational model of clinical factors was compared against new models incorporating volumetric measurements [model 1 with PV and model 2 with transitional/peripheral zone volume (TZV/PZV)]. The efficiency of PCC and PCA in model building was assessed with total variance. The total variance is first calculated for all N features in the dataset. The total variance is the sum of the variances of each feature. The variance is defined as follows: , where is the value of the i-th feature for the j-th sample, is the mean of the i-th feature, and M is the number of samples. The total variance is the sum of the variances of all features: . Second, for the selected k features, their variances are similarly calculated and summed as follows: . Here the sum is for the selected k features. Finally, the variance contribution is calculated by comparing the sum of the variances of the selected features with the total variance: . Performance in discrimination between csPC and insPC was assessed via the area under the curve (AUC) with the receiver operating characteristic (ROC). P<0.05 indicated a significant difference.
Results
Demographics and segmentation accuracy
The median age within our cohort was 68 years (IQR, 63–72 years), and the median PSA level was 9.02 ng/mL (IQR, 5.97–12.90 ng/mL). The median value of PV was 43.46 mL (IQR, 27.94–50.35 mL), with median values of 23.15 mL for the TZV (IQR, 16.30–37.18 mL) and 11.02 mL for the PZV (IQR, 8.80–13.68 mL) (Table 2). Tumors were predominantly located in the PZV (194 patients) and the TZV (134 patients), with 232 cases exhibiting involvement of both zones. For gland segmentation, the DSC was 0.93 for the whole prostate, 0.82 for the TZ, and 0.85 for the PZ, representing results superior to those of the traditional Vnet (P<0.001) (Figure 4).
Table 2
| Variable | Value |
|---|---|
| Age (years) | 68 [63–72] |
| PSA (ng/mL) | 9.02 [5.97–12.90] |
| PV (mL) | 43.46 [27.94–50.35] |
| TZV (mL) | 23.15 [16.30–37.18] |
| PZV (mL) | 11.02 [8.80–13.68] |
| PI-RADS score | |
| 2 | 70 (12.5) |
| 3 | 139 (24.8) |
| 4 | 266 (47.5) |
| 5 | 85 (15.2) |
Data are presented as median [IQR] or number of cases (%). IQR, interquartile range; PSA, prostate serum antigen; PV, prostate volume; TZV, transitional zone volume; PZV, peripheral zone volume; PI-RADS, Prostate Imaging Reporting and Data System.

Model performances in predicting cancer significance
We used clinical factors including age, PSA, PI-RADS, positivity in TZ or PZ biopsy, and number of positive prostate biopsy needles as the foundational model, which was followed by the addition of PV to the foundational model to form model 1 and finally the addition of TZV/PZV to model 1 to form model 2. The ability of the three models to discriminate between csPC and insPC was evaluated on the test dataset. With the PCC method, the performance of risk stratification model increased with the addition of AI-assisted PV metrics. PI-RADS, PV, TZV, positivity in TZ biopsy, and number of biopsy-positive needles were significant independent risk factors, while and TZV and positivity in TZ biopsy were nonsignificant in model 2 (Table 3). According to PCA, the AUC of the foundational model was 0.698, with an accuracy of 0.724. Model 1 scored an AUC of 0.712, with an accuracy of 0.742, while model 2 yielded an AUC of 0.730, with an accuracy of 0.751. Model 2 demonstrated superior classification performance over its predecessors (model 2 vs. model 1: P=0.0447; model 2 vs. foundational model: P=0.0054), with the ROC curves displayed in Figure 5.
Table 3
| Characteristic | OR (95% CI) | P value |
|---|---|---|
| Age (years) | 1.014 (0.984–1.046) | 0.357 |
| PI-RADS | 1.472 (1.172–1.848) | 0.001 |
| PSA (ng/mL) | 1.018 (0.992–1.044) | 0.181 |
| PV (mL) | 1.041 (0.994–1.091) | 0.089 |
| TZV (mL) | 0.950 (0.907–0.994) | 0.028 |
| PZV (mL) | 0.966 (0.909–1.026) | 0.259 |
| Positivity in TZ biopsy | 1.867 (1.100–3.169) | 0.021 |
| Positivity in PZ biopsy | 0.717 (0.380–1.353) | 0.305 |
| Number of biopsy-positive needles | 1.134 (0.981–1.312) | 0.090 |
OR, odds ratio; CI, confidence interval; PI-RADS, Prostate Imaging Reporting and Data System; PSA, prostate serum antigen; PV, prostate volume; TZV, transitional zone volume; PZV, peripheral zone volume; TZ, transitional zone; PZ, peripheral zone; csPC, clinically significant prostate cancer.
