
Radiation-induced aberrant structural covariance networks in patients with nasopharyngeal carcinoma: a source-based morphometry study
Introduction
Nasopharyngeal carcinoma (NPC) is a common malignancy in the nasopharyngeal cavity. It is biologically invasive and geographically restricted (1-3). Although radiotherapy (RT) has made considerable improvements in disease control and survival (2), radiation-induced brain injury (RBI), a delayed radiation-induced complication, could result in severe psychological and cognitive problems and seriously impact the patient’s quality of life (4). Hence, elucidating the pathogenesis of RBI is of great clinical significance.
Structural magnetic resonance imaging (sMRI) studies were conducted to investigate morphological alterations at different stages of RBI showing a more distributed structural abnormality as the duration of illness increase. Using diffusion tensor imaging (DTI) and voxel-based morphometry (VBM), a recent meta-analyses study reported consistent structural changes in temporal regions (5). Further structural studies also revealed abnormal grey matter (GM) volume and cortical thickness in various brain regions such as the cerebellum, inferior parietal, and occipital regions (6,7). These voxel or surface-based structural studies shed light on the insufficient understanding of the topographic changes of RBI, although far less attention has been paid to the radiation-induced abnormalities in the integration of spatial information (covariation) of distant brain regions or voxels/vertices (6,8-12).
Recently, source-based morphometry (SBM) has offered a novel means for examining alterations in whole-brain structural covariance networks. This multivariate and data-driven approach employs independent component analysis (ICA) to segment GM images and extract clusters of GM voxels that covary among participants, thereby serving as structural networks (13). SBM could identify covarying networks across distinct voxels and is widely used in brain network analysis (14). Previous studies have demonstrated that the structural covariance patterns of brain regions are highly correlated with functional networks, as indicated by their significant spatial overlap (15,16). Recently, researchers found that structural networks were enriched with brain local molecular and cellular metadata and could provide more nuanced representations of functional networks and properties (17). SBM has extensively characterized variations in structural brain networks, not only in healthy cases but also across a spectrum of cognitive and psychiatric conditions, including those influenced by intelligence and gender (18,19), schizophrenia (20,21), and depression (22,23). These studies collectively underscore the utility of SBM for elucidating structural covariance patterns in the brain, highlighting its efficacy in both normative populations and various neurological disorders. However, there are few SBM studies investigating the radiation-induced abnormalities of structural covariance networks in patients with NPC.
In this study, our aim was to explore brain structural abnormalities within the structural covariance networks of patients with NPC. An innovative integration of the SBM with the VBM was employed to derive structural covariance networks from GM data. Given its merits of not requiring region of interest (ROI) predefinition, and accurate acquisition of brain signals, we assume that SBM would effectively detect subtle changes in the structural covariance networks in patients with NPC after RT, which could provide a better understanding of the neural mechanisms underlying RBI. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-270/rc).
Methods
Cases
The study population comprised 131 patients with NPC, 47 of whom were in the pre-RT group, and 84 in the post-RT group. Tumors were staged utilizing the American Joint Committee on Cancer tumor-node-metastasis (AJCC TNM) classification, 7th edition [2009], ranging from T1N0M0 to T4N3M0 for both pre- and post-RT cohorts, with the latter also including T1N1M0. Intensity-modulated RT (IMRT) (24) and two-dimensional conventional RT (2D-CRT) (25) were performed on all the patients in the RT group. The detailed data of the RT regimen can be found in our previous works; RT irradiation was also performed as shown in Figure S1. For NPC patients classified as stages IIb to IVa–b, concurrent chemoradiotherapy, optionally supplemented with neoadjuvant or adjuvant chemotherapy, was recommended. This treatment strategy was administered 1–3 months before or after RT. The chemotherapy agents possibly included cisplatin, nedaplatin, paclitaxel, and fluorouracil. Since morphological changes in GM are most prominent during the delayed radiation reaction phase in NPC following RT (11,26), we chose to collect magnetic resonance imaging (MRI) data at least 6 months post-RT to better capture the enduring effects of RT on brain structure. This prospective study was approved by the Medical Research Ethics Committee of Xiangya Hospital (No. 201101006) and written informed consent was provided by all participants. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
MRI acquisition and image assessment
MRI data collection was conducted prospectively utilizing a 3.0-T Siemens Magnetom Tim Trio scanner (Siemens, Erlangen, Germany) with a 32-channel head coil. The protocol included routine imaging sequences: axial T1-weighted, T2-weighted, and T2-fluid-attenuated inversion recovery (FLAIR), for the detection of subclinical lesions. For high-resolution whole-brain structural assessment, a T1-weighted three-dimensional (3D) magnetization-prepared rapid acquisition with gradient echo (MPRAGE) sequence was applied, generating 176 sagittal slices at 1.0 mm thickness with no interslice gap, a 256×256 matrix, a 256 mm × 256 mm field of view (FOV), a repetition time (TR) of 2,300 ms, an echo time (TE) of 2.98 ms, a 9-degree flip angle, and isotropic voxels measuring 1.0 mm3.
