
Development and validation of a multi-parametric MRI deep-learning model for preoperative lymphovascular invasion evaluation in rectal cancer
Introduction
In the late 1990s, colorectal cancer was the fourth leading cause of cancer-related death in individuals under the age of 50, but it is now the leading cause and the second leading cause of cancer-related death in men and women in the same age group, respectively (1). Rectal cancer (RC) represents one-third of all colorectal cancers, and is a significant cause of morbidity and mortality (2). Surgical resection in conjunction with neoadjuvant chemoradiotherapy remains the standard treatment for RC (3,4). Lymphovascular invasion (LVI) in RC is defined as the infiltration of tumor cells into the luminal space lined by endothelial cells or as the destruction of the lymphovascular wall by tumor cells (5). This histological feature represents a crucial aspect of RC pathology, indicating the potential for tumor dissemination beyond the primary site (6,7). A recent study showed that LVI, rather than depth of invasion, is the key risk factor for metastases in early colorectal cancer (8). This new finding has significant clinical implications for prognosticating postoperative recurrence, metastasis, and overall survival in RC patients. However, traditional detection methods that rely on invasive biopsies limit preoperative staging and prevent timely intervention. To address this challenge, preoperative techniques urgently need to be developed to enhance the identification of LVI in patients with RC.
High-resolution magnetic resonance imaging (MRI) is a principal imaging modality used for the preoperative and postoperative assessment of RC (9). T2-weighted imaging (T2WI) and diffusion-weighted imaging (DWI) have yielded reliable results in the detection and staging of RC (10). MRI has shown moderate sensitivity and high specificity in detecting larger vessels; however, it cannot identify vessels with a diameter of <3 mm, including micro-arteriovenous and lymphatic vessels. The accurate preoperative imaging evaluation of LVI in RC continues to be a highly challenging task (11). With the advancement of quantitative image analysis methods in the past decade, it has become feasible to automatically extract quantitative features from medical images using computers (12,13). Several studies have used classic handcrafted radiomics on MRI scans to assess LVI in RC, and achieved notable results (14-17). However, precise tumor labeling in radiomics is laborious and time consuming, which has limited its clinical application, Moreover, the preselection of morphologic features restricts the information that can be extracted from the images.
In recent years, deep learning (DL) has emerged as a promising technique in the field of machine learning (18). DL, particularly its convolutional neural networks (CNNs), has garnered considerable interest in clinical applications due to its proficiency in executing automated image analysis tasks. It has demonstrated immense potential in RC, including tumor screening, the evaluation of treatment responses, and genetic profiling (19-21). Notably, the three-dimensional (3D) residual network (ResNet) architecture has shown remarkable effectiveness in handling spatial 3D datasets, especially in the realm of medical imaging, including MRI and computed tomography scans (22,23). However, to the best of our knowledge, currently, no DL methods for evaluating the LVI status have been evaluated, and some of the more advanced technologies, such as the 3D ResNet, have yet to be used.
This study sought to develop and validate a 3D ResNet DL model that integrates dual-parameter MRI data and clinical factors for the non-invasive assessment of LVI status, and to test its performance at an external center. We present this article in accordance with the TRIPOD+AI reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-789/rc).
Methods
Patients
The institutional review boards of The Second Affiliated Hospital of Harbin Medical University (No. YJSKY2024-062) and the Xinjiang Production and Construction Corps Tenth Division Beitun Hospital (No. BTKY2021-005) approved this retrospective multicohort study, and waived the requirement for written informed consent due to the retrospective nature of this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
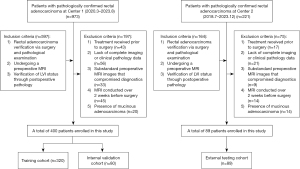
In total, the data of 489 patients with rectal adenocarcinoma, definitively confirmed by surgical intervention and subsequent pathological examinations, were retrospectively collected from The Second Affiliated Hospital of Harbin Medical University (Center 1) from March 2020 to August 2023, and from the Xinjiang Production and Construction Corps Tenth Division Beitun Hospital (Center 2) from July 2018 to December 2023. To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) have a diagnosis of rectal adenocarcinoma verified by surgery and follow-up pathological examination; (II) have undergone a preoperative MRI; and (III) have had their LVI status verified by postoperative pathology. Patients were excluded from the study if they met any of the following exclusion criteria: (I) lacked complete imaging or clinical pathology data; (II) had substandard preoperative MRI images that compromised diagnostics; (III) had undergone MRI over 2 weeks before surgery; (IV) had mucinous adenocarcinoma; and/or (V) had received preoperative treatment. Such treatment can significantly alter tumor and surrounding tissue pathology (e.g., reduce tumor size or eliminate LVI), which would have affected our assessment of the initial tumor characteristics. Ultimately, the study cohort comprised 400 patients from Center 1 and 89 from Center 2. The patients from Center 1 were divided into a training cohort and an internal validation cohort at a 4:1 ratio using a stratified random-sampling technique, and those from Center 2 comprised the independent external testing cohort (Figure 1).
