
Segmental atrophy of the liver in a child: a case description and literature analysis
Introduction
Segmental atrophy of the liver (SAL) is an exceedingly uncommon, yet often overlooked, benign condition that can resemble liver tumors (1-3). Fewer than 30 cases of SAL have been documented in the literature to date (1-3). Unlike traditional liver atrophy that affects entire lobes or significant segments, SAL affects a specific histological segment of the liver. Vascular injury has been proposed as a possible cause of SAL; however, its exact cause remains poorly understood (1,2). Histopathological examinations have revealed various stages of SAL, characterized by different features, such as parenchymal collapse, nodular elastosis, and nodular fibrosis. These lesions frequently appear to have mass-like features in imaging studies, posing substantial diagnostic challenges for healthcare professionals. SAL is particularly rare in children, with few reported cases, which has contributed to a general lack of awareness of this condition among pediatricians. This report details an unusual case of SAL in a 3-year-old patient, presenting as a large cystic-solid liver mass, an observation not previously documented. Through this case, we aim to enhance the understanding of the imaging characteristics of SAL in pediatric patients, and enrich existing knowledge of this condition.
Case presentation
A 3-year-old male was admitted to the West China Hospital of Sichuan University for further management of a liver tumor. He had a 2-month history of progressively worsening right upper abdominal pain, without accompanying fever, chills, nausea, vomiting, or bloody stool. Preliminary ultrasound at an external clinic suggested a liver mass, prompting further evaluation. The patient had no other notable systemic symptoms or chronic medical conditions, and no history of trauma was reported. Diagnostic evaluations, including liver function tests, alpha-fetoprotein (AFP), routine blood tests, and parasite screening, yielded no abnormal results. A subsequent abdominal computerized tomography (CT) scan revealed a large, heterogeneous, and hypodense mass measuring 9.2 cm × 7.4 cm × 13.3 cm in the right lobe of the liver without visible calcification, characterized by multilocular cystic and solid components. The solid components and septations showed mild enhancement post-contrast, while the cystic area showed no enhancement (Figure 1). The adjacent liver parenchyma was normal. There was no evidence of lymphadenopathy or ascites. Based on the imaging findings, the patient was diagnosed with either a primary benign/malignant liver tumor or hydatid disease.

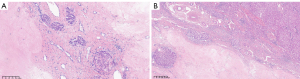
Due to the large size of the lesion and the potential risk of malignancy, a right hemihepatectomy was performed. The resected specimen had a cystic-solid structure and contained clear fluid; no abnormalities were observed in the surrounding liver parenchyma. The histological analysis revealed hepatocyte atrophy, fibrous tissue proliferation accompanied by collagenization, visible vasculature, and occasional dilated bile ducts (Figure 2). Immunohistochemical staining was positive for endothelial cluster of differentiation (CD)34 and glutamine synthetase, and negative for glypican-3. Additionally, it was negative for CD10, kappa, and lambda. No clonal amplification peaks for immunoglobulin heavy chain (IgH) or immunoglobulin kappa chain (IgK) were observed, and Congo red staining was negative. A final diagnosis of SAL was established. Given the benign nature of SAL, the patient did not undergo further therapeutic interventions. At the 12-month follow-up, the patient continued to show a favorable health status, and a follow-up ultrasound revealed no signs of recurrence. To date, he continues to undergo regular follow-up.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the child’s parents for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
SAL, which was first described by Singhi et al. in 2011, is an extremely rare and under-recognized benign disorder, currently considered a hepatic pseudotumor (1,3). Due to its rarity, the clinical and imaging characteristics of SAL remain inadequately documented, resulting in a lack of awareness of this condition among clinicians and radiologists. SAL predominantly occurs in middle-aged or older individuals, who have been reported to have an average age of approximately 60.5 years, and shows a modest female predominance (1). SAL is exceedingly rare in children; to date, only one other case has been reported (4). The symptoms of SAL are nonspecific, and often manifest as right upper abdominal pain or remain asymptomatic, and many cases have been discovered incidentally. Further, laboratory results and biomarkers generally fall within normal limits, complicating the diagnosis process.
While it is similar to traditional atrophy affecting entire lobes or large anatomic segments, which are associated with hepatobiliary diseases (5), SAL has a distinct etiology and distinct anatomical characteristics. SAL has a histological segmental distribution rather than an anatomic segmental distribution (6). SAL lesions are typically confined to a limited number of hepatic lobules, and are generally smaller than anatomical segments. The precise pathogenesis of SAL remains incompletely understood, but several vascular injury mechanisms that lead to hepatocyte loss and ischemic atrophy, such as thrombosis, fibrosis, and recanalization, have been proposed.
Histopathologically, SAL may progress through multiple developmental stages (1,2,7). In the early stages, SAL is characterized by collapsed hepatic parenchyma with intermittent islands of residual hepatocytes, accompanied by chronic inflammation and pronounced bile duct proliferation. As the disease progresses, ductal proliferation decreases, chronic inflammation subsides, and elastosis becomes more prominent. The third stage is marked by elastotic fibers interspersed with small islands of residual hepatocytes and portal tracts. Ultimately, the lesion may evolve into a nodule with dense fibrosis. Additionally, abnormally thickened blood vessels have been observed in almost all cases, suggesting that the pathogenesis may be related to vascular injury. Some cases may also present with bile duct dilation and bile duct cysts. Based on these criteria, in our case, SAL appears to have been in the third stage of development.