VBM processing
Structural MRI data were preprocessed using Computational Anatomy Toolbox (CAT12; http://dbm.neuro.uni-jena.de/cat) and SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) based on the MATLAB 2014a operating environment (MathWorks, Natick, MA, USA). In brief, the T1-weighted images were initially segmented into GM and white matter compartments within their native space. Subsequently, these images underwent modulation and normalization to align with the standardized Montreal Neurological Institute (MNI) space, employing the Diffeomorphic Anatomical Registration Through Exponential Lie Algebra (DARTEL) algorithm. After that, GM images were resampled to 1.5 mm × 1.5 mm × 1.5 mm voxels. The modulated and resampled GM images were then smoothed using an 8 mm full-width at half maximum Gaussian kernel. Finally, the smoothed GM images were entered into a two-tailed t-test in SPM12 to identify voxels showing significant between-group GM differences [false discovery rate (FDR) corrected with P<0.05]. Age, gender, and GM volume were included as covariates.
SBM analysis
Based on the preprocessed GM images, SBM analysis was performed using the GIFT Toolbox (https://trendscenter.org/software). In this study, we consulted prior literature and selected 15 as the number of independent components (ICs) for the subsequent analysis (18). Next, ICA was performed using the Infomax algorithm. The ICASSO algorithm was applied iteratively to the process, resulting in 10 repetitions to ascertain the stability and reliability of the component outputs. More information on ICASSO can be found at http://research.ics.aalto.fi/ica/icasso/. Each GM image was converted into a one-dimensional vector and arrayed into a 131 (cases) row by GM matrix. The matrix was then decomposed into a mixing matrix (cases by components) and source matrix (components by voxels). The process involved calculating “loading coefficients” to assess the contribution of each structural component to the GM characteristics across 131 cases. Subsequently, these coefficients were converted to z-scores for subsequent analysis. The source matrix, detailing the associations between ICs and voxels, was instrumental for visualizing the components. For brain mapping, this matrix was reformatted into a 3D brain image, scaled to unit standard deviations to generate Z maps.
Statistical analyses
VBM analysis
The VBM analyses were conducted utilizing SPM12 to compare whole-brain GM volumes between the two study groups, employing a two-sample t-test with adjustment for age, gender, and total GM volume as covariates. This analysis resulted in statistical parametric maps, which underwent multiple comparison corrections using an FDR method (P<0.05).
SBM analysis
We performed SBM utilizing the dedicated SBM toolbox within the Matlab 2014a environment and compared the loading coefficients between the pre- and post-RT groups using two-sample t-tests, with age, gender, and GM volume serving as covariates. The obtained results were subjected to corrections using the FDR method, with a significance level set at P<0.05.
Association with clinical characteristics
We conducted a partial correlation analysis to determine the link between clinical characteristics and post-RT structural variations in NPC patients. This analysis evaluated the correlation between clinical parameters—specifically, mean and maximum radiation doses—and changes in GM volume, in addition to the loading coefficients of ICs that showed significant differences between groups. The analysis controlled for age, gender, and total GM volume, with statistical significance set at P<0.05.
To ascertain the potential correlations between clinical characteristics and the emergence of brain structural anomalies in patients with post-RT, we used partial correlation analysis to evaluate the relationship between clinical data (the mean and max radiation doses), altered GM volume, and the loading coefficients of ICs with significant group differences. Age, gender, and GM volume were included as covariates. Statistical significance was set at P<0.05.
Results
Demographic and clinical data analyses
The general clinical and demographic data are displayed in Table 1. The mean age of the post-RT group was significantly higher than that of the pre-RT group (P=0.023). No significant between-group differences were found in gender (P=0.427) and clinical staging (P=0.223).