Clinical variables and pathological variables
Clinical data for all patients, including age, gender, and pre-treatment levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), were systematically collected from the electronic medical record system. Elevated levels of CEA and CA19-9 exceeding the normal upper thresholds established by our institution (5 ng/mL for CEA and 37 U/mL for CA19-9) were considered clinically significant elevations. The T2WI and DWI characteristics of RC, including tumor length, location, magnetic resonance-predicted tumor (mrT) stage, and magnetic resonance-predicted lymph node (mrN) stage, were the key features analyzed. All MRI data were meticulously extracted from the Picture Archiving and Communication System for comprehensive analysis. These features were first independently evaluated and then subsequently validated by a senior radiologist with over 20 years of experience in abdominal imaging. In RC, the LVI outcomes were derived from the pathological reports following the histopathological examinations.
MR imaging acquisition and image segmentation
Each patient underwent a preoperative MRI examination (see Appendix 1 for details of the protocols and imaging processes). For rectal adenocarcinoma, the specific MRI features are as follows: tumor regions with signal intensity higher than the muscularis propria but lower than the submucosa on T2WI; and lesions showing markedly higher signal intensity than normal rectal wall tissue on high b-value DWI (24). The tumor contour was outlined layer by layer on the T2WI and DWI images using ITK-SNAP (version 4.0.2) (Figure 2). Two physicians with 3 and 5 years of radiology experience independently delineated the tumors. A radiologist with over 20 years of experience in abdominal imaging then reviewed ambiguous cases to ensure accurate tumor contouring. The radiologists were blinded to the clinical and pathological outcomes to ensure an unbiased imaging analysis.

DL models development
The following three DL models were developed in this study: (I) a pure image model using only T2WI; (II) a pure image model using only DWI; and (III) a combined model that integrated T2WI, DWI, and clinical factors. The neural networks employed were pre-trained on natural images from the ImageNet dataset and multiple medical image datasets from RadImageNet (25). The 3D ResNet-18 served as the backbone of the DL models, enabling the enhanced handling of 3D data and more effective spatial information capture. Similar to other ResNet architectures, the 3D ResNet-18 used residual connections to train the networks effectively, thus avoiding issues such as vanishing gradients, and enhancing parameter efficiency (26). For models (I) and (II), a 3D ResNet-18 model equipped with fully connected layers was used to extract high-dimensional features from the imaging data and to directly evaluate the probability of LVI. In relation to the combined model, the clinical factors, having undergone both univariate and multivariate logistic regression analyses, were transformed into a 3D vector via fully connected layers, which then was concatenated with the extracted 512-dimensional features from the T2WI and DWI images. Subsequently, the resulting 1,027-dimensional vector, containing image and clinical information, was used to assess the probability of LVI (Figure 3). In MRI images of RC, the features are usually located in the fat space around the tumor. Therefore, the input dimension can be reduced by cropping the sub-region of the image. A rectangle was used to crop the image from the center of the region of interest, and to re-sample and normalize the image to 112×112×12 pixels, and then input the DL model. Additionally, given the relatively small sample size for DL, dynamic data augmentation techniques, including random horizontal flipping and cropping, were employed to augment the training data 16-fold. The Adaptive Moment Estimation optimizer was used to update the model parameters, starting with an initial learning rate of 0.001, and decaying according to the cosine annealing scheduler over 200 epochs, and a batch size of 64.

Model interpretability
To improve the transparency and comprehensibility of our DL models, particularly in terms of their decision-making processes, we incorporated gradient-weighted class activation mapping (27). This technique used gradient information flowing into the final convolutional layer of the CNNs. By performing a weighted fusion of these gradients, a class activation map was produced that visually emphasized the critical areas in the MRI images that influenced the classification decision. In the combined model, the relative weights of the clinical variables for individual patients were displayed in conjunction with the corresponding saliency maps derived from the MRI images.
