
Stenting versus medical treatment for idiopathic intracranial hypertension based on a propensity score matching method
Introduction
Idiopathic intracranial hypertension (IIH) is a syndrome characterized by unexplained increased intracranial pressure (ICP), and has an incidence rate of 0.9–2.2/100,000 (1). Its primary clinical manifestations include headache, papilledema, visual disturbance, tinnitus, and sixth nerve palsy (1,2). Conventional treatments for IIH include medical treatment (MT), bariatric surgery, lifestyle modifications, lumbar puncture (LP), cerebrospinal fluid (CSF) shunt procedures, and optic nerve sheath fenestration (ONSF) (3). For the majority of IIH patients, a combination of MT and weight loss is sufficient to alleviate symptoms and prevent progression. However, some patients whose symptoms continue to worsen despite conservative treatment require surgical intervention. In the past, patients who did not respond to MT were typically referred for ONSF or CSF shunt procedures (4). While these treatments have some efficacy, approximately 40% of patients ultimately experience disease progression or recurrence within 6–10 years (5,6).
The pathophysiological mechanisms of IIH remain unclear, but may involve disturbances in cerebral venous outflow dynamics, impaired CSF absorption, altered venous pressure gradients, metabolic and endocrine effects, and a genetic predisposition (7). The latest research indicates that approximately 90% of IIH patients exhibit venous sinus stenosis (VSS) (8). Controversy surrounds the causal relationship between ICP and VSS. Studies have shown that stenting may be a promising therapeutic choice (9); however, most of these studies employed a single-arm design and did not compare stenting with MT. Thus, this study sought to assess the difference in efficacy between stenting and MT in treating patients with ICP and VSS. This observational study used propensity score matching (PSM) to reduce the impact of confounding factors due to differences in the distribution of baseline characteristics. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1417/rc).
Cautionary statement: stenting is appropriate, but only for those patients who have severe visual loss (i.e., a visual field greater than −7 dB in mean deviation), or those who despite weight loss and resolution of papilledema with MT continue to have transverse sinus stenosis and symptom/sign recurrence. The complications that these patients with a stent may face in future years are likely to develop late.
Methods
Study design and participants
This study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committees of The First Affiliated Hospital of Zhengzhou University (ethical review No. 2024-KY-0605). The requirement of informed consent was waived due to the retrospective nature of the study. A retrospective analysis was conducted on the clinical data of individuals diagnosed with IIH and VSS from January 2018 to June 2023 at The First Affiliated Hospital of Zhengzhou University. Patient data, including demographics, clinical signs and symptoms, risk factors, imaging data, ophthalmologic examination results, medication history, surgical records, follow-up and complications, were collected from an electronic case management system. The flowchart for patient selection is shown in Figure 1.

To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria (10): (I) have papilledema; (II) other than cranial nerve abnormalities, have normal neurological examination results; (III) have undergone neuroimaging showing normal brain parenchyma without hydrocephalus, mass, or structural lesion, and show no abnormal meningeal enhancement or venous sinus thrombosis on magnetic resonance imaging (MRI) or magnetic resonance venography (MRV); if MRI was unavailable, contrast-enhanced computed tomography (CT) was used; (IV) have a normal CSF composition; (V) have raised lumbar puncture opening pressure (LPOP, >250 mmH2O in lateral decubitus); and (VI) be aged ≥18 years. A diagnosis of IIH could be made without papilledema, provided that criteria II and III aligned with either unilateral or bilateral adductor nerve palsy. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had concomitant venous sinus thrombosis; (II) had a treatment regimen change during therapy; (III) had an inability to tolerate the side effects of acetazolamide; (IV) had incomplete clinical data; and/or (V) had incomplete follow-up data or had been lost in follow-up.
Manometry and treatment strategies
Venography and manometry were performed in all patients. Each patient fasted from food and water for 6 h before surgery, the inguinal and perineal areas were routinely disinfected, and sterile sheets were spread after the patient was positioned supine. A 5-F arterial sheath and 8-F venous sheath (Merit Medical Systems, Inc., USA) were inserted after right femoral artery and femoral vein puncture under local anesthesia. A 5-F vertebral artery catheter (Cordis, Co., Florida, USA) combined with a 0.035-in guidewire (TERUMO Co., Tokyo, Japan) was used for total cerebral angiography to determine the extent and degree of VSS. An 8-F MACH 1 guiding catheter (Boston Scientific, Marlborough, MA, USA) was then introduced into the corresponding internal jugular vein, and pressure measurements and recordings were conducted at the following seven locations using a Rebar-27 microcatheter (Neurovascular, Irvine, CA, USA) connected pressure-measuring device: the posterior third of the superior sagittal sinus, the torcular sinus, the transverse sinus, the distal part of the stenosis, the proximal part of the stenosis, the sigmoid sinus, and the internal jugular vein. The pressure gradient was defined as the difference in the pressure between the distal and proximal stenotic segments, which played a crucial role in determining the necessity of stenting for a patient (11,12). Previous studies have demonstrated the safety and efficacy of stenting in cases in which the pressure gradient of stenosis was ≥8 mmHg (13). The choice of treatment for all patients was not randomized; each specific treatment strategy was selected by the supervising physician in consultation with the patient or their legal representatives based on the venography and manometry results. Some patients opted for conservative medication due to concerns about stenting hazards and a reluctance to continue oral antiplatelet treatment post-procedure, while others opted for stenting because they had difficulty tolerating their current symptoms. According to the treatment approaches, the patients were divided into the following two groups: the stenting group (Group S), and the MT group (Group M). All patients signed informed consent forms for the treatment. Additionally, they were subjected to fundus and LP examinations before standard MT or stenting.
