Selective arterial embolization of symptomatic and asymptomatic renal angiomyolipomas: a retrospective study of safety, outcomes and tumor size reduction
Introduction
Angiomyolipoma (AML) is a benign renal tumor and the most common benign hamartomatous neoplasm accounting for 0.3% to 3% of all renal masses (1-3). They are more accurately characterized as perivascular epithelioid cell neoplasms (PEComas) in pathology (4). They are composed of fat, blood vessels and smooth muscle in varying quantities and are commonly divided into sporadic and non-sporadic groups (5,6). The sporadic group, accounting for approximatively 80% of all cases, consists of solitary lesion which occurs predominantly in middle-aged women with a prevalence of 0.22% to 0.29% in females and 0.02% to 0.1% in males (7-10). The non-sporadic group is related to tuberous sclerosis complex (TSC), an autosomal dominant multi-organ phacomatosis predisposing to benign tumor formation with activation of the mammalian target of rapamycin (mTOR) pathway, and is also more frequent in women (11). Within this group, AMLs develop in 50% of all patients during their life time, are often larger, multiple, bilateral, symptomatic and lesions are likely to widespread (7,8,12,13). TSC may also be associated with lymphangioleiomyomatosis, a progressive disease related to tuberous sclerosis, which usually affects the lungs of young women (14,15). Although a large majority of AMLs with focal epithelioid components are benign, pure epithelioid renal AML (EAML), a rare subgroup characterized by the presence of epithelial cells staining strongly for melanoma-associated markers, may become locally invasive and spread by affecting renal veins and inferior vena cava (16). Those aggressive epithelioid “carcinoma-like” variants are highly cellular with very little or no fat, tend to be larger and may become symptomatic for the majority (17,18).
As a benign lesion, most renal AMLs are asymptomatic, have a slow and consistent growth rate and minimal morbidity (19). They often do not require any intervention. However, they can cause flank pain or palpable mass but the main complications are retroperitoneal bleeding or bleeding into the urinary collection system, which can be life threatening. The bleeding tendency is related to the angiogenic component of the tumor that includes irregular and/or aneurysmal blood vessels (20). The major risk factors for bleeding are tumor size, grade of the angiogenic component, and the presence of TSC (8,20). The European Association of Urology recommends active surveillance for most AMLs and treatment in case of pain, bleeding, or suspected malignancy. Prophylactic invasive procedures such as selective arterial embolization (SAE) and nephron sparing surgery (NSS) can be considered in large tumors, females of childbearing age as AMLs tend to grow in size and rupture during pregnancy because of their hormone sensitivity (21,22) and when access to emergency care may be inadequate (23). SAE can be used for prophylaxis of high-risk tumor, for acute management of tumor bleeding, or as a preoperative adjunct treatment for surgery to prevent intraoperative blood loss (8,20,24,25). This minimally invasive interventional radiology technique has become the primary treatment for AMLs since several years because it is less invasive than a surgical intervention and enables targeted treatment of bleeding vessels with a low risk of severe complications (20,24-41). The objective of this retrospective mono-centric study was to present our 11-year experience with planned prophylactic and emergency SAE of renal AMLs by reviewing our institution’s series of 23 patients and evaluating complications, outcomes and tumor size reduction (TSR) after SAE.
Methods
Patients
All imaging studies and medical records of patients with AMLs treated with SAE in our institution were reviewed over an 11-year period between January 2005 and July 2016. An electronic search of our imaging database was performed and the initial patient list consisted of all report queries of patients treated at our hospital who matched for the words “angiomyolipoma(s)”, “hemorrhage” and/or “embolization”. From this first list, patients who underwent prophylactic or emergency SAE for AMLs with follow-up imaging and clinical follow-up were included. Patients without follow-up or who underwent arteriography without embolization were excluded of the statistical analysis; it concerned only the patients for whom another therapeutic strategy was decided and not because of a technical failure. EAMLs confirmed by histology were also excluded as this tumor has totally different components and behavior.
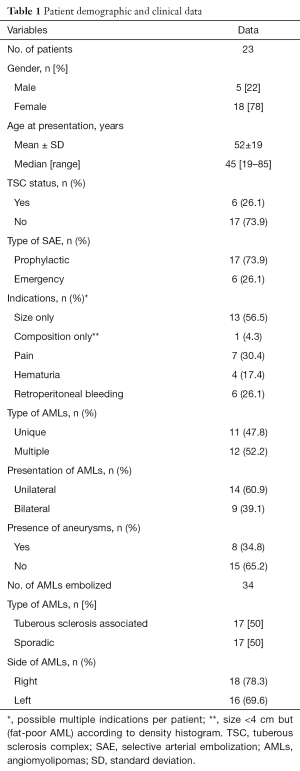
Demographic, clinical and biological data
We reviewed the medical records of all subjects and recorded demographic data (age and gender), AML type (sporadic or TSC-related), location and number of lesions, intervention type (prophylactic or emergency), clinical symptoms and complications before and within 4 weeks after SAE (according to the Society of Interventional Radiology classification system for complications) (42), recurrence (defined as recurrent symptoms or increase in tumor size of >2 cm on follow-up images requiring re-intervention) (27) and the need for further treatment independently of the reason. Serum creatinine levels were measured before SAE and during follow-up with Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) method for clearance calculation. TSC patients suitable for a trial with mTOR inhibitor (everolimus) were also considered.
Diagnosis, tumor size measurement and embolization technique
All AMLs were diagnosed with computed tomography (CT) and/or magnetic resonance imaging (MRI) on the basis of the presence of intra-tumoral fat without calcification or necrosis (43-45). Macroscopic fat appeared as areas with a density less than −20 Hounsfield units (HU) on non-enhanced CT or with cancellation of high signal intensity on T1-weighted MR images with fat saturation. In and opposed-phased chemical shift sequences were useful in low fat AMLs in which the aforementioned typical T1 findings were not readily apparent (46). Two cases were confirmed at histologic diagnosis after percutaneous needle biopsy because of lack of macroscopic fat within the lesion and one of them was an epithelioid variant.