Statistical analysis
All statistical analyses were performed using R (version 3.4.0, R Foundation), and SPSS 22.0 (IBM Corp, Armonk, NY, USA). The Mann-Whitney U test was used to compare differences between the continuous variables, while the chi-square test or Fisher’s exact test was used to compare the categorical variables between two groups. Model performance was evaluated by receiver operating characteristic (ROC) analysis to determine the area under the curve (AUC). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) at the optimal threshold, which was determined based on the maximum Youden index, were calculated. The DeLong test was used to compare the AUC values of different models across the validation and testing sets. A calibration curve analysis and decision curve analysis (DCA) were conducted to assess the goodness-of-fit and the clinical relevance of the models through net benefit calculations, respectively.
Results
Patient characteristics
Table 1 displays the clinical characteristics of the patients. Our study included 263 (48.9%) patients with LVI and 226 (51.1%) patients without LVI. Subsequently, the patients were divided into the LVI (+) and LVI (−) groups. No significant differences were observed among the two groups in terms of gender, age, mrN stage, tumor length, and tumor location in the training cohort, internal validation cohort, and external testing cohort (P>0.05). However, significant differences were observed among the groups in terms of the CEA and CA19-9 levels in the training cohort and the external testing cohort (P<0.05), but not in the internal validation cohort. Additionally, significant differences were observed among the groups in terms of the mrT stage in the training cohort, but not in the internal validation cohort and external testing cohort.
Table 1
| Characteristics | Training cohort (n=320) | Internal validation cohort (n=80) | External testing cohort (n=89) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LVI (−) | LVI (+) | P | LVI (−) | LVI (+) | P | LVI (−) | LVI (+) | P | |||
| Age (years) | 66 (58.0, 71.0) | 64 (57.0, 71.0) | 0.352 | 61 (54.3, 70.8) | 62 (56.5, 68.8) | 0.874 | 65 (57.0, 74.0) | 66 (58.8, 71.3) | 0.921 | ||
| Gender | 0.864 | 0.104 | 0.877 | ||||||||
| Female | 55 (31.98) | 46 (31.08) | 18 (45.00) | 11 (27.5) | 14 (27.45) | 11 (28.95) | |||||
| Male | 117 (68.02) | 102 (68.92) | 22 (55.00) | 29 (72.5) | 37 (72.55) | 27 (71.05) | |||||
| mrT stage | 0.001** | 0.809 | 0.104 | ||||||||
| T1–T2 | 61 (35.47) | 33 (22.30) | 13 (32.50) | 12 (30.00) | 14 (27.45) | 5 (13.16) | |||||
| T3–T4 | 111 (64.53) | 115 (77.70) | 27 (67.50) | 28 (70.00) | 37 (72.55) | 33 (86.84) | |||||
| mrN stage | 0.094 | 0.852 | 0.237 | ||||||||
| N0 | 23 (13.37) | 15 (10.14) | 15 (37.50) | 15 (37.50) | 23 (45.10) | 11 (28.95) | |||||
| N1 | 70 (40.70) | 47 (31.76) | 13 (32.50) | 11 (27.50) | 21 (41.18) | 18 (47.37) | |||||
| N2 | 79 (45.93) | 86 (58.11) | 12 (30.00) | 14 (35.00) | 7 (13.73) | 9 (23.68) | |||||
| Tumor length (cm) | 4.20 (3.5, 5.4) | 4.20 (3.9, 5.3) | 0.261 | 4.00 (3.3, 5.0) | 4.00 (3.5, 5.0) | 0.716 | 4.50 (3.0, 5.5) | 4.35 (3.5, 5.1) | 0.822 | ||
| Tumor location | 0.878 | 0.062 | 0.093 | ||||||||
| High | 43 (25.00) | 34 (22.97) | 14 (35.00) | 6 (15.00) | 22 (43.14) | 10 (26.32) | |||||
| Middle | 96 (55.81) | 83 (56.08) | 13 (32.50) | 22 (55.00) | 19 (37.25) | 23 (60.53) | |||||
| Low | 33 (19.19) | 31 (20.95) | 13 (32.50) | 12 (32.00) | 10 (19.61) | 5 (13.16) | |||||
| CEA | 0.007** | 0.431 | 0.003** | ||||||||
| Normal | 135 (78.49) | 96 (64.86) | 32 (80.00) | 29 (72.50) | 42 (82.35) | 20 (52.63) | |||||
| Elevated | 37 (21.51) | 52 (35.14) | 8 (20.00) | 11 (27.50) | 9 (17.65) | 18 (47.37) | |||||
| CA19-9 | 0.002** | 0.288 | 0.001** | ||||||||
| Normal | 161 (93.60) | 122 (82.43) | 37 (92.50) | 34 (85.00) | 48 (94.12) | 25 (65.79) | |||||
| Elevated | 11 (6.40) | 26 (17.57) | 3 (7.50) | 6 (15.00) | 3 (5.88) | 13 (34.21) | |||||
Data are expressed as the number of participants (percentage) or median (interquartile range). **, P<0.01. mrT stage, MR-predicted tumor stage; mrN stage, MR-predicted lymph node stage; LVI, lymphovascular invasion; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; MR, magnetic resonance.