Group M: the patients were advised to eat a diet low in sodium for weight loss and take acetazolamide (250 mg); the initial dosage was 1 g daily in two separate doses, with weekly increments of 250 mg up to a maximum of 4 g/d. In the course of MT, if the patient’s symptoms showed significant improvement, the dose escalation was halted; conversely, if the symptoms worsened, dose escalation was continued until it reached 4 g/d. In cases in which the maximally tolerated doses of acetazolamide (4 g/d, divided) were inadequate for symptom improvement, furosemide and other diuretics were used as second-line agents (14). If multiple medications were being administered, regular blood potassium monitoring was conducted to prevent hypokalemia caused by diuretic usage leading to increased potassium excretion. Further, appropriate symptomatic treatment was administered based on the patient’s condition, and a repeat ophthalmoscopic examination and LPOP examination were conducted before discharge to assess improvement.
Group S: the patient began undergoing dual antiplatelet therapy (aspirin 100 mg/d and clopidogrel 75 mg/d) 3 days prior to surgery. If the stenosis pressure gradient was ≥8 mmHg and the patient and their family consented to the procedure, all venous sinus stenting procedures were performed under general anesthesia and full intravenous heparinization by interventional neuroradiologists with more than 10 years of experience. The Synchro-14 microguidewire (Boston Scientific Corp., Natick, MA, USA) and Rebar-27 microcatheter (eV3) were used in conjunction to reach the stenosis site, and a suitable size Wallstent (Boston Scientific Corp.) or Protégé (eV3, Neurovascular) was introduced along the microcatheter and released with the narrowing area as the center. The decision to perform balloon angioplasty was contingent on the successful deployment of stenting by the surgeon. After stenting, venous angiography was performed again, and a pressure measurement device was connected to measure the pressure at both ends of the stenosis. The imaging of two cases are shown in Figures 2,3. After postoperative refinement of the Dyna CT scan to rule out bleeding complications, the patient was given a subcutaneous injection of low-molecular-weight heparin 5,000 IU. The patient’s vital signs were strictly monitored for 24 hours after the operation. Before discharge, a follow-up fundus examination was conducted to assess any alleviation in the papilledema Frisén grade, and an LP examination was conducted to evaluate any decrease in intracranial hypertension. After surgery, dual antiplatelet therapy was recommended for at least 3 months, and the medication was then adjusted based on the imaging results at follow-up.
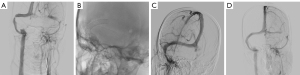
Follow-up and assessment of therapeutic efficacy
The patients were followed up through outpatient or inpatient procedures at 6 and 12 months after discharge to assess the efficacy of the treatment. The relevant imaging findings [MRV and digital subtraction angiography (DSA)] were interpreted by two experienced neurointerventional radiologists (L.Y. and Y.W., with 15 and 10 years of experience, respectively). The papilledema Frisén grade was assessed by two experienced neuro-ophthalmologists (Yue Chen and Yudi Xu, with 20 and 5 years of experience, respectively). If disagreements arose, a third, more senior physician (S.S., with 30 years of experience) was involved in the final evaluation. The long-term effectiveness of the two treatment modalities, which was the primary outcome, were assessed by evaluating any improvement in clinical symptoms and signs at discharge, and at 6 and 12 months post-discharge. The papilledema Frisén grade and the LPOP at admission and discharge were used as secondary outcomes to assess the short-term efficacy of the two treatment methods. The safety results included surgical complications and adverse reactions to oral medications.
For the purpose of the statistical analysis, the changes in clinical outcomes were categorized as follows: (I) asymptomatic: all clinical symptoms had returned to normal; (II) improved: there was a significant improvement compared to before treatment, and no need for further interventional treatment; (III) unchanged: there was no change compared to before treatment, and further interventional treatment was needed; (IV) worsened: there was a worsening compared to before treatment, and further interventional treatment was needed (15). In this research, the papilledema grading system was selected as the objective metric to ensure a more dependable evaluation of function. The Frisén scale for papilledema grading includes the following six stages: stage 0, a normal optic disc; stage 1, excessive blurring of the nasal border of the optic disc, with disruption of the normal radial arrangement of nerve fiber bundles; stage 2, blurring of all of the temporal margin, with elevation of the nasal circumference of the nerve head; stage 3, a clearly increased diameter of the nerve head, with elevation of the temporal circumference; stage 4, elevation of the whole nerve head in combination with obliteration of the optic cup; and stage 5, complete or partial obscuration of all vessels of the disc, with protrusion of the optic nerve head as a dome (16).