Post-processing was performed on a syngo®.via workstation (Siemens Healthcare, Erlangen, Germany). All pre-SAE and follow-up images were reviewed by an experienced radiologist and each embolized tumor was measured using maximum diameter as previously applied in the literature (32,47). For measurement, we used the last available imaging exam performed before SAE (CT or MRI). Depending on availability, three-dimensional (3D) reconstructions with maximum diameter, 3D volume and density histograms calculation were performed for the prophylactic group diagnosed and followed-up with the CT modality by using MM Oncology software (Siemens Healthcare). Intralesional aneurysms were measured on angiographic images, not only with automatic calibration but also with reference to guiding catheter and microcatheter sizes. When a patient was found to have multiple aneurysms, the largest one was selected for evaluation.
The treatment plan was based on multidisciplinary proposal by interventional radiologists, urologists and/or nephrologists. SAE was performed under local anesthesia through the common femoral artery using 4–5 Fr angiographic catheters and coaxial microcatheters. An aortogram was first obtained to locate the renal arteries and to determine the presence of accessory renal arteries or extra-renal feeding vessels. After aortography, selective renal angiography was performed to assess AML circulation and extension of tumoral vessels outside the normal nephrogram, aneurysms, active extravasation and vascular displacements by the tumor or retro-peritoneal hematoma in emergency cases. Next, supra-selective catheterization of AML feeding vessels was performed using a coaxial microcatheter in order to spare as much renal parenchyma as possible. Various embolic materials were used alone or in combination as calibrated particles, liquid embolic agents and microcoils including: tris-acryl gelatin and polyphosphazene microspheres 100–700 µm (Embosphere®/EmboGold®, Biosphere Medical Inc.-Merit Medical Inc., Rockland, MA, USA; Embozene®, CeloNova-Boston Scientific, Marlborough, Massachusetts, USA), detachable microcoils (Detach-18®, Cook Medical Technologies LLC, William Cook Europe A/S; Interlock-18 Coil®, Boston Scientific Corporation), acrylic glue (N-butyl 2-cyanoacrylate, Glubran 2®, GEM Srl, Viareggio, Italy) mixed with ethiodized oil (Lipiodol® Ultra-Fluid, Guerbet, Aulnay-sous-Bois, France) in a 1:3 to 1:6 ratio, and Onyx® 18 (Covidien, Mansfield, MA, USA). One covered stent (Fluency® Plus Endovascular Stent Graft 7–40 mm, C.R. Bard, Inc., New Jersey, USA) was used to exclude a small bleeding adrenal branch from the origin of a renal artery in one patient. Embolic materials were injected under fluoroscopic guidance and coils were used to occlude large aneurysmal formations that would have been unsuitable for particle embolization alone. Additional supplying branches were selectively catheterized and embolized until total devascularization. Technical success was defined as stasis of flow in arteries feeding the tumor and lack of opacification of AML on post-SAE angiogram (35,41).
After the procedure, patients embolized for preventive purpose were transferred to a urology department at our institution or in a referring hospital for 24–48 h stay and usually discharged the day after embolization. Vital signs were monitored and post-embolization syndrome (PES) (low-grade fever, pain and vomiting 3–7 days after SAE) routinely treated with non-steroidal anti-inflammatory drugs (NSAID). Antibiotic prophylaxis was not given. Patients from the TSC group were admitted to the nephrology department which was the best qualified to monitor them. Finally, patients embolized in emergency were then referred to various departments, especially intensive care and surgical units.
Follow-up
Medical records, outpatient charts, follow-up visits and imaging were reviewed. All the patients without follow-up at our institution were contacted by phone or e-mail to evaluate their current medical status and the need for additional treatment. They also provided their latest biology and imaging follow-up. Most of the outpatients underwent follow-up by the urology and nephrology teams 3–6 months after the procedure and once a year thereafter. CT or MR follow-up was recommended 3 months after SAE and annually if no change or decrease in size was noted. Images were obtained at different institutions with different equipments and protocols. For measurement, we used the last imaging exam available (CT or MRI or US).
Statistical analyses
Qualitative variables were described as percentages and compared using exact Fisher test. Quantitative variables were described using means with standard deviations (SDs) and medians with ranges. They were compared using the non-parametric Mann and Whitney test. Correlation between the tumor reduction and initial tumor size was determined using Pearson coefficient.
P values less than 0.05 were considered as significant. Analyses were performed using SAS® 9.3 (Statistical Analysis Software).
Results
Figure 1 represents flow chart of cohort construction for statistical analyses. The demographic and clinical data are presented in Table 1. During an 11-year study period, 23 patients underwent SAE in emergency to treat bleeding AMLs (n=6) (Figure 2) or as prophylactic treatment for high-risk AMLs (tumor size >4 cm, abnormal vasculature on CT, other symptoms) (n=17) and could be followed-up. One small sporadic asymptomatic AML was not embolized because its feeding vessels could not be identified during the arteriography; a percutaneous radiofrequency ablation was performed instead (48,49). We preferred a conservative approach by active surveillance for a second sporadic asymptomatic AML because of its very small angiomyomatous component. One embolized patient was excluded because he died from heart failure before the first imaging control. We decided to treat a small epithelioid variant with SAE as this sub-group tends to be locally invasive and become symptomatic and another small AML on the same patient due to its high vascular composition (40,50). Additional percutaneous thermal ablation with microwaves was decided for the epithelioid variant because of its uncertain evolution. The first tumor was excluded from statistical analysis according to exclusion criteria (tumors, n=34) (Figure 3). One patient had a single right kidney before treatment. As it is widely accepted that lesions over 4 cm are more likely to become symptomatic (>90%) and more likely to bleed (>50%), almost all sporadic lesions were embolized on the basis of the size only (51).
Full table
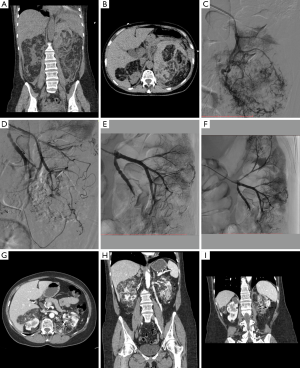
AMLs were successfully embolized for 22 patients in the first instance (96%) with immediate and complete devascularization on post-procedural aortogram and 4 re-embolizations were necessary during the follow-up because of incomplete embolization (n=1) or recurrence (2 bleeding AMLs and 1 re-growth). Microspheres and glue were the most frequent embolic agents used. Aneurysms were found in eight tumors (Figure 4). In our cohort, total nephrectomy was performed for one renal AML 9 months after the primary treatment with SAE therapy because of poor functional parenchyma on follow-up.