Performance of the DL models
Univariate and multivariate logistic regression analyses were conducted, and the following three variables were found to be correlated with the assessment of LVI, and subsequently integrated into the combined model: (I) mrT stage (a polytomous variable; T1 = 0, T2 = 1, T3 = 2, T4 = 3); (II) CEA level (a dichotomous variable; <5 ng/mL = 0, ≥5 ng/mL = 1); and (III) CA19-9 (a dichotomous variable; <37 U/mL = 0, ≥37 U/mL = 1). For further details of the selection procedure, see Tables S1,S2, and Figures S1,S2.
The T2WI-based model demonstrated a robust capacity for identifying LVI, with AUC values of 0.865 [95% confidence interval (CI), 0.825–0.905], 0.795 (95% CI, 0.693–0.897), and 0.764 (95% CI, 0.630–0.898) in the training, internal validation, and external testing cohorts, respectively. The DWI-based model exhibited enhanced performance compared to that of the T2WI-based model, with AUC values of 0.904 (95% CI, 0.871–0.936) in the training cohort, 0.822 (95% CI, 0.721–0.922) in the internal validation cohort (P<0.05 versus the T2WI-based model), and 0.825 (95% CI, 0.735–0.916) in the external testing cohort (P=0.408 versus to the T2WI-based model). The combined model, which integrates T2WI, DWI, and clinical factors, achieved the optimal performance among the three models, with AUC values of 0.983 (95% CI, 0.973–0.993) in the training cohort, 0.848 (95% CI, 0.758–0.938) in the internal validation cohort (P<0.05 versus the DWI image model), and 0.899 (95% CI, 0.829–0.968) in the external testing cohort (P<0.05 versus the DWI-based model). Figure 4 illustrates the results of the ROC analysis.

Table 2 presents a comprehensive comparison of the diagnostic capabilities of the T2WI-based model, DWI-based model, and combined model for evaluating LVI. In the testing cohort, the combined model showed superior evaluative capabilities for LVI compared with the T2WI-based model (sensitivity 0.769, specificity 0.825, accuracy 0.809) and the DWI-based model (sensitivity 0.885, specificity 0.762, accuracy 0.798), exhibiting comparable sensitivity (0.731), enhanced specificity (0.905), and greater accuracy (0.854). The calibration curve analysis revealed good concordance between the evaluated probabilities of LVI and the actual probabilities for the three models (Figure 5). The DCA results confirmed the clinical utility of all three models, among which, the combined and DWI-based models demonstrated a more pronounced overall net benefit in evaluating LVI compared to the T2WI-based model (Figure 6).
Table 2
| Model | AUC (95% CI) | Accuracy | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| ResNet18_DWI | ||||||
| Training | 0.904 (0.831–0.936) | 0.841 | 0.831 | 0.850 | 0.847 | 0.834 |
| Internal validation | 0.822 (0.721–0.922) | 0.812 | 0.900 | 0.744 | 0.766 | 0.879 |
| External testing | 0.825 (0.735–0.916) | 0.798 | 0.885 | 0.762 | 0.605 | 0.941 |
| ResNet18_T2 | ||||||
| Training | 0.865 (0.825–0.905) | 0.809 | 0.869 | 0.750 | 0.777 | 0.851 |
| Internal validation | 0.795 (0.623–0.898) | 0.787 | 0.725 | 0.850 | 0.829 | 0.756 |
| External testing | 0.764 (0.630–0.898) | 0.809 | 0.769 | 0.825 | 0.645 | 0.897 |
| ResNet18_combine | ||||||
| Training | 0.983 (0.973–0.993) | 0.931 | 0.887 | 0.975 | 0.973 | 0.897 |
| Internal validation | 0.848 (0.759–0.938) | 0.812 | 0.825 | 0.800 | 0.805 | 0.821 |
| External testing | 0.899 (0.829–0.968) | 0.854 | 0.731 | 0.905 | 0.760 | 0.891 |
AUC, area under the curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; DWI, diffusion-weighted imaging.