Statistical analysis
SPSS software (IBM Corp., New York, USA) was used to perform all statistical analyses. The normally distributed continuous variables are presented as the mean ± standard deviation, and were compared between the two groups using the Student’s t-test. The ordinal variables are presented as the median with the range (non-normalized variables), and were compared using the Wilcoxon rank-sum test. The categorical variables are expressed as the number (percentage), and were compared using either the Pearson Chi-squared (χ2) test or Fisher’s exact test. Statistical significance was defined as a two-sided P value <0.05. PSM was performed to improve comparability between the two groups. The covariates used to generate the propensity scores included patient variables that were significantly associated with the treatment method (MT vs. stenting), including age, sex, time from onset to treatment, headache, papilledema, visual disturbances, stenosis rate, stenosis length and preoperative papilla edema grade, trans-stenotic pressure gradient, LPOP. The data were matched 1:1 without replacement (using a greedy matching algorithm) at a caliper width of ≤0.02.
Results
Baseline data and clinical outcomes
A total of 128 patients were enrolled in the study. The patients had an average age of 40.0±11.1 years (range, 18–61 years) and a body mass index (BMI) of 27.5±3.3 kg/m2 (range, 20.0–40.0 kg/m2), and 73.43% were female (68 in Group S and 60 in Group M). Compared with the patients in Group M, those in Group S had a longer median time from onset to treatment (2 vs. 1 month, P=0.026), a higher proportion of papilledema (85.3% vs. 68.3%, P=0.033), a higher median pretreatment stenosis rate (80% vs. 70%, P=0.005), a larger median pretreatment trans-stenotic pressure gradient (15.5 vs. 11.0 mmHg, P=0.001), and a larger median pretreatment LPOP (391.1 vs. 350.5 mmH2O, P=0.006). There were no statistically significant differences between the two groups in terms of age, gender, symptoms and signs (headache, visual disturbance, sixth nerve palsy, and tinnitus), obesity and metabolic disorders (BMI, hypertension, diabetes, hyperlipidemia, and thyroid-related disorders), female-related factors (anemia, uterine fibroids, polycystic ovary syndrome, oral contraceptive use, and pregnancy/miscarriage), other factors (smoking history, anxiety or depression, and autoimmune-related conditions), site, type and degree of stenosis, transverse sinus dominance, and papilledema Frisén grade at admission (all P>0.05, Table 1). In addition, at discharge, the median papilledema Frisén grade was lower (1 vs. 2, P<0.001), and the average of LPOP was also lower (210.2 vs. 245.8 mmHg, P=0.001) in Group S than Group M. During the follow-up period, both cohorts of patients exhibited varying degrees of improvement in clinical symptoms and signs. However, the overall improvement was more pronounced in Group S than Group M (all P<0.001), and Group S had a higher proportion of asymptomatic or improved patients at discharge (85.3% vs. 43.3%), and at 6 months (89.7% vs. 53.3%) and 12 months (89.7% vs. 55.0%) after discharge than Group M (Table 2).
Table 1
| Variables | Pre-matched (N=128) | Post-matched (N=56) | |||||
|---|---|---|---|---|---|---|---|