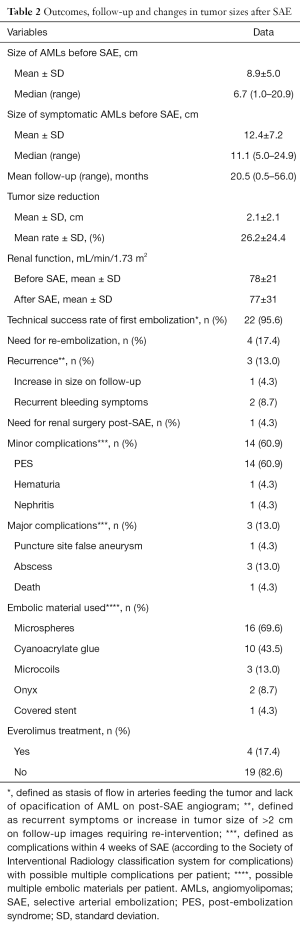
Patient outcomes, follow-up times and changes in tumor sizes are presented in Table 2. The mean size of AMLs was 8.9 cm and the median size was 7.7 cm. Minor complications affected 14 patients with PES treated with conservative measures (n=14), persistent hematuria (n=1) and pyelonephritis (n=1). Major complications affected three patients with two renal abscesses formations 17 and 21 days after the procedure and one femoral false-aneurysm associated to renal abscess during hospitalization. Drainage of renal collections was proposed to the first and the second patient; they recovered well and were discharged after 1 week. A few days after embolization of femoral false-aneurysm and abscess drainage, the third patient unfortunately died from hemodynamic failure. One TSC patient deceased from tumoral brain hemorrhage 4 years after SAE and one patient from the sporadic AML group had a total contralateral nephrectomy for concomitant renal cell carcinoma (RCC). Recurrence affected three patients during follow-up and all of them were successfully re-embolized with good results. The efficacy of embolization was determined over a mean follow-up of 20.5 months (range, 0.5–56 months). No patients were lost to follow-up. For measurement of tumor sizes before SAE, CT was used for 18 patients and MRI was used for 5 patients. For measurement of tumor sizes after SAE, CT was used for 15 patients, MRI was used for 6 patients and ultrasound was used for 2 patients. Seventeen patients had pre-existent reduction of glomerular filtration rate (GFR) with mild reduction for 13, moderate reduction for 3 and severe for 1 according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) classification for chronic kidney disease (CKD) (Table S1). There was no change in creatinine mean level after SAE. Changes in AMLs size after SAE are summarized in Table 3; the mean AMLs size reduction was 2.1 cm (26.2%) (Table 2) and only non-TSC status was significantly associated with better shrinkage of the tumors (P=0.022).
Full table

Full table
Full table
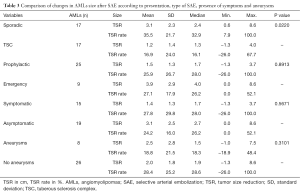
Hemorrhagic presentation was significantly more frequent in patients with intralesional aneurysms (P=0.0086) (Table 4), with presence of aneurysms in 62.5% of patients embolized in emergency for hemorrhage and only one hemorrhagic case without aneurysm found on arteriography.

Full table
Discussion
Prior to 1976, more than 90% of sporadic AMLs were treated with total nephrectomy, as a malignant lesion could not be excluded (48). With improvement of cross sectional imaging and even in cases of low-fat tumors, AMLs can now be confidently diagnosed with an MRI specificity of up to 99% (52,53). Since the first report by Adler et al. in 1984 (54), transarterial embolization has increasingly been used to become the new standard for preventive or emergency treatment of AMLs with minimally invasive selective targeting of small arterial feeders (25-27,36). Literature comparing nephrectomy and NSS with SAE in the management of AMLs is limited (3,8,55,56) with medical and economic analyses favoring embolization in symptomatic renal AMLs or AMLs at risk of complications. Endovascular therapy for AMLs has less postoperative morbidity (6.9%) compared to partial nephrectomy (12%), with minimal invasiveness and shorter hospitalization (54,57). It also allows rapid stabilization in cases of acute hemorrhage with optimal sparing of the normal renal parenchyma to maintain maximal renal function which is especially important in TSC patients. Surgery allows complete resection of the tumor and pathologic analysis to confirm diagnosis but remains quite difficult for AMLs in some cases with complex vascular anatomy, hilar location or for TSC-associated AMLs because of the multiplicity of the lesions (47). In the case of failure of primary or repeat SAE, nephron-sparing surgery might be considered (20,58). In our series, only one patient had surgical resection decided by urologists after successful SAE because of poor residual functional parenchyma.
Literature about laparoscopic and percutaneous ablative therapies for AMLs is also limited and largely restricted to small and asymptomatic tumors. Radiofrequency ablation series demonstrate good results with few to no retreatments, minor complications and no recurrences (median follow up of up to 45 months) (48,49). To date, we have performed six radiofrequency ablations for asymptomatic AMLs in our center and one of them was considered due to lack of abnormal vessels on arteriography. Reports for the use of cryoablation for AMLs are actually limited to small series (59-61).