The interpretability of DL models
Visualizations, including highlighted heatmaps generated from T2WI and DWI images, were used to assess the interpretability of the models. The attention of the models was predominantly focused on the peripheral and internal areas of the tumors, while the normal intestinal walls and adjacent tissues showed minimal engagement. A weighted-evaluation analysis was conducted to compare the relative importance of each clinical MRI factor (Figure 7).

Discussion
A novel DL model combining T2WI, DWI, and clinical factors was developed to evaluate the presence of LVI in RC patients. The results indicated that the combined model surpassed the T2WI- and DWI-based models in identifying LVI. In the external testing cohort, the combined model demonstrated superior performance in terms of AUC, accuracy, specificity, and PPV. Further, the model exhibited excellent calibration and clinical utility, suggesting its potential as a powerful tool in guiding personalized treatment for RC patients. This study may herald new directions in the development of medical imaging technology and offer more precise guidance for the treatment of RC patients.
In our external testing cohort, the AUC of the DWI-based model surpassed that of the T2WI-based model. This outcome likely stems from the enhanced sensitivity and precision of DWI in tracking water molecule movement in tumors and adjacent tissues. Such capability allows for the quantitative evaluation of cellular-level details, thereby reflecting the morphological and metabolic alterations in RC extramural blood vessels (28). Fornell-Perez et al. conducted a study involving 100 biopsy-confirmed RC patients using both T2WI and DWI sequences to assess extramural vascular invasion (EMVI) (29), and found that integrating DWI with T2WI improved the sensitivity and specificity of EMVI diagnosis by 11.1% and 8.6%, respectively. Similarly, Chen et al.’s volumetric analysis of 50 patients established a correlation between tumor volume measured by DWI and T2WI techniques and LVI (30). Notably, DWI was shown to have a higher evaluative efficiency for LVI than T2WI. This superiority may be linked to the limited ability of T2WI to differentiate tumors from surrounding fibrotic reaction bands, a challenge DWI overcomes by distinguishing high-signal tumors from low-signal fibrotic reactions.
Previous studies have reported inconsistencies in terms of the risk factors for LVI. Zhang et al. observed significant inconsistencies in the pathological tumor and node stage between LVI (+) and LVI (−) (17). Additionally, Zhang et al. concluded that significant differences existed in CA19-9 levels between LVI (+) and LVI (−) groups (14). However, Tong et al. found no such significant differences between these two groups (16). These inconsistencies might be attributed to variations in patient demographics. Further, the composition of the study populations might have also influenced these outcomes. In the present study, significant differences were found between the LVI (+) and LVI (−) groups in terms of mrT stage, and CEA, and CA19-9 levels. Our cohort included a significant number of advanced RC patients, some of whom had already developed metastasis. Previous research has indicated that CEA CA19-9 levels, and LVI are critical in prognosticating early distant metastasis in RC (31-33). Upon the invasion of lymphatic and blood vessels by RC, tumor cells may enter the lymphatic system or bloodstream, resulting in an increased release of CEA and CA19-9 tumor markers into the blood (34). Thus, the invasion of these vessels is associated with elevated CEA and CA19-9 levels.
Previous research on LVI in RC relied on classical radiomics, yielding an AUC value of approximately 0.8–0.9 in an internal validation set at a single institution (14-17). Our 3D ResNet model not only exhibits comparable efficacy but also features the most substantial sample size among similar studies to date. Moreover, we used an independent external cohort to examine the effectiveness of the model. DL networks, characterized as multi-layer feedforward neural networks, enable end-to-end training in supervised approaches, facilitating the learning of highly discriminative image features, and removing the need to manually extract radiomic features (35).
This study made several advancements. First, unlike previous studies that have used traditional two-dimensional (2D) CNNs, this study employed a 3D ResNet. The input data also comprised 3D segmentation data of the entire tumor, eschewing the limitations of 2D segmentation data. This innovative approach yielded richer internal tumor information, facilitating the identification of small diameter arteries and lymphatic vessels unobservable directly on images, thereby markedly enhancing evaluation accuracy (36,37). Second, we incorporated clinical factors, and T2WI and DWI data into a combined model developed using a multi-layer perceptron. This approach considerably aided in enhancing the evaluation of LVI status. Integrating these key data into the model enabled a more comprehensive consideration of multiple factors, thus improving the accuracy of LVI status evaluation. Third, among the patients enrolled in the study, the ratio of LVI (+) to LVI (−) groups was approximately 1:1. This balanced ratio was advantageous for model training, and effectively mitigated the risk of overfitting. Fourth, in relation to the interpretability of the images, we aligned the visualization of key image regions and the relative weights of individual patients’ clinical variables. This bifurcated approach not only clarified what the model interpreted in MRI scans but also delineated the influence of each patient’s clinical data on the final diagnostic outcome. This methodology increases the transparency and interpretability in machine-learning models, empowering healthcare professionals to grasp the foundation of model evaluations, and thus enhancing the trust and practicality of such models in clinical applications. Finally, this study was conducted across multiple medical centers, using images from two types of scanning devices. This variance in data sources significantly strengthened the robustness and generalizability of model, which in turn could broaden its application and adoption. The use of data from multiple centers enabled a better simulation of real clinical scenarios, bolstering the credibility of the research findings.