Table 1
| Clinical characteristics | Pre-RT group (n=47) | Post-RT groups (n=84) | P value |
|---|---|---|---|
| Age (years) | 47±9.57 | 42.7±10.55 | 0.023* |
| Gender | 0.427 | ||
| Male | 35 (74.47) | 57 (67.86) | |
| Female | 12 (25.53) | 27 (32.14) | |
| AJCC stage | 0.223 | ||
| I/II | 14 (29.79) | 34 (40.48) | |
| III/IV | 33 (70.21) | 50 (59.52) |
Data are presented as mean ± SD or n (%). *, P<0.05. RT, radiation therapy; AJCC, American Joint Committee on Cancer; SD, standard deviation.
VBM analyses
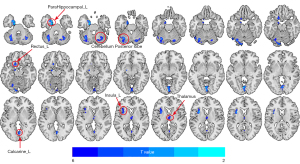
Compared with the pre-RT NPC group, the post-RT NPC patients exhibited notable reductions in GM volume in several brain regions. Specifically, these reductions were observed in the left temporal lobe, the left parahippocampus, and left uncus, as well as in the cerebellum (involving bilateral cerebellar Crus I, bilateral cerebellar Crus II, bilateral cerebellar VI, bilateral cerebellar VIIb, and the left cerebellar VIII). Additional reductions in GM volume were seen in the bilateral thalamus, left rectus, left insular, and various regions of the occipital lobe (including bilateral lingual gyrus and left calcarine). The involved brain regions are illustrated in Figure 1 and their detailed information are recorded in Table S1. Notably, no increased clusters of GM volume were found in the post-RT NPC patients. All results were reported using FDR corrected (P<0.05).
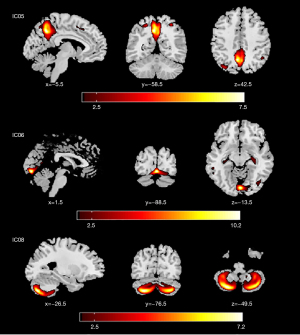
SBM analysis
Significant between-group differences were observed in three ICs, namely IC05, IC06, and IC08 (Figure 2, Tables S2,S3). The brain regions within IC06 included the bilateral hippocampus, parahippocampal gyrus, left middle temporal gyrus, and bilateral lingual gyrus. The brain regions within IC08 primarily localized in the cerebellar posterior lobe. Similarly, the brain regions within IC05 included bilateral precuneus, right inferior parietal lobule, posterior cingulate, and bilateral superior parietal lobe.
The average z-scores of IC06 and IC08 in the post-RT NPC group (0.014±0.004 and 0.012±0.006) were found to be significantly lower than those of the pre-RT NPC group (0.017±0.005 and 0.014±0.005). Additionally, the average z-score of IC05 in the post-RT NPC group (0.014±0.005) was significantly higher than that of the pre-RT NPC group (0.013±0.005).
Correlational results
Association of loading coefficients of the structural covariance network with clinical variables
A significant negative correlation was observed between the z-scores of the loading coefficients in the IC08 network (n=42) and the mean radiation doses received in the right temporal lobe region (r=−0.349, P=0.027). However, in the IC05 and IC06 networks, such correlations could not be observed.
Association of GM volumes with clinical variables
After adjusting the covariates of age and gender, a significant negative correlation was found between the maximum radiation doses of the brainstem region and the left parahippocampal gyrus (r=−0.380, P=0.014). Furthermore, a negative correlation was observed between the mean radiation doses of the brainstem region and both the right lingual gyrus (r=−0.471, P=0.002) and the left parahippocampal gyrus (r=−0.400, P=0.01).
Association of GM volumes with loading coefficients of the structural covariance network
After adjusting for the covariates of age, gender, and total GM volume, a positive correlation between the GM volumes of cerebellar clusters and the z-scores of the loading coefficients in the IC08 network could be observed in post-RT NPC group (r=0.516, 0.718, 0.785, all P<0.001). Notably, no significant correlation was observed between the GM volumes and loading coefficients in the IC05 and IC06 networks.