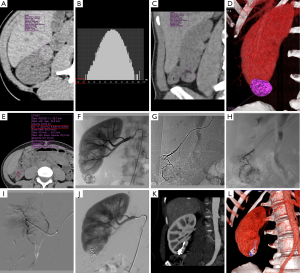
In every case, discussion between interventional radiologists and urologists is essential to determine the optimal management. For many years, the threshold diameter for prophylactic treatment has been 4 cm (7,8,48) since Oesterling et al. (51) estimated that in lesions greater than 4 cm, 82–94% were symptomatic and 50–60% bled spontaneously. However, this historical threshold has since been discussed by authors recommending treatment for asymptomatic tumors larger than 6 (62) or 8 cm (8,63) as the rate of symptomatic AML >4 cm seems to have been over-evaluated in old series (64). Hocquelet et al. (40) identified the percentage of fat content before SAE as a predictive factor of volume decrease in their series with a volume reduction significantly more important for AMLs with less than 50% of fat than for those with more than 50% of fat (84% vs. 50%; P<0.00001). In their multivariate analysis, the best predictive model for volume decrease included only the percentage fat content (R2=0.61; P<0.0001). Unfortunately, the small number of unenhanced CT scans available to calculate density histograms did not allow us to confirm this hypothesis. However, we believe that in addition to size, composition of AMLs should play an important role in the embolization indication and results because the angiomyogenic component is the main cause of bleeding complications and the target of embolization. Planché et al. (50) observed that the angiomyogenic components disappeared faster after embolization than the fatty components, and that “fat-poor” AMLs exhibited a larger volume reduction after SAE compared to “fat-rich” AMLs. In our series, we took into consideration the amount of angiomyogenic component and decided to embolize a “fat-poor” AML <4 cm with less than 5% of fat. It was the best result of our study with total disappearance of the mass on follow-up imaging (Figure 2).
In the same patient, we also decided to embolize an epithelioid AML diagnosed by pathology. This independent rare subtype of AML has malignant potential and imaging characteristics similar to RCC. Some cases of epithelioid AML metastasized to the lung, liver or bone have been previously reported but our patient wasn’t metastatic and was asymptomatic (65-67). As the proper treatment for epithelioid AML is still discussed, we decided to perform an additional thermal ablation of the tumor while other authors recommend a more aggressive strategy using surgery with or without chemotherapy (68).
Several studies have recently recommended active surveillance as initial treatment for asymptomatic or mildly symptomatic AMLs, even if they are >4 cm (59). Ouzaid et al. (69) found that only 13 out of 38 patients with AML >4 cm discontinued active surveillance after 4 years mean follow-up, arguing that treatment for all tumors >4 cm would have resulted in a 65% over-treatment rate and that 67% of symptomatic patients were managed with active surveillance without complications. Bhatt et al. (64) found in their series of 447 patients up to 70% of asymptomatic lesions >4 cm with no difference in growth rates for lesions >4 and <4 cm. They support the hypothesis that a small sub-group of “fast growers” tend to be significantly more symptomatic than “slow” or “non-growers” and should be aware of the risk of progression and bleeding. Recent studies have suggested that although tumor size is important, size of associated aneurysms may be more significant. Yamakado et al. (27,70) found using the conventional cut-off of 4 cm has significantly poorer specificity (38%) than aneurysm of 5 mm or larger (86%) and aneurysm size was the only factor significantly linked to rupture in their multiple regression analysis (P=0.001). Aneurysms may be seen on CT scans but small ones are more easily assessed by conventional angiography (71). In our series, aneurysms were significantly more frequent in hemorrhagic patients (P=0.0086), confirming the importance of tumor composition.
A recent strategy for managing AML in patients with TSC is based on the use of targeted therapeutics focused on inhibition of the mTOR pathway to stop tumor progression and even promote regression of tumors. The first mTOR inhibitor tried was sirolimus, also known as rapamycin, initially developed as an immunosuppressive agent in solid organ transplantations. Everolimus is a rapamycin derivative that has been recently studied with data from two randomized controlled trials suggesting safety and efficacy (72,73). EXIST-2, a double-blind-randomized controlled trial that included 118 patients with 3 cm or larger AMLs in the setting of TSC, randomized everolimus and placebo (67). At a median interval of 38 weeks there was a 42% response rate for the everolimus group [95% confidence interval (CI), 31–53%] versus 0% for the placebo group (95% CI, 0–9%) with a response defined as a 50% or greater reduction in total AMLs volume. Median time to response for everolimus was 2.9 months and median exposure was 8.7 months. Progression-free rates for everolimus and placebo, respectively, were 92% (95% CI, 65–98%) and 25% (95% CI, 1–64%) at 12 months and no patients who achieved a response in AML volume reduction progressed. A longer-term analysis of everolimus treatment demonstrated stability of everolimus effects over time with an improvement in response rate to 54% (median exposure, 28.9 months) (74). AML shrinkage continued over time and no hemorrhage occurred. Six patients (5.4%) presented AML progression at any time of the study.
Four of the six TSC patients in our SAE series received everolimus and it is difficult to determine which treatment had the greatest impact on the change in tumor size. Among those patients, one patient presented a 17% increasing size of tumors (sum of maximal diameters) after a 56 months follow-up and the three others presented respectively a 36%, 44% and 13% shrinkage (49-, 36- and 1-month follow-up). The first patient had severe renal impairment before treatments with stage 4 CKD and unfortunately progressed to stage 5. The second patient had stage 2 CKD before treatments and progressed to stage 3 with one single kidney before any therapeutics whereas the third maintained stage 2 CKD. The fourth patient did not have any renal impairment before and after treatments. Severe renal impairments (GFR <30 mL/min/1.73 m2) were observed in 7 (6.3%) patients at least once post-baseline in EXIST-2 trial but all of these patients had compromised renal function (GFR <60 mL/min/1.73 m2) prior to everolimus initiation. No patients had stage 3/4 elevated serum creatinine, but 15 (13.4%) patients had stage 1/2 elevations.
Though these trials suggest safety and efficacy of everolimus in decreasing AML tumor and preventing progression in TSC population, its role remains to be investigated. Indeed, there is no study to our knowledge comparing SAE and mTOR only treatment. Moreover, the role of mTOR in the management of non-TSC associated AML remains to be determined.
In our series, two TSC patients presenting large AMLs were managed with SAE only. A 13% and 15% decrease was respectively observed with stage 3 and 2 CKD before treatment. No CKD progression was noticed for the first patient and the second patient presented a surprising renal function improvement after SAE. We explain these changes by the fact that we used only two renal function values for reference: the last available value before treatment and the last available value during the follow-up. Renal clearance is a complex variable influenced by many exogenous and endogenous events and it is hard to determine whether the treatment or this multi-organ disease had the most influence on renal function in our limited study population. In their 16 TSC-patients-only AML embolization series, Williams et al. (30) found no statistically significant change in GFR after embolization with a mean interval between the procedure and the most recent renal function measurement of 23 months. Mean GFR before embolization was 95.75 mL/min/1.73 m2. Their study suggest efficiency and safety of SAE to treat AML in TSC patient by preserving renal function which is very important in this population but the evolution of GFR seems to be uncertain once it has begun to deteriorate. Villalta et al. (39) found no difference in tumor reduction size in patients with or without TSC. We observed significantly better tumors shrinkage in non-TSC patients after SAE (P=0.022) than in TSC-patients, suggesting the importance of combining different therapeutic strategies to obtain similar results in TSC patients.