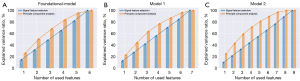
In model 2, the PCA coefficients for PV, TZV, and positivity in TZ biopsy were 0.010, 0.054, and 0.142, respectively. In contrast, in the foundational model, the PCA coefficient for positivity in the TZ biopsy was 0.352. In model 1, the PCA coefficients for PV and positivity in TZ biopsy were −0.272 and −0.411, respectively. Compared to PCC, the PCA algorithm for the foundational model yielded markedly higher AUC values in the test dataset (PCA vs. PCC: 0.698 vs. 0.640; P=0.0060). The AUC value of the PCA dimensionality reduction algorithm for model 1 was significantly higher in the test dataset compared to PCC (PCA vs. PCC: 0.712 vs. 0.683; P=0.0045). The PCA dimensionality reduction algorithm for model 2 had dramatically higher AUC values in the test dataset than did PCC (PCA vs. PCC: 0.730 vs. 0.700; P=0.0061). This indicates that PCA provides superior interpretability of the dataset as compared to PCC, which was corroborated by the results of the variance contribution rate (Figure 6).
Discussion
In this study, we developed an AI model capable of accurately measuring the volume of specific subareas within the prostate. Our findings suggest that combining AI-derived measurements of the TZV/PZV with clinical factors can enhance the precision of distinguishing between csPC and insPC in patients with biopsy-confirmed prostate cancer with a GS 6.
PSA mainly leaks from the TZ (14), and accurate measurement of PV is crucial for various clinical applications. The traditional approach of approximating the prostate as an ellipsoid has limitations, especially for irregularly shaped, enlarged prostates that do not conform to this assumption (22-24). Previous studies have demonstrated the effectiveness of AI-based diagnosis in automatic prostate segmentation (18,25). However, challenges persist, particularly in subregion segmentation, and many studies have struggled to achieve satisfactory results in segmenting the TZ. In our study, we encountered similar segmentation issues with our initial model, but the performance significantly improved in our cascade model, resulting in a satisfactory outcome for practical use. This suggests that a cascaded model approach can be a reliable method for segmenting subareas within the prostate, with each step conferring cumulative benefit. Furthermore, our study demonstrated that the improved model exhibited excellent generalization across different scanning devices, emphasizing the strengths of a cascaded model in this regard.
The majority of patients with biopsy-proven prostate cancer and a GS 0 to 6 opt are suggested to undergo active surveillance in clinic, but the definitive diagnosis often relies on prostate biopsy, which may underestimate the risk of csPC. According to the literature, a certain percentage (44–87%) of patients may experience disease progression within 2 years (26-28). There has been limited research focused on differentiating between csPC and insPC in patients with biopsy-proven prostate cancer and a GS of 6. In our study, we found that 67.3% patients already had csPC, highlighting the importance of stratifying patients before the initiated of active surveillance. Our findings indicate that achieving effective differentiation can potentially provide better stratification of the patient’s risk in the selection of the appropriate treatment method.