Limitations
This study had a number of limitations. First, it sought to optimize preoperative assessment strategies to provide a reliable basis for clinical treatment at the initial diagnosis; however, this necessitated a focus on the characteristics of primary tumors that had not undergone clinical intervention, leading us to exclude patients who received neoadjuvant therapy from this study. This is a very important group, and in our future research, we intend to further analyze the efficacy of neoadjuvant therapy and the changes observed in different imaging assessment tools before and after treatment. Second, in terms of the number of participants, while our study sample comprised 489 RC patients, which exceeds the sample size used in previous radiomic studies for evaluating RC LVI, this number remains relatively small for DL applications. Additionally, the sample sizes of both the internal validation and external testing cohorts were small, which might have introduced inherent biases and potential confounding factors. Third, in terms of data collection, all data were collected retrospectively. Consequently, larger prospective cohort studies need to be conducted for validation. Fourth, in terms of technological methodologies, this study used a technique of tracing the tumor contour during the contouring process. Unlike the radiomics segmentation method, which necessitates the meticulous manual annotation of the tumor and the exclusion of bowel contents, air, and necrotic areas, this approach is simpler and more efficient, and saves considerable time. Conversely, manual segmentation is time consuming and depends on the subjective experience of observers. Consequently, in the future, we intend to develop accurate automated segmentation methods for RC. Finally, other prognostic factors such as lymph node metastasis, perineural invasion, and surgical margin status are also important. In our future research, we intend to collaborate with more hospitals and computer workstations to better assist clinicians to evaluate patient prognosis and formulate personalized treatment plans.
Conclusions
To the best of our knowledge, this was the first study to use DL technology for the preoperative assessment of histological LVI status in patients with RC. We showed that T2WI- and DWI-based models exhibited good evaluative performance, but incorporating clinical factors into a combined model with T2WI and DWI data significantly enhanced the model’s efficacy. Consequently, this approach could serve as an adjunctive method for the non-invasive evaluation of LVI in patients with RC, thereby facilitating the precision treatment for these individuals.
Acknowledgments
Funding: This research was supported by a grant from
Footnote
Reporting Checklist: The authors have completed the TRIPOD+AI reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-789/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-789/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The institutional review boards of The Second Affiliated Hospital of Harbin Medical University (No. YJSKY2024-062) and the Xinjiang Production and Construction Corps Tenth Division Beitun Hospital (No. BTKY2021-005) approved this retrospective multicohort study and waived the requirement for written informed consent due to the retrospective nature of this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. [Crossref] [PubMed]
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Morris VK, Kennedy EB, Baxter NN, Benson AB 3rd, Cercek A, Cho M, et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J Clin Oncol 2023;41:678-700. [Crossref] [PubMed]
- Inchingolo R, Maino C, Cannella R, Vernuccio F, Cortese F, Dezio M, Pisani AR, Giandola T, Gatti M, Giannini V, Ippolito D, Faletti R. Radiomics in colorectal cancer patients. World J Gastroenterol 2023;29:2888-904. [Crossref] [PubMed]