We encountered low complication rates as it has been described in the past series (Table S2). Only three patients presented severe complications. One false-aneurysm of the common femoral artery was successfully embolized and three abscesses formation following necrosis and liquefaction of tumors after SAE were managed with percutaneous drainage (75). As expected, PES, an inflammatory response causing pain and fever that can last for several days following SAE, was the main minor complication. Every patient pain and/or fever was easily managed with analgesics. Bissler et al. (76) described the use of a short-term tapering dose of prednisone over a 2-week period after SAE of AMLs instead of acetaminophen and it appeared to reduce PES and improved patient comfort.
Full table
Since Han et al. (24) and Lee et al. (25) mostly used iodized oil mixed with absolute ethanol in their 90’s series, many authors adopted this approach until Rimon et al. (13) reached a 94% clinical success rate by adding polyvinyl alcohol (PVA) at completion. In our series, we used various embolic agents since studies comparing smaller and larger embolic agents for AMLs embolization did not find difference in tumor reduction size or need for repeat embolization (39). However, we’re using more and more N-butyl 2-cyanoacrylate glue (Glubran 2®) for SAE these last years. Property of glue for arterial occlusion is mostly seen in lesions supplied by end-arteries; as a consequence, intra-parenchymal tumors or vascular lesions with end-artery feeders such as AMLs are suitable for glue application, with the expectation of a sclerosis of the tumor and a total devascularization of the vascular bed (77). Advantages of glue are that it works instantly, provides fast and distal embolization with high dilution of lipiodol (ratio, glue:lipiodol >1:5), completely occludes vessels and is permanent (78). It is also cheap and easy to use mixed with ethiodized oil (lipiodol), making easier to see the progression of glue in the vascular feeders and to visualize reflux along the microcatheter, which can reduce the risk of gluing the tip of the microcatheter and injecting glues into normal renal parenchyma. No series have described the use of glue in such a setting whereas it combines many advantages over other embolic agents, even if a learning curve is mandatory.
This study has some limitations. First, this is a retrospective study with a small size cohort due to the rarity of large and/or symptomatic AMLs. Our population was heterogeneous with sporadic cases (n=17), TSC patients (n=6) and different presentations (preventive vs. emergency embolization). Second, as in other studies, we encountered difficulty to measure with accuracy large AMLs associated with retroperitoneal hemorrhage, especially as several modalities were used (US, CT and MRI). In particular, data were difficult to measure in cases of AML ruptures, which was often the mode of revelation of emergency cases. In addition, the follow-up images of some patients were obtained from other hospitals with different equipments than that available in our hospital. However, no patients were lost during follow-up in this study. Variable follow-up [mean follow-up of 20.5 months (range, 0.5–56 months)] should not impact the findings of our study since the majority of AMLs shrinkage occurs within the first years following SAE (79). This was confirmed for the last two patients of our series with only short term follow-up images available; the first one presented a 8% and 9% tumors shrinkage 1 month after treatment and the second an impressive 48% decreased 2 months after embolization. With 13% of complications, 13% of recurrence and 17% of repeated embolization, our results are comparable to those previously reported in the literature and summarized in Table S2.
Conclusions
SAE is an effective technique to manage AMLs preventively or in emergency to treat bleeding, with substantial reduction in tumor size, low recurrence rates and acceptable complications. We found a larger tumor reduction size for sporadic AMLs, stressing the importance of a multidisciplinary approach to AMLs associated to TSC which are usually bigger, multiple and consequently harder to manage. This study emphasizes the role of tumor composition and especially the presence of intratumoral aneurysms which was significantly more frequent in hemorrhagic tumors. As a consequence, we believe that in addition to size, the initial composition of the tumor should be considered in treatment decision. Further studies are needed to clarify the precise role of all therapeutic modalities currently available, especially in TSC patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethics committee approval of the institution was waived because the endovascular procedures were routinely performed as part of clinical practice. Written informed consent was obtained from all patients.