Due to the current limited understanding of this disease, SAL is frequently misdiagnosed as metastasis, leading to significant mismanagement. Thus, early and accurate diagnosis is crucial for patients with SAL. Improving understanding of the imaging features associated with this disease is critical for enhancing diagnostic accuracy. In the literature, SAL has typically presented as a solitary subcapsular mass on various radiographic examinations, ranging in size from 1.8 to over 10 cm. These lesions commonly appeared as well-defined, hypodense, non-enhancing masses on CT scans, and demonstrated T1 hypointensity, T2 hyperintensity, and diffusion-weighted hyperintensity on magnetic resonance imaging. Further, SAL has been shown to exhibit iso-metabolism compared to the background hepatic parenchyma on 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT, suggesting a benign nature. However, in the present case, the lesion manifested as a large multilocular cystic-solid mass; a previously undocumented presentation. While previous pathological findings indicated microscopic, small-to-large bile duct dilation and biliary cysts in some SAL lesions (1,8), there have been no previous reports of multiple macroscopic cystic lesions as observed in our case. We speculate that these cystic components may represent biliary retention cysts, which is supported by the presence of some dilated bile ducts in the histological examinations. Nevertheless, elucidating the mechanism behind such multilocular cystic component remains challenging and requires further research. Given that radiological findings are rooted in histological appearances, it is important to recognize the broad spectrum of histological changes in SAL, which may result in diverse radiological presentations.
In the present case, the lesion had to be differentiated from other cystic-solid masses that occur in children, including infantile hepatic hemangioma, hepatic mesenchymal hamartoma, hepatoblastoma, and hepatic hydatid disease (3,9). Certain features, such as a lack of enhancement, no invasion on imaging, a lack of FDG uptake on PET/CT, and negative biomarkers, such as AFP, are useful for differential diagnosis; however, the limited awareness of SAL among clinicians, radiologists, and pediatricians makes it challenging to recognize and differentiate this condition.
At present, there are no established treatment protocols for SAL. Considering the huge size of the lesion and the potential malignant risk, our patient underwent right hemihepatectomy. Due to the benign nature of SAL, the patient did not receive additional therapeutic interventions. At the 12-month follow-up, the patient continued to show a favorable health status. A subsequent ultrasound, performed 12 months after the surgery, revealed no evidence of recurrence. The prognosis of SAL remains unclear in limited samples. As our case showed, complete resection may be the optimal therapeutic approach for such large lesions.
In summary, our case enriches the sparse documentation of SAL occurrences in pediatric populations, thereby advancing the understanding of the disease profile in this demographic. It also presents a novel contribution to the understanding of the imaging characteristics of this condition. SAL is extremely rare, but it can mimic the morphology of tumors clinically, and thus must be differentiated from other liver tumors to prevent overtreatment.
Acknowledgments
Funding: This study was financially supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1324/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the child’s parents for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singhi AD, Maklouf HR, Mehrotra AK, Goodman ZD, Drebber U, Dienes HP, Torbenson M. Segmental atrophy of the liver: a distinctive pseudotumor of the liver with variable histologic appearances. Am J Surg Pathol 2011;35:364-71. [Crossref] [PubMed]
- Spolverato G, Anders R, Kamel I, Pawlik TM. Segmental atrophy of the liver: an uncommon and often unrecognized pseudotumor. Dig Dis Sci 2014;59:3122-5. [Crossref] [PubMed]
- Assarzadegan N, Montgomery E. Uncommon Benign Neoplasms and Pseudotumors of the Liver. Arch Pathol Lab Med 2023;147:390-402. [Crossref] [PubMed]
- Bedada AG, Sreekumaran MI, Azzie G. Segmental atrophy of the liver in a child: Case report and review of the literature. South African Journal of Child Health 2019;13:100-1.
- Ham JM. Lobar and segmental atrophy of the liver. World J Surg 1990;14:457-62. [Crossref] [PubMed]
- Garg I, Graham RP, VanBuren WM, Goenka AH, Torbenson MS, Venkatesh SK. Hepatic segmental atrophy and nodular elastosis: imaging features. Abdom Radiol (NY) 2017;42:2447-53. [Crossref] [PubMed]
- Ishizaki Y, Mizuno T, Hara K, Kawasaki S. Advanced segmental atrophy of the liver with marked elastosis. Surgery 2015;157:826-7. [Crossref] [PubMed]
- Findeis-Hosey JJ, Zhou Z, Gonzalez RS. Hepatic sclerosing cavernous haemangioma can mimic the nodular elastosis stage of segmental atrophy. Histopathology 2019;75:876-81. [Crossref] [PubMed]
- Lucas B, Ravishankar S, Pateva I. Pediatric Primary Hepatic Tumors: Diagnostic Considerations. Diagnostics (Basel) 2021;11:333. [Crossref] [PubMed]