- Poornakala S, Prema NS. A study of morphological prognostic factors in colorectal cancer and survival analysis. Indian J Pathol Microbiol 2019;62:36-42. [Crossref] [PubMed]
- Al-Sukhni E, Attwood K, Gabriel EM, LeVea CM, Kanehira K, Nurkin SJ. Lymphovascular and perineural invasion are associated with poor prognostic features and outcomes in colorectal cancer: A retrospective cohort study. Int J Surg 2017;37:42-9. [Crossref] [PubMed]
- Zhang F, Chen H, Luo D, Xiong Z, Li X, Yin S, Jin L, Chen S, Peng J, Lian L. Lymphovascular or perineural invasion is associated with lymph node metastasis and survival outcomes in patients with gastric cancer. Cancer Med 2023;12:9401-8. [Crossref] [PubMed]
- Rönnow CF, Arthursson V, Toth E, Krarup PM, Syk I, Thorlacius H. Lymphovascular Infiltration, Not Depth of Invasion, is the Critical Risk Factor of Metastases in Early Colorectal Cancer: Retrospective Population-based Cohort Study on Prospectively Collected Data, Including Validation. Ann Surg 2022;275:e148-54. [Crossref] [PubMed]
- Jayaprakasam VS, Alvarez J, Omer DM, Gollub MJ, Smith JJ, Petkovska I. Watch-and-Wait Approach to Rectal Cancer: The Role of Imaging. Radiology 2023;307:e221529. [Crossref] [PubMed]
- Lord AC, D'Souza N, Shaw A, Rokan Z, Moran B, Abulafi M, Rasheed S, Chandramohan A, Corr A, Chau I, Brown G. MRI-Diagnosed Tumor Deposits and EMVI Status Have Superior Prognostic Accuracy to Current Clinical TNM Staging in Rectal Cancer. Ann Surg 2022;276:334-44. [Crossref] [PubMed]
- Gu C, Yang X, Zhang X, Zheng E, Deng X, Hu T, Wu Q, Bi L, Wu B, Su M, Wang Z. The prognostic significance of MRI-detected extramural venous invasion, mesorectal extension, and lymph node status in clinical T3 mid-low rectal cancer. Sci Rep 2019;9:12523. [Crossref] [PubMed]
- Li R, Li L, Xu Y, Yang J. Machine learning meets omics: applications and perspectives. Brief Bioinform 2022;23:bbab460. [Crossref] [PubMed]
- Danishuddin Khan S, Kim JJ. From cancer big data to treatment: Artificial intelligence in cancer research. J Gene Med 2024;26:e3629. [Crossref] [PubMed]
- Zhang K, Ren Y, Xu S, Lu W, Xie S, Qu J, Wang X, Shen B, Pang P, Cai X, Sun J. A clinical-radiomics model incorporating T2-weighted and diffusion-weighted magnetic resonance images predicts the existence of lymphovascular invasion / perineural invasion in patients with colorectal cancer. Med Phys 2021;48:4872-82. [Crossref] [PubMed]
- Wong C, Liu T, Zhang C, Li M, Zhang H, Wang Q, Fu Y. Preoperative detection of lymphovascular invasion in rectal cancer using intravoxel incoherent motion imaging based on radiomics. Med Phys 2024;51:179-91. [Crossref] [PubMed]
- Tong P, Sun D, Chen G, Ni J, Li Y. Biparametric magnetic resonance imaging-based radiomics features for prediction of lymphovascular invasion in rectal cancer. BMC Cancer 2023;23:61. [Crossref] [PubMed]
- Zhang Y, He K, Guo Y, Liu X, Yang Q, Zhang C, Xie Y, Mu S, Guo Y, Fu Y, Zhang H. A Novel Multimodal Radiomics Model for Preoperative Prediction of Lymphovascular Invasion in Rectal Cancer. Front Oncol 2020;10:457. [Crossref] [PubMed]
- Wong C, Fu Y, Li M, Mu S, Chu X, Fu J, Lin C, Zhang H. MRI-Based Artificial Intelligence in Rectal Cancer. J Magn Reson Imaging 2023;57:45-56. [Crossref] [PubMed]
- Wei Y, Wang H, Chen Z, Zhu Y, Li Y, Lu B, Pan K, Wen C, Cao G, He Y, Zhou J, Pan Z, Wang M. Deep Learning-Based Multiparametric MRI Model for Preoperative T-Stage in Rectal Cancer. J Magn Reson Imaging 2024;59:1083-92. [Crossref] [PubMed]
- Song K, Zhao Z, Ma Y, Wang J, Wu W, Qiang Y, Zhao J, Chaudhary S. A multitask dual-stream attention network for the identification of KRAS mutation in colorectal cancer. Med Phys 2022;49:254-70. [Crossref] [PubMed]