References
- Hajdu SI, Foote FW. Angiomyolipoma of the kidney: report of 27 cases and review of the literature. J Urol 1969;102:396-401. [PubMed]
- Soulen MC, Faykus MH Jr, Shlansky-Goldberg RD, Wein AJ, Cope C. Elective embolization for prevention of hemorrhage from renal angiomyolipomas. J Vasc Interv Radiol 1994;5:587-91. [Crossref] [PubMed]
- Koo KC, Kim WT, Ham WS, Lee JS, Ju HJ, Choi YD. Trends of presentation and clinical outcome of treated renal angiomyolipoma. Yonsei Med J 2010;51:728-34. [Crossref] [PubMed]
- Zhao Y, Bui MM, Spiess PE, Dhillon J. Sclerosing PEComa of the kidney: clinicopathologic analysis of 2 cases and review of the literature. Clin Genitourin Cancer 2014;12:e229-32. [Crossref] [PubMed]
- Morgan GS, Straumfjord JV, Hall EJ. Angiomyolipoma of the kidney. J Urol 1951;65:525-7. [PubMed]
- Sherman JL, Hartman DS, Friedman AC, Madewell JE, Davis CJ, Goldman SM. Angiomyolipoma: computed tomographic-pathologic correlation of 17 cases. AJR Am J Roentgenol 1981;137:1221-6. [Crossref] [PubMed]
- Steiner MS, Goldman SM, Fishman EK, Marshall FF. The natural history of renal angiomyolipoma. J Urol 1993;150:1782-6. [PubMed]
- Nelson CP, Sanda MG. Contemporary diagnosis and management of renal angiomyolipoma. J Urol 2002;168:1315-25. [Crossref] [PubMed]
- Pfister C, Thoumas D, Fauquet I. Diagnostic and therapeutic approach of angiomyolipoma. Prog Urol 2002;12:108-13. [PubMed]
- Fujii Y, Ajima J, Oka K, Tosaka A, Takehara Y. Benign renal tumors detected among healthy adults by abdominal ultrasonography. Eur Urol 1995;27:124-7. [PubMed]
- Bissler JJ, Kingswood JC. Renal angiomyolipomata. Kidney Int 2004;66:924-34. [Crossref] [PubMed]
- Harabayashi T, Shinohara N, Katano H, Nonomura K, Shimizu T, Koyanagi T. Management of renal angiomyolipomas associated with tuberous sclerosis complex. J Urol 2004;171:102-5. [Crossref] [PubMed]
- Rimon U, Duvdevani M, Garniek A, Golan G, Bensaid P, Ramon J, Morag B. Large renal angiomyolipomas: digital subtraction angiographic grading and presentation with bleeding. Clin Radiol 2006;61:520-6. [Crossref] [PubMed]
- Rumancik WM, Bosniak MA, Rosen RJ, Hulnick D. Atypical renal and pararenal hamartomas associated with lymphangiomyomatosis. AJR Am J Roentgenol 1984;142:971-2. [Crossref] [PubMed]
- Tobino K, Johkoh T, Fujimoto K, Sakai F, Arakawa H, Kurihara M, Kumasaka T, Koike K, Takahashi K, Seyama K. Computed tomographic features of lymphangioleiomyomatosis: evaluation in 138 patients. Eur J Radiol 2015;84:534-41. [Crossref] [PubMed]
- Warakaulle DR, Phillips RR, Turner GD, Davies D, Protheroe AS. Malignant monotypic epithelioid angiomyolipoma of the kidney. Clin Radiol 2004;59:849-52. [Crossref] [PubMed]
- Islam AH, Ehara T, Kato H, Hayama M, Kashiwabara T, Nishizawa O. Angiomyolipoma of kidney involving the inferior vena cava. Int J Urol 2004;11:897-902. [Crossref] [PubMed]
- Tsai CC, Wu WJ, Li CC, Wang CJ, Wu CH, Wu CC. Epithelioid angiomyolipoma of the kidney mimicking renal cell carcinoma: a clinicopathologic analysis of cases and literature review. Kaohsiung J Med Sci 2009;25:133-40. [Crossref] [PubMed]
- Mues AC, Palacios JM, Haramis G, Casazza C, Badani K, Gupta M, McKiernan J, Benson MC, Landman J. Contemporary experience in the management of angiomyolipoma. J Endourol 2010;24:1883-6. [Crossref] [PubMed]
- Ramon J, Rimon U, Garniek A, Golan G, Bensaid P, Kitrey ND, Nadu A, Dotan ZA. Renal angiomyolipoma: long-term results following selective arterial embolization. Eur Urol 2009;55:1155-61. [Crossref] [PubMed]
- Preece P, Mees B, Norris B, Christie M, Wagner T, Dundee P. Surgical management of haemorrhaging renal angiomyolipoma in pregnancy. Int J Surg Case Rep 2015;7C:89-92. [Crossref] [PubMed]
- Boorjian SA, Sheinin Y, Crispen PL, Lohse CM, Kwon ED, Leibovich BC. Hormone receptor expression in renal angiomyolipoma: clinicopathologic correlation. Urology 2008;72:927-32. [Crossref] [PubMed]
- Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L, Merseburger AS, Mulders P, Powles T, Staehler M, Volpe A, Bex A. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67:913-24. [Crossref] [PubMed]
- Han YM, Kim JK, Roh BS, Song HY, Lee JM, Lee YH, Chung GH, Kim CS, Sohn MH, Choi KC. Renal angiomyolipoma: selective arterial embolization--effectiveness and changes in angiomyogenic components in long-term follow-up. Radiology 1997;204:65-70. [Crossref] [PubMed]
- Lee W, Kim TS, Chung JW, Han JK, Kim SH, Park JH. Renal angiomyolipoma: embolotherapy with a mixture of alcohol and iodized oil. J Vasc Interv Radiol 1998;9:255-61. [Crossref] [PubMed]
- Ewalt DH, Diamond N, Rees C, Sparagana SP, Delgado M, Batchelor L, Roach ES. Long-term outcome of transcatheter embolization of renal angiomyolipomas due to tuberous sclerosis complex. J Urol 2005;174:1764-6. [Crossref] [PubMed]
- Kothary N, Soulen MC, Clark TW, Wein AJ, Shlansky-Goldberg RD, Stavropoulos SW. Renal angiomyolipoma: long-term results after arterial embolization. J Vasc Interv Radiol 2005;16:45-50. [Crossref] [PubMed]
- Rimon U, Duvdevani M, Garniek A, Golan G, Bensaid P, Ramon J, Morag B. Ethanol and polyvinyl alcohol mixture for transcatheter embolization of renal angiomyolipoma. AJR Am J Roentgenol 2006;187:762-8. [Crossref] [PubMed]
- Dabbeche C, Chaker M, Chemali R, Perot V, El Hajj L, Ferriere JM, Ballanger P, Chabbert V, Cimpean A, Otal P, Huyghe E, Grenier N, Joffre F. Role of embolization in renal angiomyolipomas. J Radiol 2006;87:1859-67. [Crossref] [PubMed]