The ability of PI-RADS to discriminate csPC from insPC in patients with biopsy-proven prostate cancer with a GS 6 can still be improved. It is thus worth considering multiple factors in the differentiation process, as relying on a single factor is problematic. The results of a regression analysis using the PCC confirmed the value of adding both AI-assisted PV metrics and clinical factors in distinguishing between csPC and insPC. Furthermore, the inclusion of subarea volume metric of prostate can further improve the diagnostic accuracy. It is worth noting that previous research has also indicated the potential of PV in evaluating prostate cancer. Our findings align with these prior studies, indicating that subregion-based TZV and PZV are valuable factors for distinguishing between csPC and insPC. As TZ and PZ present different histological structures and could have distinct contributions to particular pathophysiological processes (29) and correlations with PSA levels. This finding underscores the significance of utilizing different subarea volume metric features in clinical applications. Using TZV and PZV as substitutes for PV may serve as promising imaging markers, particularly for accurately segmenting the prostate gland. The accuracy our AI model in prostate gland segmentation suggests its considerable potential in providing robust and comparable parameters. AI-assisted PV measurement is more accessible and uniform, thus enabling the determination of the actual PV and foregoing the dependence on estimations.
Additionally, we discovered that the performance obtained from PCA algorithms with various models outperformed those obtained from PCC. PCA, which is commonly used in atmospheric science and referred to as empirical orthogonal function (EOF) analysis, was initially proposed by Obukhov (30) and Lorenz (31). It has been employed for diverse purposes, such as for identifying patterns in large datasets of meteorological or oceanographic data, reducing data dimensionality, and extracting signals from noisy data. In our study, we believe that PCA generated new composite variables and reduced dimensionality while preserving essential information, thereby enhancing the performance and efficiency in constructing diagnostic models.
However, several limitations in this study should be acknowledged. First, despite the large sample size of our study, it is essential to recognize that we employed a retrospective design carried out at a single center. Second, our models primarily focused on the volume information extracted from MRI. We recognize that mining image data could offer a promising avenue to further enhance the performance of differential diagnosis. Ultimately, previous studies have used neural network architectures different from ours for gland segmentation, with some relatively simple structures achieving remarkable results (32-34). We speculate that the variability in research populations and segmentation approaches may lead to discrepant outcomes. Based on our sample, we recommend employing cascade strategies to improve enhance segmentation performance. In the future, we plan to integrate our strategy with networks used in other studies to assess whether further improvements in segmentation performance can be achieved.
Conclusions
Our findings support the integration of AI-derived volumetric measurements with clinical factors in the prediction of csPC, confirming the potential of AI-assisted diagnostic systems in clinical risk stratification and the management of prostate cancer.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD + AI reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1015/rc
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1015/coif). Y.S. is an employee from Siemens Healthineers Ltd. and provided MR and technical support in a collaboration with Siemens but did not receive any payment and had no personal concern regarding this study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and received approval from the Ethics Committee of Renji Hospital (No. KY2018-212-s). The institutional ethics committee waived the requirement for written informed consent due to the retrospective nature of the analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Tomlins SA, Mehra R, Rhodes DR, Cao X, Wang L, Dhanasekaran SM, Kalyana-Sundaram S, Wei JT, Rubin MA, Pienta KJ, Shah RB, Chinnaiyan AM. Integrative molecular concept modeling of prostate cancer progression. Nat Genet 2007;39:41-51. [Crossref] [PubMed]