Discussion
To our knowledge, this is the first study combining both VBM and SBM to investigate alterations in structural covariance networks in NPC patients after RT. Compared with the pre-RT group, post-RT NPC patients showed reduced GM volume in several brain regions in the left temporal lobe, cerebellum, bilateral thalamus, left insular, and occipital lobe. Our SBM analyses revealed increased z-scores in brain regions within IC05, including the bilateral precuneus, right inferior parietal lobule, posterior cingulate, and bilateral superior parietal lobe, which topographically overlap with the posterior area of the default mode network (DMN). In contrast, we observed decreased z-scores in the temporal-occipital network and cerebellar network in post-RT NPC patients when compared to the pre-RT group. In addition, the significant correlation between radiation doses of the brainstem and the z-score of the cerebellar network suggested that the altered structural covariance network was mainly induced by RT. Our detection of aberrations within the structural covariance networks provided enhanced insights into the neural mechanisms underlying RBI.
Our VBM findings of reduced GM volume in the left temporal lobe, cerebellum, bilateral thalamus, left insular, and occipital lobe in post-RT NPC patients were in good agreement with previous functional and sMRI studies showing reduced GM volume in bilateral hippocampus, cortical thinning in bilateral inferior temporal gyri and insular gyrus, inward atrophy and decreased regional brain activities in bilateral thalamus, as well as decreased functional connectivity (FC) in the occipital lobe (7,8,27,28). The reported structural abnormalities in the cerebellum were, however, inconsistent due to the small sample employed and the varying disease duration (9,12,29). For example, some studies reported decreased cerebellar GM volume in NPC patients following RT (29), whereas others found opposite results (9,12). In this regard, our findings of decreased GM volume of posterior cerebellum provided further evidence for the radiation-induced cerebellar atrophy in NPC. As cortical volume is indicative of neuronal cell morphology and distribution (11,30), the observed GM volumetric reduction may suggest the occurrence of substantial pathological processes. Specifically, during RT for NPC patients, brain regions such as temporal lobes and cerebellum receive an intense radiation dose and have been associated with microcirculatory dysfunction, neuronal vulnerability, and localized inflammatory events (31-34), ultimately causing neuronal degeneration and apoptosis that are reflected in the macroscopic cortical volume loss.
The SBM analyses identified a total of 15 structural covariance networks, with significant differences detected in three of these networks. We found a decreased source matrix coefficient of the temporal-occipital network and cerebellar network in post-RT NPC patients. Our finding of the impaired temporal-occipital network could be partially supported by two graph-theory-based brain network studies showing that the node efficiency of frontotemporal network was decreased in NPC patients after RT (35,36). The temporal-occipital network (including the bilateral hippocampus, right middle temporal gyrus, and bilateral lingual gyrus) is functionally relevant to a series of biological processes such as emotional processing, cognitive control, and visual regulation (37). In fact, social and emotional disorders, neurocognitive deficits, and visual impairments are common clinical features in NPC patients with RBI and have been well-reported in previous neuropsychological studies (4,38,39). We therefore speculate that the observed abnormalities in the temporal-occipital network may be the structural substrates for the clinical symptoms in RBI. Our finding of decreased source matrix coefficient in the cerebellar network indicated that cerebellar function was impaired in NPC patients after RT, which is congruent with two previous functional MRI studies showing abnormal cerebellar perfusion and altered FC between subregions within the cerebellum (40,41). One possible explanation for this result is that the regionally decreased cerebellar GM volume may contribute to disrupting the structural covariation of different cerebellar subregions. This inner relationship is believed to be mediated mainly by radiation dose. These assumptions are supported by our finding of correlation analyses showing that the reduced GM volume was associated with the decreased source matrix coefficient of the cerebellar covariation network, and the mean and maximum radiation doses of the brainstem were negatively correlated with the z-score of the cerebellar network. As is well-known, the cerebellum plays an important role in controlling motor functions (such as balance and posture) as well as non-motor functions (such as cognition and emotion) (42,43), and the presenting impaired cerebellar network function signifies a series of motor and non-motor symptoms such as dysphagia and cognitive decline, which have been well-reported in NPC patients following RT (4,7,44).