- Williams JM, Racadio JM, Johnson ND, Donnelly LF, Bissler JJ. Embolization of renal angiomyolipomata in patients with tuberous sclerosis complex. Am J Kidney Dis 2006;47:95-102. [Crossref] [PubMed]
- Lenton J, Kessel D, Watkinson AF. Embolization of renal angiomyolipoma: immediate complications and long-term outcomes. Clin Radiol 2008;63:864-70. [Crossref] [PubMed]
- Lee SY, Hsu HH, Chen YC, Huang CC, Wong YC, Wang LJ, Chuang CK, Yang CW. Embolization of renal angiomyolipomas: short-term and long-term outcomes, complications, and tumor shrinkage. Cardiovasc Intervent Radiol 2009;32:1171-8. [Crossref] [PubMed]
- Sooriakumaran P, Gibbs P, Coughlin G, Attard V, Elmslie F, Kingswood C, Taylor J, Corbishley C, Patel U, Anderson C. Angiomyolipomata: challenges, solutions, and future prospects based on over 100 cases treated. BJU Int 2010;105:101-6. [Crossref] [PubMed]
- Tillou X, Boutemy F, Remond A, Petit J. Contribution of curative and preventive embolization for renal angiomyolipomas treatment. Prog Urol 2010;20:627-32. [Crossref] [PubMed]
- Bishay VL, Crino PB, Wein AJ, Malkowicz SB, Trerotola SO, Soulen MC, Stavropoulos SW. Embolization of giant renal angiomyolipomas: technique and results. J Vasc Interv Radiol 2010;21:67-72. [Crossref] [PubMed]
- Chick CM, Tan BS, Cheng C, Taneja M, Lo R, Tan YH. Long-term follow-up of the treatment of renal angiomyolipomas after selective arterial embolization with alcohol. BJU Int 2010;105:390-4. [Crossref] [PubMed]
- Chan CK, Yu S, Yip S, Lee P. The efficacy, safety and durability of selective renal arterial embolization in treating symptomatic and asymptomatic renal angiomyolipoma. Urology 2011;77:642-8. [Crossref] [PubMed]
- Stoica G, Kheir C, Schöenig A, Chabrot P, Cassagnes L, Ravel A, Boiteux JP, Guy L, Boyer L. Preventive and emergency embolization of angiomyolipomas: our experience. Prog Urol 2011;21:514-20. [Crossref] [PubMed]
- Villalta JD, Sorensen MD, Durack JC, Kerlan RK, Stoller ML. Selective Aarterial embolization of angiomyolipomas: a comparison of smaller and larger embolic agents. J Urol 2011;186:921-7. [Crossref] [PubMed]
- Hocquelet A, Cornelis F, Le Bras Y, Meyer M, Tricaud E, Lasserre AS, Ferrière JM, Robert G, Grenier N. Long-term results of preventive embolization of renal angiomyolipomas: evaluation of predictive factors of volume decrease. Eur Radiol 2014;24:1785-93. [Crossref] [PubMed]
- Duan XH, Zhang MF, Ren JZ, Han XW, Chen PF, Zhang K, Jia ZL. Urgent transcatheter arterial embolization for the treatment of ruptured renal angiomyolipoma with spontaneous hemorrhage. Acta Radiol 2016;57:1360-5. [Crossref] [PubMed]
- Omary RA, Bettmann MA, Cardella JF, Bakal CW, Schwartzberg MS, Sacks D, Rholl KS, Meranze SG, Lewis CA, Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol 2003;14:S293-5. [Crossref] [PubMed]
- Simpson E, Patel U. Diagnosis of angiomyolipoma using computed tomography-region of interest < or = -10 HU or 4 adjacent pixels < or = -10 HU are recommended as the diagnostic thresholds. Clin Radiol 2006;61:410-6. [Crossref] [PubMed]
- Davenport MS, Neville AM, Ellis JH, Cohan RH, Chaudhry HS, Leder RA. Diagnosis of renal angiomyolipoma with hounsfield unit thresholds: effect of size of region of interest and nephrographic phase imaging. Radiology 2011;260:158-65. [Crossref] [PubMed]
- Kim JK, Kim SH, Jang YJ, Ahn H, Kim CS, Park H, Lee JW, Kim S, Cho KS. Renal angiomyolipoma with minimal fat: differentiation from other neoplasms at double-echo chemical shift FLASH MR imaging. Radiology 2006;239:174-80. [Crossref] [PubMed]
- Israel GM, Hindman N, Hecht E, Krinsky G. The use of opposed-phase chemical shift MRI in the diagnosis of renal angiomyolipomas. AJR Am J Roentgenol 2005;184:1868-72. [Crossref] [PubMed]
- Seyam RM, Bissada NK, Kattan SA, Mokhtar AA, Aslam M, Fahmy WE, Mourad WA, Binmahfouz AA, Alzahrani HM, Hanash KA. Changing trends in presentation, diagnosis and management of renal angiomyolipoma: comparison of sporadic and tuberous sclerosis complex-associated forms. Urology 2008;72:1077-82. [Crossref] [PubMed]
- Castle SM, Gorbatiy V, Ekwenna O, Young E, Leveillee RJ. Radiofrequency ablation (RFA) therapy for renal angiomyolipoma (AML): an alternative to angio-embolization and nephron-sparing surgery. BJU Int 2012;109:384-7. [Crossref] [PubMed]
- Tan YK, Best SL, Olweny E, Park S, Trimmer C, Cadeddu JA. Radiofrequency ablation of incidental benign small renal mass: outcomes and follow-up protocol. Urology 2012;79:827-30. [Crossref] [PubMed]
- Planché O, Correas JM, Mader B, Joly D, Méjean A, Hélénon O. Prophylactic embolization of renal angiomyolipomas: evaluation of therapeutic response using CT 3D volume calculation and density histograms. J Vasc Interv Radiol 2011;22:1388-95. [Crossref] [PubMed]
- Oesterling JE, Fishman EK, Goldman SM, Marshall FF. The management of renal angiomyolipoma. J Urol 1986;135:1121-4. [PubMed]
- Lienert AR, Nicol D. Renal angiomyolipoma. BJU Int 2012;110:25-7. [Crossref] [PubMed]
- Sasiwimonphan K, Takahashi N, Leibovich BC, Carter RE, Atwell TD, Kawashima A. Small (<4 cm) renal mass: differentiation of angiomyolipoma without visible fat from renal cell carcinoma utilizing MR imaging. Radiology 2012;263:160-8. [Crossref] [PubMed]
- Adler J, Greweldinger J, Litzky G. “Macro” aneurism in renal angiomyolipoma: two cases, with therapeutic embolization in one patient. Urol Radiol 1984;6:201-3. [Crossref] [PubMed]