| MT (N=60) | Stenting (N=68) | P value | MT (N=28) | Stenting (N=28) | P value | ||
| Age (years, mean ± SD) | 39.4±11.0 | 40.6±11.2 | 0.521 | 38.3±10.5 | 38.4±11.7 | 0.971 | |
| Female, n (%) | 41 (68.3) | 53 (77.9) | 0.236 | 22 (78.6) | 22 (78.6) | >0.99 | |
| Onset to treatment time (months, IQR) | 1 (0.5, 3) | 2 (1, 6) | 0.026* | 1 (0.3, 2) | 2 (1, 6) | 0.083 | |
| Symptoms and signs, n (%) | |||||||
| Headache | 35 (58.3) | 47 (69.1) | 0.268 | 19 (67.9) | 18 (64.3) | >0.99 | |
| Papilledema | 41 (68.3) | 58 (85.3) | 0.033* | 21 (75.0) | 22 (78.6) | >0.99 | |
| Visual disturbances | 53 (88.3) | 58 (85.3) | 0.795 | 21 (75.0) | 22 (78.6) | >0.99 | |
| Sixth nerve palsy | 8 (13.3) | 11 (16.2) | 0.804 | 3 (10.7) | 7 (25.0) | 0.295 | |
| Tinnitus | 6 (10.0) | 15 (22.1) | 0.093 | 3 (10.7) | 7 (25.0) | 0.295 | |
| Obesity and metabolism disorder | |||||||
| BMI (kg/m2, mean ± SD) | 27.9±3.1 | 27.2±3.5 | 0.273 | 27.6±3.7 | 27.5±3.8 | 0.971 | |
| Hypertension, n (%) | 17 (28.3) | 22 (32.4) | 0.702 | 7 (25.0) | 8 (28.6) | >0.99 | |
| Diabetes mellitus, n (%) | 6 (10.0) | 7 (10.3) | >0.99 | 0 | 2 (7.1) | 0.491# | |
| Hyperlipidemia, n (%) | 6 (10.0) | 12 (17.6) | 0.309 | 3 (10.7) | 4 (14.3) | >0.99# | |
| Thyroid-related diseases, n (%) | 3 (5.0) | 4 (5.9) | >0.99 | 3 (10.7) | 1 (3.6) | 0.611# | |
| Female-related factors, n (%) | |||||||
| Anemia | 7 (11.7) | 5 (7.4) | 0.546 | 3 (10.7) | 1 (3.6) | 0.611# | |
| Uterine myoma | 1 (1.7) | 4 (5.9) | 0.370 | 1 (3.6) | 3 (10.7) | 0.611# | |
| Polycystic ovary syndrome | 4 (6.7) | 3 (4.4) | 0.705 | 2 (7.1) | 1 (3.6) | >0.99# | |
| Contraceptive drug | 1 (1.7) | 2 (2.9) | >0.99 | 0 | 0 | – | |
| Pregnancy/abortion | 1 (1.7) | 1 (1.5) | >0.99 | 1 (3.6) | 0 | >0.99# | |
| Other factors, n (%) | |||||||
| Smoking history | 7 (11.7) | 10 (14.7) | 0.795 | 1 (3.6) | 3 (10.7) | 0.611# | |
| Anxiety or depression | 1 (1.7) | 4 (5.9) | 0.370 | 0 | 2 (7.1) | 0.491# | |
| Immunity related factors | 1 (1.7) | 1 (1.5) | >0.99 | 0 | 0 | – | |
| Stenosis location, n (%) | 0.055# | 0.436# | |||||
| Superior sagittal sinus | 2 (3.3) | 1 (1.5) | 2 (7.1) | 0 | |||
| Transverse sinus | 16 (26.7) | 8 (11.8) | 6 (21.4) | 4 (14.3) | |||
| Sigmoid sinus | 7 (11.7) | 5 (7.4) | 4 (14.3) | 3 (10.7) | |||
| Transverse sigmoid sinus | 35 (58.3) | 54 (79.4) | 16 (57.1) | 21 (75.0) | |||
| Stenosis type, n (%) | 0.666 | 0.885 | |||||
| Intrinsic | 19 (31.7) | 24 (35.3) | 11 (39.6) | 10 (35.7) | |||
| Extrinsic | 32 (53.3) | 31 (45.6) | 12 (42.9) | 11 (39.3) | |||
| Mixed | 9 (15.0) | 13 (19.1) | 5 (17.9) | 7 (25.0) | |||
| Transverse sinus dominance | 0.845 | >0.99 | |||||
| Unilateral dominance, n (%) | 43 (71.7) | 50 (73.5) | 20 (71.4) | 19 (67.9) | |||
| Codominance, n (%) | 17 (28.3) | 18 (26.5) | 8 (28.6) | 9 (32.1) | |||
| Stenosis rate (%, IQR) | 70 (70, 75) | 80 (70, 90) | 0.005* | 71 (70, 75) | 75 (70, 87.5) | 0.175 | |
| Stenosis length (mm, IQR) | 23 (20.3, 26) | 25 (21.3, 25) | 0.059 | 24.5 (21, 27.8) | 25 (20, 25) | 0.368 | |
| Stenosis pressure gradient (mmHg, IQR) | 11 (8.0, 17.8) | 15.5 (12.3, 22.8) | 0.001* | 12 (8, 21) | 15 (10.3, 24.5) | 0.136 | |
#, using Fisher’s exact probability method; *, statistically significant P values. N, number; MT, medical treatment; SD, standard deviation; IQR, interquartile range; BMI, body mass index.