The increased source matrix coefficient of the posterior DMN in post-RT NPC patients is of particular interest. This result aligns with prior research from an SBM study, which reported greater cortical thickness in the posterior cingulate cortex, inferior parietal cortex, and precuneus (core components of DMN) in the late-delayed stage RBI patients as compared to pre-RT NPC patients (7). Several structural and functional MRI studies, however, have reported reduced node efficiency, and decreased FC and local brain activity in DMN-related brain regions in NPC patents after RT (36,45), a finding that appears to conflict with our observations. This divergence could stem from variations in demographic parameters, analytical strategies, and/or disease stage of the patients included, and multicenter large sample studies are warranted for confirmative results. Our finding of a negative correlation between the source matrix coefficient of posterior DMN and that of the temporal-occipital network implies that the decreased structural covariations of temporal-occipital regions are associated with increased region-to-region structural connectivity of posterior DMN. Considering that the temporal-occipital network and DMN are jointly involved in various domains of cognitive and social processing (46-48), it is tempting to speculate that the observed increased structural covariations of posterior DMN regions may be a compensatory response for the functional impairments caused by damaged structural covariation of the temporal-occipital network. Nonetheless, the underlying basis of this phenomenon remains elusive and requires further research. Our SBM results are tightly linked to the present VBM findings. The potential implications of this statement are twofold. First, the spatial overlap between the brain regions with reduced GM volume (such as the left temporal lobe, cerebellum, and occipital lobe) and the involved areas of identified temporal-occipital and cerebellar network suggests that the radiation-induced regional cortical atrophy may be responsible for the abnormal structural covariation of the two networks. Second, after close inspection of the spatial distribution differences between these two approaches, we found that SBM could potentially be more sensitive than VBM in uncovering the radiation-induced morphologic abnormalities in NPC patients, as evidenced by the additional discovery of posterior DMN in SBM rather than VBM. As previously reported, in terms of utilizing the interrelationship among voxels, spatially filtering artificial sources, capturing the covariation of specific sources, and minimizing the number of comparisons, SBM is superior to VBM (13). Previous studies have shown that SBM may be more sensitive than VBM in detecting structural abnormalities, although opposite findings are occasionally reported (49-51). Taken together, the SBM and VBM are disparate methods in detecting abnormalities for the different structural domains, and simultaneous applications of both techniques can complement each other’s advantages to obtain different dimensional information and an accurate understanding of the neural mechanisms underlying RBI.
Several limitations in this study need to be acknowledged. First, it is important to note that this study was cross-sectional in nature and therefore could not establish causal relationships. Further longitudinal studies are necessary to validate these findings. Second, given the interaction of chemotherapy with RT, the chemotherapy-associated neurotoxic effects on the observed radiation-induced abnormalities in the brain covariance network could not be eliminated. Animal models are warranted to further clarify this issue. Third, detailed cognitive function was not performed, which weakens the interpretability of our findings. Fourth, subgroup analyses were not allowed due to the small sample size, making the present study unable to obtain the disease stage-dependent changes of the structural covariance network.