- Hu Y, Ahmed HU, Carter T, Arumainayagam N, Lecornet E, Barzell W, Freeman A, Nevoux P, Hawkes DJ, Villers A, Emberton M, Barratt DC. A biopsy simulation study to assess the accuracy of several transrectal ultrasonography (TRUS)-biopsy strategies compared with template prostate mapping biopsies in patients who have undergone radical prostatectomy. BJU Int 2012;110:812-20. [Crossref] [PubMed]
- Tefilli MV, Gheiler EL, Tiguert R, Sakr W, Grignon DJ, Banerjee M, Pontes JE, Wood DP Jr. Should Gleason score 7 prostate cancer be considered a unique grade category? Urology 1999;53:372-7. [Crossref] [PubMed]
- Eggener SE, Scardino PT, Walsh PC, Han M, Partin AW, Trock BJ, Feng Z, Wood DP, Eastham JA, Yossepowitch O, Rabah DM, Kattan MW, Yu C, Klein EA, Stephenson AJ. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol 2011;185:869-75. [Crossref] [PubMed]
- Epstein JI, Amin MB, Reuter VE, Humphrey PA. Contemporary Gleason Grading of Prostatic Carcinoma: An Update With Discussion on Practical Issues to Implement the 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 2017;41:e1-7. [Crossref] [PubMed]
- Capitanio U, Ahyai S, Graefen M, Jeldres C, Shariat SF, Erbersdobler A, Schlomm T, Haese A, Steuber T, Heinzer H, Perrotte P, Péloquin F, Pharand D, Arjane P, Huland H, Karakiewicz PI. Assessment of biochemical recurrence rate in patients with pathologically confirmed insignificant prostate cancer. Urology 2008;72:1208-11; discussion 1212-3. [Crossref] [PubMed]
- Sengupta S, Blute ML, Bagniewski SM, Inman B, Leibovich BC, Slezak JM, Myers RP, Zincke H. After radical retropubic prostatectomy 'insignificant' prostate cancer has a risk of progression similar to low-risk 'significant' cancer. BJU Int 2008;101:170-4. [Crossref] [PubMed]
- Hashimoto Y, Okamoto A, Imai A, Yoneyama T, Hatakeyama S, Yoneyama T, Koie T, Kaminura N, Ohyama C. Biochemical outcome of small-volume or insignificant prostate cancer treated with radical prostatectomy in Japanese population. Int J Clin Oncol 2012;17:119-23. [Crossref] [PubMed]
- Ting F, van Leeuwen PJ, Delprado W, Haynes AM, Brenner P, Stricker PD. Tumor volume in insignificant prostate cancer: Increasing the threshold is a safe approach to reduce over-treatment. Prostate 2015;75:1768-73. [Crossref] [PubMed]
- King CR. Patterns of prostate cancer biopsy grading: trends and clinical implications. Int J Cancer 2000;90:305-11. [Crossref] [PubMed]
- Dong F, Jones JS, Stephenson AJ, Magi-Galluzzi C, Reuther AM, Klein EA. Prostate cancer volume at biopsy predicts clinically significant upgrading. J Urol 2008;179:896-900; discussion 900. [Crossref] [PubMed]
- Porten SP, Whitson JM, Cowan JE, Cooperberg MR, Shinohara K, Perez N, Greene KL, Meng MV, Carroll PR. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J Clin Oncol 2011;29:2795-800. [Crossref] [PubMed]
- Castro HAS, Iared W, Santos JEM, Solha RS, Shigueoka DC, Ajzen SA. Impact of PSA density of transition zone as a potential parameter in reducing the number of unnecessary prostate biopsies in patients with psa levels between 2.6 and 10.0 ng/mL. Int Braz J Urol 2018;44:709-16. [Crossref] [PubMed]
- Wang C, Wang YY, Wang SY, Ding JX, Ding M, Ruan Y, Wang XH, Jing YF, Han BM, Xia SJ, Jiang CY, Zhao FJ. Peripheral zone PSA density: a predominant variable to improve prostate cancer detection efficiency in men with PSA higher than 4 ng ml(-1). Asian J Androl 2021;23:415-20. [Crossref] [PubMed]
- Roehrborn CG, Girman CJ, Rhodes T, Hanson KA, Collins GN, Sech SM, Jacobsen SJ, Garraway WM, Lieber MM. Correlation between prostate size estimated by digital rectal examination and measured by transrectal ultrasound. Urology 1997;49:548-57. [Crossref] [PubMed]