Table 2
| Variables | Pre-matched (N=128) | Post-matched (N=56) | |||||
|---|---|---|---|---|---|---|---|
| MT (N=60) | Stenting (N=68) | P value | MT (N=28) | Stenting (N=28) | P value | ||
| Papilledema Frisén grade (IQR) | |||||||
| At admission | 3 (1, 3.8) | 3 (3, 3) | 0.361 | 3 (0.3, 3.8) | 3 (3, 3) | 0.725 | |
| At discharge | 2 (1, 2) | 1 (0, 1) | <0.001* | 2 (0, 2) | 1 (0, 1) | 0.002* | |
| LPOP (mmH2O, mean ± SD) | |||||||
| At admission | 350.5±73.9 | 391.1±87.8 | 0.006* | 367.9±94.3 | 374.8±100.9 | 0.791 | |
| At discharge | 245.8±53.4 | 210.2±60.3 | 0.001* | 259.8±47.8 | 213.0±65.4 | 0.003* | |
| Symptoms and signs at discharge | <0.001#* | 0.019#* | |||||
| Asymptomatic | 3 (5.0) | 15 (22.1) | 1 (3.6) | 7 (25.0) | |||
| Improved | 23 (38.3) | 43 (63.2) | 11 (39.3) | 15 (53.6) | |||
| Unchanged | 28 (46.7) | 7 (10.3) | 12 (42.9) | 4 (14.3) | |||
| Worsened | 6 (10.0) | 3 (4.4) | 4 (14.3) | 2 (7.1) | |||
| Symptoms and signs at 6 months | <0.001* | 0.011#* | |||||
| Asymptomatic | 14 (23.3) | 38 (55.9) | 6 (21.4) | 16 (57.1) | |||
| Improved | 18 (30.0) | 23 (33.8) | 10 (35.7) | 9 (32.1) | |||
| Unchanged | 20 (33.3) | 4 (5.9) | 8 (28.6) | 1 (3.6) | |||
| Worsened | 8 (13.3) | 3 (4.4) | 4 (14.3) | 2 (7.1) | |||
| Symptoms and signs at 12 months | <0.001* | <0.001#* | |||||
| Asymptomatic | 12 (20.0) | 48 (70.6) | 5 (17.9) | 20 (71.4) | |||
| Improved | 21 (35.0) | 13 (19.1) | 12 (42.9) | 4 (14.3) | |||
| Unchanged | 19 (31.7) | 5 (7.4) | 6 (21.4) | 3 (10.7) | |||
| Worsened | 8 (13.3) | 2 (2.9) | 5 (17.9) | 1 (3.6) | |||
#, using Fisher’s exact probability method; *, statistically significant P values. N, number; MT, medical treatment; IQR, interquartile range; LPOP, lumbar puncture opening pressure; SD, standard deviation.
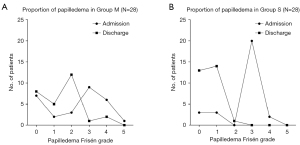
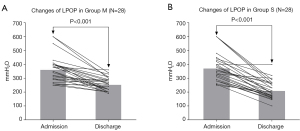
PSM and score-adjusted analysis
Both groups of patients were matched 1:1 according to their baseline characteristics (Table 1). After PSM, 28 patients were enrolled in each group. These patients had an average age of 38.3±11.0 years (range, 18.0–61.0 years) and a BMI of 27.5±3.7 kg/m2 (range, 21.2–41.2 kg/m2). The distribution of papilledema Frisén grades for the two groups at admission and discharge are shown in Figure 4, and changes in LPOP for the patients at admission and discharge are shown in Figure 5. There were no statistically significant differences in the baseline data between the two groups (all P>0.05, Table 1). At discharge, compared with Group M, the median papilledema Frisén grade in Group S was lower (1 vs. 2, P=0.002), as was the LPOP average (213.0 vs. 259.8 mmHg, P=0.003). Further, the symptom improvement was more obvious at discharge (P=0.019), and at 6 months (P=0.011) and 12 months (P<0.001) after discharge in Group S than Group M (Table 2).
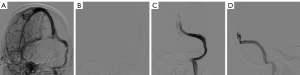
Analysis of complication outcomes
During the follow-up period, complications occurred in (6/68, 8.8%) in Group S, including a single instance of pseudoaneurysm development at the site of puncture, which improved after receiving symptomatic therapy; a thrombus inside the stent in one patient and at the stent’s distal end in another patient, both which improved after thrombolytic treatment; one case of stent occlusion, which showed poor relief after balloon angioplasty, but the subsequent reimplantation of another stent resulted in a favorable outcome; and distal stent narrowing in two patients, both of which improved following the placement of a new stent. While in Group M, adverse drug reactions occurred in six patients (6/60, 10.0%), three of whom experienced transient paresthesia in their limbs, and three of whom experienced nausea and vomiting upon increasing the drug dose. No fatalities were observed in either group.
Discussion
The incidence of IIH, which is characterized by unexplained ICP, is low. However, the underlying pathophysiological mechanisms driving ICP are poorly understood (17). The management of IIH involves the protection of optic nerve function, a decrease in ICP, and symptom control. The first consensus guidelines outline key management strategies, including weight loss, MT with carbonic anhydrase inhibitors, and indications for surgical management (18). Earlier research has predominantly focused on the overproduction or impaired absorption of CSF, as well as metabolic dysfunction and hormonal imbalances (19). However, recent angiography studies have revealed that 90% of IIH patients exhibit VSS (8), which may be attributed to intrinsic obstruction from enlarged arachnoid granulations or exogenous compression due to ICP (20,21). VSS leads to a decrease in venous outflow and an increase in pressure, which alters the pressure gradient between CSF and venous circulation (22). A causal relationship between VSS and ICP has not yet been established (23); however, studies have shown that stenting can quickly and sustainably reduce ICP and alleviate clinical symptoms by relieving VSS (24,25). A recent meta-analysis reported a 79.6% improvement rate in headache, a 93.7% improvement rate in papilledema, and a 90.3% improvement rate in pulsatile tinnitus following treatment (26). In general, stenting has shown satisfactory results in patients with IIH who are not amenable to MT. We hypothesized that addressing VSS, regardless of the cause or consequence, would help reduce ICP and improve symptoms. Therefore, venous sinus stenting represented an appealing therapeutic target. This minimally invasive procedure presents a novel method for restoring normal venous drainage and alleviating the signs and symptoms of ICP (27). However, most previous studies were conducted as single-arm trials and lacked comparative analyses with conventional MT.