- Huyghe E, Dechier MC, Mottier ML, Rischmann P, Otal P, Soulie M, Plante P, Joffre F. Management of renal angiomyolipoma: medical and cost effectiveness comparison of selective embolization and surgery. J Urol 2010;183:e527. [Crossref]
- Faddegon S, So A. Treatment of angiomyolipoma at a tertiary care centre: the decision between surgery and angioembolization. Can Urol Assoc J 2011;5:E138-41. [Crossref] [PubMed]
- Boorjian SA, Frank I, Inman B, Lohse CM, Cheville JC, Leibovich BC, Blute ML. The role of partial nephrectomy for the management of sporadic renal angiomyolipoma. Urology 2007;70:1064-8. [Crossref] [PubMed]
- De Luca S, Terrone C, Rossetti SR. Management of renal angiomyolipoma: a report of 53 cases. BJU Int 1999;83:215-8. [Crossref] [PubMed]
- Byrd GF, Lawatsch EJ, Mesrobian HG, Begun F, Langenstroer P. Laparoscopic cryoablation of renal angiomyolipoma. J Urol 2006;176:1512-6. [Crossref] [PubMed]
- Krummel T, Garnon J, Lang H, Gangi A, Hannedouche T. Percutaneous cryoablation for tuberous sclerosis-associated renal angiomyolipoma with neoadjuvant mTOR inhibition. BMC Urol 2014;14:77. [Crossref] [PubMed]
- Trelborg K, Nielsen TK, Østraat EØ, Olsen LH. Laparoscopic cryoablation of angiomyolipomas in adolescents and young adults: A report of four cases associated with tuberous sclerosis and 1 case of sporadic origin. J Pediatr Urol 2016;12:384.e1-384.e6. [Crossref] [PubMed]
- Kuusk T, Biancari F, Lane B, Tobert C, Campbell S, Rimon U, D'Andrea V, Mehik A, Vaarala MH. Treatment of renal angiomyolipoma: pooled analysis of individual patient data. BMC Urol 2015;15:123. [Crossref] [PubMed]
- Dickinson M, Ruckle H, Beaghler M, Hadley HR. Renal angiomyolipoma: optimal treatment based on size and symptoms. Clin Nephrol 1998;49:281-6. [PubMed]
- Bhatt JR, Richard PO, Kim NS, Finelli A, Manickavachagam K, Legere L, Evans A, Pei Y, Sykes J, Jhaveri K, Jewett MA. Natural History of Renal Angiomyolipoma (AML): Most Patients with Large AMLs >4cm Can Be Offered Active Surveillance as an Initial Management Strategy. Eur Urol 2016;70:85-90. [Crossref] [PubMed]
- Lau SK, Marchevsky AM, McKenna RJ, Luthringer DJ. Malignant monotypic epithelioid angiomyolipoma of the retroperitoneum. Int J Surg Pathol 2003;11:223-8. [Crossref] [PubMed]
- Huang KH, Huang CY, Chung SD, Pu YS, Shun CT, Chen J. Malignant epithelioid angiomyolipoma of the kidney. J Formos Med Assoc 2007;106:S51-4. [Crossref] [PubMed]
- Guo B, Song H, Yue J, Li G. Malignant renal epithelioid angiomyolipoma: A case report and review of the literature. Oncol Lett 2016;11:95-8. [PubMed]
- Vicens RA, Jensen CT, Korivi BR, Bhosale PR. Malignant renal epithelioid angiomyolipoma with liver metastasis after resection: a case report with multimodality imaging and review of the literature. J Comput Assist Tomogr 2014;38:574-77. [Crossref] [PubMed]
- Ouzaid I, Autorino R, Fatica R, Herts BR, McLennan G, Remer EM, Haber GP. Active surveillance for renal angiomyolipoma: outcomes and factors predictive of delayed intervention. BJU Int 2014;114:412-7. [PubMed]
- Yamakado K, Tanaka N, Nakagawa T, Kobayashi S, Yanagawa M, Takeda K. Renal angiomyolipoma: relationships between tumor size, aneurism formation, and rupture. Radiology 2002;225:78-82. [Crossref] [PubMed]
- Yip KH, Peh WC, Tam PC. Spontaneous rupture of renal tumours: the role of imaging in diagnosis and management. Br J Radiol 1998;71:146-54. [Crossref] [PubMed]
- Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, Sauter M, Nonomura N, Brakemeier S, de Vries PJ, Whittemore VH, Chen D, Sahmoud T, Shah G, Lincy J, Lebwohl D, Budde K. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2013;381:817-24. [Crossref] [PubMed]
- Kingswood JC, Jozwiak S, Belousova ED, Frost MD, Kuperman RA, Bebin EM, Korf BR, Flamini JR, Kohrman MH, Sparagana SP, Wu JY, Brechenmacher T, Stein K, Berkowitz N, Bissler JJ, Franz DN. The effect of everolimus on renal angiomyolipoma in patients with tuberous sclerosis complex being treated for subependymal giant cell astrocytoma: subgroup results from the randomized, placebo-controlled, Phase 3 trial EXIST-1. Nephrol Dial Transplant 2014;29:1203-10. [Crossref] [PubMed]
- Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, Sauter M, Nonomura N, Brakemeier S, de Vries PJ, Berkowitz N, Miao S, Segal S, Peyrard S, Budde K. Everolimus for renal angiomyolipoma in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: extension of a randomized controlled trial. Nephrol Dial Transplant 2016;31:111-9. [Crossref] [PubMed]
- Ozkara H, Ozkan B, Solok V. Management of renal abscess formation after embolization due to renal angiomyolipomas in two cases. Int Urol Nephrol 2006;38:427-9. [Crossref] [PubMed]
- Bissler JJ, Racadio J, Donnelly LF, Johnson ND. Reduction of postembolization syndrome after ablation of renal angiomyolipoma. Am J Kidney Dis 2002;39:966-71. [Crossref] [PubMed]
- Ginat DT, Saad WE, Turba UC. Transcatheter renal artery embolization: clinical applications and techniques. Tech Vasc Interv Radiol 2009;12:224-39. [Crossref] [PubMed]
- Vaidya S, Tozer KR, Chen J. An overview of embolic agents. Semin Intervent Radiol 2008;25:204-15. [Crossref] [PubMed]
- Patatas K, Robinson GJ, Ettles DF, Lakshminarayan R. Patterns of renal angiomyolipoma regression post embolisation on medium- to long-term follow-up. Br J Radiol 2013;86:20120633. [Crossref] [PubMed]