- Jiang X, Zhao H, Saldanha OL, Nebelung S, Kuhl C, Amygdalos I, Lang SA, Wu X, Meng X, Truhn D, Kather JN, Ke J. An MRI Deep Learning Model Predicts Outcome in Rectal Cancer. Radiology 2023;307:e222223. [Crossref] [PubMed]
- Yang Y, Zhang Q. Multiview framework using a 3D residual network for pulmonary micronodule malignancy risk classification. Biomed Mater Eng 2020;31:253-67. [Crossref] [PubMed]
- Yang B, Huang J, Wu G, Yang J. Classifying the tracing difficulty of 3D neuron image blocks based on deep learning. Brain Inform 2021;8:25. [Crossref] [PubMed]
- Gürses B, Böge M, Altınmakas E, Balık E. Multiparametric MRI in rectal cancer. Diagn Interv Radiol 2019;25:175-82. [Crossref] [PubMed]
- Mei X, Liu Z, Robson PM, Marinelli B, Huang M, Doshi A, Jacobi A, Cao C, Link KE, Yang T, Wang Y, Greenspan H, Deyer T, Fayad ZA, Yang Y. RadImageNet: An Open Radiologic Deep Learning Research Dataset for Effective Transfer Learning. Radiol Artif Intell 2022;4:e210315. [Crossref] [PubMed]
- Lin YH, Lin CT, Chang YH, Lin YY, Chen JJ, Huang CR, Hsu YW, You WC. Development and Validation of a 3D Resnet Model for Prediction of Lymph Node Metastasis in Head and Neck Cancer Patients. J Imaging Inform Med 2024;37:679-87. [Crossref] [PubMed]
- Zhang H, Ogasawara K. Grad-CAM-Based Explainable Artificial Intelligence Related to Medical Text Processing. Bioengineering (Basel) 2023;10:1070. [Crossref] [PubMed]
- Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics 2019;39:367-87. [Crossref] [PubMed]
- Fornell-Perez R, Perez-Alonso E, Porcel-de-Peralta G, Duran-Castellon A, Vivas-Escalona V, Aranda-Sanchez J, Gonzalez-Dominguez MC, Rubio-Garcia J, Aleman-Flores P, Lozano-Rodriguez A, Orihuela-de-la-Cal ME, Loro-Ferrer JF. Primary and post-chemoradiotherapy staging using MRI in rectal cancer: the role of diffusion imaging in the assessment of perirectal infiltration. Abdom Radiol (NY) 2019;44:3674-82. [Crossref] [PubMed]
- Chen XL, Chen GW, Pu H, Yin LL, Li ZL, Song B, Li H. DWI and T2-Weighted MRI Volumetry in Resectable Rectal Cancer: Correlation With Lymphovascular Invasion and Lymph Node Metastases. AJR Am J Roentgenol 2019;212:1271-8. [Crossref] [PubMed]
- Zhang LN, OuYang PY, Xiao WW, Yu X, You KY, Zeng ZF, Xu RH, Gao YH. Elevated CA19-9 as the Most Significant Prognostic Factor in Locally Advanced Rectal Cancer Following Neoadjuvant Chemoradiotherapy. Medicine (Baltimore) 2015;94:e1793. [Crossref] [PubMed]
- Shan J, Gu B, Shi L, Wang X, Ye W, Zhou W, Sun X. Prognostic value of CEA and CA19-9 in patients with local advanced rectal cancer receiving neoadjuvant chemoradiotherapy, radical surgery and postoperative chemotherapy. Transl Cancer Res 2021;10:88-98. [Crossref] [PubMed]
- Hogan J, Chang KH, Duff G, Samaha G, Kelly N, Burton M, Burton E, Coffey JC. Lymphovascular invasion: a comprehensive appraisal in colon and rectal adenocarcinoma. Dis Colon Rectum 2015;58:547-55. [Crossref] [PubMed]
- Berek JS, Matias-Guiu X, Creutzberg C, Fotopoulou C, Gaffney D, Kehoe S, Lindemann K, Mutch D, Concin NEndometrial Cancer Staging Subcommittee, FIGO Women's Cancer Committee. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet 2023;162:383-94. Erratum in: Int J Gynaecol Obstet 2024;166:1374. [Crossref] [PubMed]
- Stahlschmidt SR, Ulfenborg B, Synnergren J. Multimodal deep learning for biomedical data fusion: a review. Brief Bioinform 2022;23:bbab569. [Crossref] [PubMed]
- Singh SP, Wang L, Gupta S, Goli H, Padmanabhan P, Gulyás B. 3D Deep Learning on Medical Images: A Review. Sensors (Basel) 2020;20:5097. [Crossref] [PubMed]
- Xiao H, Teng X, Liu C, Li T, Ren G, Yang R, Shen D, Cai J. A review of deep learning-based three-dimensional medical image registration methods. Quant Imaging Med Surg 2021;11:4895-916. [Crossref] [PubMed]