Currently, the medications used clinically to treat IIH include acetazolamide, methazolamide, topiramate, furosemide and other thiazide diuretics (28). Acetazolamide serves as a first-line medication for IIH, and is thought to work by inhibiting carbonic anhydrase. It reduces the formation of hydrogen and bicarbonate ions from carbon dioxide and water resulting in decreased sodium ion transport across the choroidal epithelium, thus reducing the formation of CSF (29). Such inhibitor medications have shown promise in improving visual acuity and other symptoms, such as headaches and papilledema (30). Research indicates that acetazolamide in combination with a low-sodium diet, which can moderately improve the clinical symptoms of certain patients (31). Due to the scarcity of research contrasting MT with stenting, clinicians must exercise judgement and engage in multidisciplinary consultations (e.g., neuro-ophthalmology, neurology, ophthalmology, neurosurgery, and interventional neuroradiology consultations) to determine the appropriate treatment for each IIH and VSS patient (28). Therefore, we conducted this 1:1 matched controlled study to evaluate the difference in effectiveness between MT and stenting, and to provide clinicians with a reference for treatment selection.
In the short term (from admission to discharge), we found that stenting was significantly more effective than MT both in terms of the degree of reduction in the papilledema Frisén grade and LPOP. The rapid recovery of cerebral venous blood flow and the subsequent decrease in venous pressure after stenting may account for this phenomenon. Conversely, it was difficult to achieve a similar therapeutic effect in the short term with MT. In addition, compared with the MT patients, the stenting patients experienced significantly faster recovery of clinical signs and symptoms, and a higher proportion had improved asymptomatic and symptomatically at the end of follow-up. These findings suggest that the early resolution of intracranial hypertension resulted in long-term clinical improvements. Prolonged ICP may interfere with lymphatic drainage and result in the accumulation of metabolic products that can cause long-term or permanent neuronal damage, both metabolically and hydrostatically (24). In this study, five patients had transient worsening headaches because of stent tension; however, these headaches usually resolved substantially within a week.
The complications of stenting are rare and are usually associated with the angiographic procedure itself. Major complications include vessel perforation, subdural hematoma, subarachnoid or parenchymal hemorrhage, stent thrombosis, and restenosis (32). In this study, Group S experienced six instances (6/68, 8.8%) of event complications, falling short of the 13.7% rate of treatment failure and post-stent complications reported in a recent meta-analysis (33). Although such “treatment failures” could be considered a part of the natural history of the disease itself rather than a procedural complication. The complication rates observed after venous sinus stenting were significantly lower than those observed after ventriculoperitoneal shunts (47.1%) (34). Moreover, the patients in Group S showed better improvements in their clinical symptoms and signs than those in Group M. These results indicate that stenting was also positive in terms of safety and long-term efficacy, as serious complications were rare (32). Consistent with a previous study (29), the patients in Group M did not show any significant complications except for six cases of adverse drug reactions. However, the problem of worsening of symptoms due to a failure to improve cerebral venous blood flow and intracranial hypertension should not be ignored. If the effect of MT is not obvious, the timely modification of the treatment strategy and proactive surgical intervention are essential. Stenting is an etiologic treatment and thus is theoretically more appropriate for IIH due to VSS.
All of our studies used carotid stents (Wallstent or Protégé). Some previous studies have also used carotid stents; however, the results of these investigations varied, with the majority suggesting that no stent design displayed superiority (35,36). Since IIH involves venous interventions, there are currently no stents specifically designed for its treatment. Consequently, the selection of stents for IIH will likely remain a subject of debate, and further research is necessary to determine the optimal choice among the available stents.
This retrospective study performed PSM in the final analysis to minimize the effect of confounding factors that may result from differences in the distribution of baseline characteristics. In addition, our results were based on real-world data, and provide additional and updated information regarding the efficacy of stenting treatment in patients with IIH and VSS. Therefore, we are of the view that stenting can resolve VSS more rapidly and effectively than MT, thereby improving patients’ clinical symptoms, and can be considered a new approach for treating IIH and VSS.
Limitations
The results of our study were encouraging; however, the study also had some limitations. First, this was a non-randomized controlled study. Despite conducting PSM in our final analysis, the present study did not assess certain unmeasured confounding factors that could potentially influence treatment outcomes, which might have caused a confounding bias. Second, the assessment of clinical symptom improvement primarily relied on subjective measures without objective evaluation indicators or scales. Additionally, venous sinus pressures fluctuate with changes in ventilation, but pressure measurements were not always taken under general anesthesia, which might have also led to some bias. Finally, the patients in this retrospective cohort study were from China; thus, there may be genetic, social, cultural, and economic preferences that might make the results of this study difficult to apply to another population. Additionally, Chinese people have a relatively lower BMI than Western populations, thus the results may differ in Western populations. Therefore, future research should focus on conducting multicenter, large-scale randomized controlled trials to validate the reliability of our results.