Conclusions
The present study revealed radiation-induced changes in structural covariance networks (including posterior DMN, temporal-occipital network, and cerebellar network) and cortical volume (mainly in the cerebellum, temporal, and occipital lobes) in patients with NPC. These findings shed some light on the neural basis of symptom patterns in RBI and may support the development of new intervention strategies to prevent progression to radiation-induced brain necrosis.
Acknowledgments
Funding: This study was supported in part by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-270/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-270/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Medical Research Ethics Committee of Xiangya Hospital (No. 201101006) and written informed consent was provided by all participants. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zeng H, Zheng R, Sun K, Zhou M, Wang S, Li L, Chen R, Han B, Liu M, Zhou J, Xu M, Wang L, Yin P, Wang B, You J, Wu J, Wei W, He J. Cancer survival statistics in China 2019-2021: a multicenter, population-based study. J Natl Cancer Cent 2024;4:203-13. [Crossref] [PubMed]
- Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet 2019;394:64-80. [Crossref] [PubMed]
- Liu H, Tang L, Li Y, Xie W, Zhang L, Tang H, Xiao T, Yang H, Gu W, Wang H, Chen P. Nasopharyngeal carcinoma: current views on the tumor microenvironment's impact on drug resistance and clinical outcomes. Mol Cancer 2024;23:20. [Crossref] [PubMed]
- Tang Y, Luo D, Rong X, Shi X, Peng Y. Psychological disorders, cognitive dysfunction and quality of life in nasopharyngeal carcinoma patients with radiation-induced brain injury. PLoS One 2012;7:e36529. [Crossref] [PubMed]
- Voon NS, Lau FN, Zakaria R, Md Rani SA, Ismail F, Manan HA, Yahya N. MRI-based brain structural changes following radiotherapy of Nasopharyngeal Carcinoma: A systematic review. Cancer Radiother 2021;25:62-71. [Crossref] [PubMed]
- Shi L, Du FL, Sun ZW, Zhang L, Chen YY, Xie TM, Li PJ, Huang S, Dong BQ, Zhang MM. Radiation-induced gray matter atrophy in patients with nasopharyngeal carcinoma after intensity modulated radiotherapy: a MRI magnetic resonance imaging voxel-based morphometry study. Quant Imaging Med Surg 2018;8:902-9. [Crossref] [PubMed]
- Lin J, Lv X, Niu M, Liu L, Chen J, Xie F, Zhong M, Qiu S, Li L, Huang R. Radiation-induced abnormal cortical thickness in patients with nasopharyngeal carcinoma after radiotherapy. Neuroimage Clin 2017;14:610-21. [Crossref] [PubMed]
- Lv XF, Zheng XL, Zhang WD, Liu LZ, Zhang YM, Chen MY, Li L. Radiation-induced changes in normal-appearing gray matter in patients with nasopharyngeal carcinoma: a magnetic resonance imaging voxel-based morphometry study. Neuroradiology 2014;56:423-30. [Crossref] [PubMed]
- Leng X, Fang P, Lin H, An J, Tan X, Zhang C, Wu D, Shen W, Qiu S. Structural MRI research in patients with nasopharyngeal carcinoma following radiotherapy: A DTI and VBM study. Oncol Lett 2017;14:6091-6. [Crossref] [PubMed]
- Lv X, He H, Yang Y, Han L, Guo Z, Chen H, Li J, Qiu Y, Xie C. Radiation-induced hippocampal atrophy in patients with nasopharyngeal carcinoma early after radiotherapy: a longitudinal MR-based hippocampal subfield analysis. Brain Imaging Behav 2019;13:1160-71. [Crossref] [PubMed]
- Zhang YM, Chen MN, Yi XP, Li L, Gao JM, Zhang JL, Yuan XR, Zhang N, Liu LZ, Cai PQ, Chen BT, Zee C, Liao WH, Zhang YC. Cortical Surface Area Rather Than Cortical Thickness Potentially Differentiates Radiation Encephalopathy at Early Stage in Patients With Nasopharyngeal Carcinoma. Front Neurosci 2018;12:599. [Crossref] [PubMed]
- Zhu W, Chen F, Yin D, Chen K, Wang S. Changes in brain gray matter volume in nasopharyngeal carcinoma patients after radiotherapy in long-term follow-up. Braz J Otorhinolaryngol 2023;89:477-84. [Crossref] [PubMed]
- Xu L, Groth KM, Pearlson G, Schretlen DJ, Calhoun VD. Source-based morphometry: the use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum Brain Mapp 2009;30:711-24. [Crossref] [PubMed]