- Jeong CW, Park HK, Hong SK, Byun SS, Lee HJ, Lee SE. Comparison of prostate volume measured by transrectal ultrasonography and MRI with the actual prostate volume measured after radical prostatectomy. Urol Int 2008;81:179-85. [Crossref] [PubMed]
- Liu G, Pan S, Zhao R, Zhou H, Chen J, Zhou X, Xu J, Zhou Y, Xue W, Wu G. The added value of AI-based computer-aided diagnosis in classification of cancer at prostate MRI. Eur Radiol 2023;33:5118-30. [Crossref] [PubMed]
- Yu L, Yang X, Chen H, Qin J, Heng PA. Volumetric ConvNets with Mixed Residual Connections for Automated Prostate Segmentation from 3D MR Images. Proceedings of the AAAI Conference on Artificial Intelligence 2017;31. doi:
10.1609/aaai.v31i1.10510 .10.1609/aaai.v31i1.10510 - Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N, Schmid HP, van der Kwast T, Wiegel T, Zattoni FEuropean Association of Urology. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol 2011;59:61-71. [Crossref] [PubMed]
- Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med 2018;378:1767-77. [Crossref] [PubMed]
- Park KJ, Kim MH, Kim JK, Cho KS. Characterization and PI-RADS version 2 assessment of prostate cancers missed by prebiopsy 3-T multiparametric MRI: Correlation with whole-mount thin-section histopathology. Clin Imaging 2019;55:174-80. [Crossref] [PubMed]
- Tosun M, Uslu H. Prebiopsy multiparametric MRI and PI-RADS version 2.0 for differentiating histologically benign prostate disease from prostate cancer in biopsies: A retrospective single-center comparison. Clin Imaging 2021;78:98-103. [Crossref] [PubMed]
- Wang W, Wang G, Wu X, Ding X, Cao X, Wang L, Zhang J, Wang P. Automatic segmentation of prostate magnetic resonance imaging using generative adversarial networks. Clin Imaging 2021;70:1-9. [Crossref] [PubMed]
- Zhu Y, Wei R, Gao G, Ding L, Zhang X, Wang X, Zhang J. Fully automatic segmentation on prostate MR images based on cascaded fully convolution network. J Magn Reson Imaging 2019;49:1149-56. [Crossref] [PubMed]
- Dall'Era MA, Klotz L. Active surveillance for intermediate-risk prostate cancer. Prostate Cancer Prostatic Dis 2017;20:1-6. [Crossref] [PubMed]
- Washington SL 3rd, Baskin AS, Ameli N, Nguyen HG, Westphalen AC, Shinohara K, Carroll PR. MRI-Based Prostate-Specific Antigen Density Predicts Gleason Score Upgrade in an Active Surveillance Cohort. AJR Am J Roentgenol 2020;214:574-8. [Crossref] [PubMed]
- Willemse PM, Davis NF, Grivas N, Zattoni F, Lardas M, Briers E, et al. Systematic Review of Active Surveillance for Clinically Localised Prostate Cancer to Develop Recommendations Regarding Inclusion of Intermediate-risk Disease, Biopsy Characteristics at Inclusion and Monitoring, and Surveillance Repeat Biopsy Strategy. Eur Urol 2022;81:337-46. [Crossref] [PubMed]
- McNeal JE. Normal histology of the prostate. Am J Surg Pathol 1988;12:619-33. [Crossref] [PubMed]
- Obukhov AM. Statistically homogeneous fields on a sphere. Usp Mat Nauk 1947;2:196-8.
- Lorenz EN. Empirical orthogonal functions and statistical weather prediction. Technical report, Statistical Forecast Project Report 1, Dept of Meteor 1956;MIT:49.
- Hamzaoui D, Montagne S, Renard-Penna R, Ayache N, Delingette H. Automatic zonal segmentation of the prostate from 2D and 3D T2-weighted MRI and evaluation for clinical use. J Med Imaging (Bellingham) 2022;9:024001. [Crossref] [PubMed]
- Jimenez-Pastor A, Lopez-Gonzalez R, Fos-Guarinos B, Garcia-Castro F, Wittenberg M, Torregrosa-Andrés A, Marti-Bonmati L, Garcia-Fontes M, Duarte P, Gambini JP, Bittencourt LK, Kitamura FC, Venugopal VK, Mahajan V, Ros P, Soria-Olivas E, Alberich-Bayarri A. Automated prostate multi-regional segmentation in magnetic resonance using fully convolutional neural networks. Eur Radiol 2023;33:5087-96. [Crossref] [PubMed]
- Hamm CA, Baumgärtner GL, Padhani AR, Froböse KP, Dräger F, Beetz NL, Savic LJ, Posch H, Lenk J, Schallenberg S, Maxeiner A, Cash H, Günzel K, Hamm B, Asbach P, Penzkofer T. Reduction of false positives using zone-specific prostate-specific antigen density for prostate MRI-based biopsy decision strategies. Eur Radiol 2024;34:6229-40. [Crossref] [PubMed]