Conclusions
The findings of our study suggest that compared to MT, stenting can provide more rapid and effective relief for papilledema and LPOP, as well as their associated symptoms and signs, and improve the prognosis of patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1417/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1417/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committees of The First Affiliated Hospital of Zhengzhou University (ethical review No. 2024-KY-0605). The requirement of informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thurtell MJ, Wall M. Idiopathic intracranial hypertension (pseudotumor cerebri): recognition, treatment, and ongoing management. Curr Treat Options Neurol 2013;15:1-12. [Crossref] [PubMed]
- Acheson JF. Idiopathic intracranial hypertension and visual function. Br Med Bull 2006;79-80:233-44. [Crossref] [PubMed]
- Li XX, Cui WN. Clinical manifestations and treatment strategies of idiopathic intracranial hypertension. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2023;58:384-8. [PubMed]
- Fonseca PL, Rigamonti D, Miller NR, Subramanian PS. Visual outcomes of surgical intervention for pseudotumour cerebri: optic nerve sheath fenestration versus cerebrospinal fluid diversion. Br J Ophthalmol 2014;98:1360-3. [Crossref] [PubMed]
- Kesler A, Hadayer A, Goldhammer Y, Almog Y, Korczyn AD. Idiopathic intracranial hypertension: risk of recurrences. Neurology 2004;63:1737-9. [Crossref] [PubMed]
- Shah VA, Kardon RH, Lee AG, Corbett JJ, Wall M. Long-term follow-up of idiopathic intracranial hypertension: the Iowa experience. Neurology 2008;70:634-40. [Crossref] [PubMed]
- Wang MTM, Bhatti MT, Danesh-Meyer HV. Idiopathic intracranial hypertension: Pathophysiology, diagnosis and management. J Clin Neurosci 2022;95:172-9. [Crossref] [PubMed]
- Kelly LP, Saindane AM, Bruce BB, Ridha MA, Riggeal BD, Newman NJ, Biousse V. Does bilateral transverse cerebral venous sinus stenosis exist in patients without increased intracranial pressure? Clin Neurol Neurosurg 2013;115:1215-9. [Crossref] [PubMed]
- McDougall CM, Ban VS, Beecher J, Pride L, Welch BG. Fifty shades of gradients: does the pressure gradient in venous sinus stenting for idiopathic intracranial hypertension matter? A systematic review. J Neurosurg 2019;130:999-1005. [Crossref] [PubMed]
- Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013;81:1159-65. [Crossref] [PubMed]
- Fargen KM, Liu K, Garner RM, Greeneway GP, Wolfe SQ, Crowley RW. Recommendations for the selection and treatment of patients with idiopathic intracranial hypertension for venous sinus stenting. J Neurointerv Surg 2018;10:1203-8. [Crossref] [PubMed]
- Garner RM, Aldridge JB, Wolfe SQ, Fargen KM. Quality of life, need for retreatment, and the re-equilibration phenomenon after venous sinus stenting for idiopathic intracranial hypertension. J Neurointerv Surg 2021;13:79-85. [Crossref] [PubMed]
- Fargen KM. A unifying theory explaining venous sinus stenosis and recurrent stenosis following venous sinus stenting in patients with idiopathic intracranial hypertension. J Neurointerv Surg 2021;13:587-92. [Crossref] [PubMed]
- Phillips PH. Pediatric pseudotumor cerebri. Int Ophthalmol Clin 2012;52:51-9. xii. [Crossref] [PubMed]
- Higgins JN, Cousins C, Owler BK, Sarkies N, Pickard JD. Idiopathic intracranial hypertension: 12 cases treated by venous sinus stenting. J Neurol Neurosurg Psychiatry 2003;74:1662-6. [Crossref] [PubMed]
- Frisén L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry 1982;45:13-8. [Crossref] [PubMed]
- Thurtell MJ, Kawasaki A. Update in the Management of Idiopathic Intracranial Hypertension. Neurol Clin 2021;39:147-61. [Crossref] [PubMed]
- Mollan SP, Davies B, Silver NC, Shaw S, Mallucci CL, Wakerley BR, Krishnan A, Chavda SV, Ramalingam S, Edwards J, Hemmings K, Williamson M, Burdon MA, Hassan-Smith G, Digre K, Liu GT, Jensen RH, Sinclair AJ. Idiopathic intracranial hypertension: consensus guidelines on management. J Neurol Neurosurg Psychiatry 2018;89:1088-100. [Crossref] [PubMed]