- Gupta CN, Turner JA, Calhoun VD. Source-based morphometry: a decade of covarying structural brain patterns. Brain Struct Funct 2019;224:3031-44. [Crossref] [PubMed]
- Zhang X, Lai H, Li Q, Yang X, Pan N, He M, Kemp GJ, Wang S, Gong Q. Disrupted brain gray matter connectome in social anxiety disorder: a novel individualized structural covariance network analysis. Cereb Cortex 2023;33:9627-38. [Crossref] [PubMed]
- Dong C, Thalamuthu A, Jiang J, Mather KA, Sachdev PS, Wen W. Brain structural covariances in the ageing brain in the UK Biobank. Brain Struct Funct 2024;229:1165-77. [Crossref] [PubMed]
- Suárez LE, Markello RD, Betzel RF, Misic B. Linking Structure and Function in Macroscale Brain Networks. Trends Cogn Sci 2020;24:302-15. [Crossref] [PubMed]
- Ge R, Liu X, Long D, Frangou S, Vila-Rodriguez F. Sex effects on cortical morphological networks in healthy young adults. Neuroimage 2021;233:117945. [Crossref] [PubMed]
- Ge R, Ching CRK, Bassett AS, Kushan L, Antshel KM, van Amelsvoort T, et al. Source-based morphometry reveals structural brain pattern abnormalities in 22q11.2 deletion syndrome. Hum Brain Mapp 2024;45:e26553. [Crossref] [PubMed]
- Kaspárek T, Marecek R, Schwarz D, Prikryl R, Vanícek J, Mikl M, Cesková E. Source-based morphometry of gray matter volume in men with first-episode schizophrenia. Hum Brain Mapp 2010;31:300-10. [Crossref] [PubMed]
- Van Assche L, Takamiya A, Van den Stock J, Van de Ven L, Luyten P, Emsell L, Vandenbulcke M. A voxel- and source-based morphometry analysis of grey matter volume differences in very-late-onset schizophrenia-like psychosis. Psychol Med 2024;54:592-600. [Crossref] [PubMed]
- Ge R, Downar J, Blumberger DM, Daskalakis ZJ, Lam RW, Vila-Rodriguez F. Structural network integrity of the central executive network is associated with the therapeutic effect of rTMS in treatment resistant depression. Prog Neuropsychopharmacol Biol Psychiatry 2019;92:217-25. [Crossref] [PubMed]
- Wang K, Hu Y, Yan C, Li M, Wu Y, Qiu J, Zhu X. Brain structural abnormalities in adult major depressive disorder revealed by voxel- and source-based morphometry: evidence from the REST-meta-MDD Consortium. Psychol Med 2023;53:3672-82. [Crossref] [PubMed]
- Zhang MX, Li J, Shen GP, Zou X, Xu JJ, Jiang R, You R, Hua YJ, Sun Y, Ma J, Hong MH, Chen MY. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: A 10-year experience with a large cohort and long follow-up. Eur J Cancer 2015;51:2587-95. [Crossref] [PubMed]
- Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, Sun Y, Lin AH, Liu MZ, Ma J. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys 2011;80:661-8. [Crossref] [PubMed]
- Lv X, Guo Z, Tang L, Li Z, Lin X, Li J, Han L, Qiu Y, Mai H. Divergent effects of irradiation on brain cortical morphology in patients with nasopharyngeal carcinoma: one-year follow-up study using structural magnetic resonance imaging. Quant Imaging Med Surg 2021;11:2307-20. [Crossref] [PubMed]
- Nan F, Gao JM, Li L, Zhang YM, Zhang Y. Interaction of chemotherapy and radiotherapy in altering the shape of subcortical structures in patients with nasopharyngeal carcinoma. Front Oncol 2022;12:952983. [Crossref] [PubMed]
- Zhang YM, Gao JM, Zhou H, Li L, Liu LZ, Han ZD, Yi XP, Liao WH. Pre-symptomatic local brain activity and functional connectivity alterations in nasopharyngeal carcinoma patients who developed radiation encephalopathy following radiotherapy. Brain Imaging Behav 2020;14:1964-78. [Crossref] [PubMed]
- Hu F, Li T, Wang Z, Zhang S, Wang X, Zhou H, Qiu S. Use of 3D-ASL and VBM to analyze abnormal changes in brain perfusion and gray areas in nasopharyngeal carcinoma patients undergoing radiotherapy. Biomed Res 2017;28:7879-85.
- Rimol LM, Nesvåg R, Hagler DJ Jr, Bergmann O, Fennema-Notestine C, Hartberg CB, Haukvik UK, Lange E, Pung CJ, Server A, Melle I, Andreassen OA, Agartz I, Dale AM. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry 2012;71:552-60.
- Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: A review. Front Oncol 2012;2:73. [Crossref] [PubMed]
- Yang L, Yang J, Li G, Li Y, Wu R, Cheng J, Tang Y. Pathophysiological Responses in Rat and Mouse Models of Radiation-Induced Brain Injury. Mol Neurobiol 2017;54:1022-32. [Crossref] [PubMed]
- Zhang YM, Kang YF, Zeng JJ, Li L, Gao JM, Liu LZ, Shi LR, Liao WH. Surface-Based Falff: A Potential Novel Biomarker for Prediction of Radiation Encephalopathy in Patients With Nasopharyngeal Carcinoma. Front Neurosci 2021;15:692575. [Crossref] [PubMed]
- Shamsesfandabadi P, Patel A, Liang Y, Shepard MJ, Wegner RE. Radiation-Induced Cognitive Decline: Challenges and Solutions. Cancer Manag Res 2024;16:1043-52. [Crossref] [PubMed]
- Fu G, Xie Y, Pan J, Qiu Y, He H, Li Z, Li J, Feng Y, Lv X. Longitudinal study of irradiation-induced brain functional network alterations in patients with nasopharyngeal carcinoma. Radiother Oncol 2022;173:277-84. [Crossref] [PubMed]
- Zhang X, Pan J, Lin Y, Fu G, Xu P, Liang J, Ye C, Peng J, Lv X, Yang Y, Feng Y. Structural network alterations in patients with nasopharyngeal carcinoma after radiotherapy: A 1-year longitudinal study. Front Neurosci 2022;16:1059320. [Crossref] [PubMed]
- Beauchamp MS. See me, hear me, touch me: multisensory integration in lateral occipital-temporal cortex. Curr Opin Neurobiol 2005;15:145-53. [Crossref] [PubMed]
- Li Z, Zhan Z, Xiao J, Lan Y. Radiation-Induced Optical Coherence Tomography Angiography Retinal Alterations in Patients With Nasopharyngeal Carcinoma. Front Med (Lausanne) 2020;7:630880. [Crossref] [PubMed]
- Chow JCH, Lee J, Lai MMP, Li S, Lau AMC, Ng BSY, Leung GGG, Li STY, Lui JCF, Cheung KM, Au KH, Wong KH, Lau AYL, Zee BCY. Multi-domain neurocognitive impairment following definitive intensity-modulated radiotherapy for nasopharyngeal cancer: A cross-sectional study. Radiother Oncol 2024;193:110143. [Crossref] [PubMed]
- Ma Q, Zeng LL, Qin J, Luo Z, Su J, Wu D, Qiu S, Hu D. Radiation-induced cerebellar-cerebral functional connectivity alterations in nasopharyngeal carcinoma patients. Neuroreport 2017;28:705-11. [Crossref] [PubMed]
- Hu F, Zheng XH, Li T, She HL, Zhang SF. Brain Perfusion Abnormalities after Radiotherapy Measured by 3-Dimensional Arterial Spin Labeling MRI and Correlations with Cognitive Impairment. Radiat Res 2022;197:324-31. [Crossref] [PubMed]
- Jacobi H, Faber J, Timmann D, Klockgether T. Update cerebellum and cognition. J Neurol 2021;268:3921-5. [Crossref] [PubMed]
- Rudolph S, Badura A, Lutzu S, Pathak SS, Thieme A, Verpeut JL, Wagner MJ, Yang YM, Fioravante D. Cognitive-Affective Functions of the Cerebellum. J Neurosci 2023;43:7554-64. [Crossref] [PubMed]
- Voon NS, Manan HA, Yahya N. Remote assessment of cognition and quality of life following radiotherapy for nasopharyngeal carcinoma: deep-learning-based predictive models and MRI correlates. J Cancer Surviv 2024;18:1297-308. [Crossref] [PubMed]
- Ding Z, Zhang H, Lv XF, Xie F, Liu L, Qiu S, Li L, Shen D. Radiation-induced brain structural and functional abnormalities in presymptomatic phase and outcome prediction. Hum Brain Mapp 2018;39:407-27. [Crossref] [PubMed]
- Rolls ET. The cingulate cortex and limbic systems for action, emotion, and memory. Handb Clin Neurol 2019;166:23-37. [Crossref] [PubMed]
- Menon V. 20 years of the default mode network: A review and synthesis. Neuron 2023;111:2469-87. [Crossref] [PubMed]
- Xiao D, Li J, Ren Z, Dai M, Jiang Y, Qiu T, Zhang H, Chen Y, Zhang Y, Zhang Y, Palaniyappan L. Association of cortical morphology, white matter hyperintensity, and glymphatic function in frontotemporal dementia variants. Alzheimers Dement 2024;20:6045-59. [Crossref] [PubMed]
- Harenski CL, Harenski KA, Calhoun VD, Kiehl KA. Source-based morphometry reveals gray matter differences related to suicidal behavior in criminal offenders. Brain Imaging Behav 2020;14:1-9. [Crossref] [PubMed]
- Pappaianni E, Siugzdaite R, Vettori S, Venuti P, Job R, Grecucci A. Three shades of grey: detecting brain abnormalities in children with autism using source-, voxel- and surface-based morphometry. Eur J Neurosci 2018;47:690-700. [Crossref] [PubMed]
- Kunst J, Marecek R, Klobusiakova P, Balazova Z, Anderkova L, Nemcova-Elfmarkova N, Rektorova I. Patterns of Grey Matter Atrophy at Different Stages of Parkinson's and Alzheimer's Diseases and Relation to Cognition. Brain Topogr 2019;32:142-60. [Crossref] [PubMed]