- Colman BD, Boonstra F, Nguyen MN, Raviskanthan S, Sumithran P, White O, Hutton EJ, Fielding J, van der Walt A. Understanding the pathophysiology of idiopathic intracranial hypertension (IIH): a review of recent developments. J Neurol Neurosurg Psychiatry 2024;95:375-83. [PubMed]
- Cheng H, Jin H, Hu Y, Chen L, Chen Z, Zhong G. Long-term efficacy of venous sinus stenting in the treatment of idiopathic intracranial hypertension. CNS Neurosci Ther 2024;30:e14356. [Crossref] [PubMed]
- Raynald Huo X, Yang H, Wang Z, Tong X, Li X, Liu L, Wang S, Miao Z, Mo D. Characteristics and Outcomes of the Idiopathic Intracranial Hypertension Treatment in Intrinsic and Extrinsic Stenosis: A Single-Center Experience in China. Neurol Ther 2021;10:1029-44. [Crossref] [PubMed]
- Markey KA, Mollan SP, Jensen RH, Sinclair AJ. Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. Lancet Neurol 2016;15:78-91. [Crossref] [PubMed]
- Durst CR, Ornan DA, Reardon MA, Mehndiratta P, Mukherjee S, Starke RM, Wintermark M, Evans A, Jensen ME, Crowley RW, Gaughen J, Liu KC. Prevalence of dural venous sinus stenosis and hypoplasia in a generalized population. J Neurointerv Surg 2016;8:1173-7. [Crossref] [PubMed]
- Raynald Yang H, Tong X, Huo X, Li X, Liu L, Sui B, Qu H, Dong K, Wang Y, Wang S, Miao Z, Mo D. Stenting versus medical treatment for idiopathic intracranial hypertension: a matched-control study. J Neurointerv Surg 2023;15:1021-6. [Crossref] [PubMed]
- Cappuzzo JM, Hess RM, Morrison JF, Davies JM, Snyder KV, Levy EI, Siddiqui AH. Transverse venous stenting for the treatment of idiopathic intracranial hypertension, or pseudotumor cerebri. Neurosurg Focus 2018;45:E11. [Crossref] [PubMed]
- Nicholson P, Brinjikji W, Radovanovic I, Hilditch CA, Tsang ACO, Krings T, Mendes Pereira V, Lenck S. Venous sinus stenting for idiopathic intracranial hypertension: a systematic review and meta-analysis. J Neurointerv Surg 2019;11:380-5. [Crossref] [PubMed]
- Kalyvas A, Neromyliotis E, Koutsarnakis C, Komaitis S, Drosos E, Skandalakis GP, Pantazi M, Gobin YP, Stranjalis G, Patsalides A. A systematic review of surgical treatments of idiopathic intracranial hypertension (IIH). Neurosurg Rev 2021;44:773-92. [Crossref] [PubMed]
- Subramanian PS. Novel Approaches to the Treatment of Idiopathic Intracranial Hypertension. Curr Neurol Neurosci Rep 2024;24:265-72. [Crossref] [PubMed]
- ten Hove MW, Friedman DI, Patel AD, Irrcher I, Wall M, McDermott MP. Safety and Tolerability of Acetazolamide in the Idiopathic Intracranial Hypertension Treatment Trial. J Neuroophthalmol 2016;36:13-9. [Crossref] [PubMed]
- Smith SV, Friedman DI. The Idiopathic Intracranial Hypertension Treatment Trial: A Review of the Outcomes. Headache 2017;57:1303-10. [Crossref] [PubMed]
- Wall M, McDermott MP, Kieburtz KD, Corbett JJ, Feldon SE, Friedman DI, Katz DM, Keltner JL, Schron EB, Kupersmith MJ. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA 2014;311:1641-51. [Crossref] [PubMed]
- Townsend RK, Jost A, Amans MR, Hui F, Bender MT, Satti SR, Maurer R, Liu K, Brinjikji W, Fargen KM. Major complications of dural venous sinus stenting for idiopathic intracranial hypertension: case series and management considerations. J Neurointerv Surg 2022;14:neurintsurg-2021-017361.
- Azzam AY, Mortezaei A, Morsy MM, Essibayi MA, Ghozy S, Elamin O, Azab MA, Elswedy A, Altschul D, Kadirvel R, Brinjikji W, Kallmes DF. Venous sinus stenting for idiopathic intracranial hypertension: An updated Meta-analysis. J Neurol Sci 2024;459:122948. [Crossref] [PubMed]
- McGovern RA, Kelly KM, Chan AK, Morrissey NJ, McKhann GM 2nd. Should ventriculoatrial shunting be the procedure of choice for normal-pressure hydrocephalus? J Neurosurg 2014;120:1458-64. [Crossref] [PubMed]
- Batista S, Palavani LB, Verly G, Ferreira MY, Sanches JPB, Silva GM, Pinheiro AC, Almeida Filho JA. Comparing open and closed cell stents in idiopathic intracranial hypertension: A comprehensive meta-analysis of clinical outcomes. Neuroradiol J 2024; Epub ahead of print. [Crossref] [PubMed]
- Bilgin C, Oliver AA, Cutsforth-Gregory JK, Chen JJ, Rammos SK, Cloft HJ, Lanzino G, Kallmes DF, Brinjikji W. Zilver stent versus Carotid Wallstent for endovascular treatment of idiopathic intracranial hypertension. J Neurointerv Surg 2023;15:1269-73. [Crossref] [PubMed]


