
Intravoxel incoherent motion diffusion-weighted imaging in nasopharyngeal carcinoma: comparison of the turbo spin-echo and echo-planar imaging techniques
Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial carcinoma originating from the mucous membrane of the nasopharynx. It is more common in the pharyngeal recess and predominantly occurs in Southeast Asia (1,2). The gold standard for diagnosing NPC remains tissue biopsy (3,4), but this is an invasive procedure unsuitable for screening and follow-up (5). Magnetic resonance imaging (MRI), a common and noninvasive method, offers high soft tissue contrast and resolution, enabling clear depiction of lesion morphology boundaries, surrounding invasion, and cervical lymph node metastasis (6,7).
In recent years, studies have explored the use of contrast-enhanced T1-weighted imaging (8,9), diffusion-weighted imaging (DWI) (10), and intravoxel incoherent motion (IVIM) (11) for diagnosing NPC and evaluating tumor response. Contrast-enhanced T1-weighted imaging requires contrast agents that some patients may be allergic to. Meanwhile, DWI is a noncontrast MRI technique that reflects the diffusion of water molecules in tissues (12), but it suffers from low resolution and is prone to image distortion. The apparent diffusion coefficient (ADC) derived from the monoexponential model cannot distinguish between perfusion and diffusion, limiting its ability to reflect water molecule diffusion accurately (13). IVIM is an advanced method for DWI analysis that uses a biexponential model to separate pure water diffusion from tissue microcirculation, overcoming some of the limitations of traditional DWI (14). Given the nasopharynx’s special anatomical location and NPC’s tendency for skull base invasion, traditional DWI can cause image distortion at the skull base, affecting diagnostic efficacy. Traditional IVIM relies on single-shot echo-planar imaging (EPI), which is prone to susceptibility artifacts and can lead to geometric distortion and signal loss, potentially reducing image quality and affecting the diagnosis or measurement of ADC and IVIM-derived parameters (15,16). In one study, IVIM DWI for NPCs was found to be unsuccessful in approximately 8.7% of cases (17), and EPI-IVIM has demonstrated limited accuracy in assessing small NPCs (18,19).
Turbo spin echo (TSE) employs radiofrequency refocusing pulses to reduce image distortion and enhance image quality. Reports indicate that TSE DWI can provide high image quality in the context of oral cancer (20), lung cancer (21), pituitary tumors (22), and even postoperatively in spines with metallic implants (23). Building on these successful applications, we aimed to apply TSE to patients with NPC to subjectively and objectively evaluate IVIM images of nasopharyngeal lesions, nasal concha, the spinal cord, and the temporal pole. Additionally, we sought to propose appropriate scanning strategies based on the interrelationships among these indicators. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1021/rc).
Methods
Patients
Thirty inpatients with pathologically confirmed NPC who underwent TSE-IVIM and EPI-IVIM examinations in the Department of Radiology of the Cancer Center of Union Hospital in Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) between March 2022 and August 2022 were included in this study. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of Union Hospital in Tongji Medical College, Huazhong University of Science and Technology (No. UHCT-IEC-SOP-016-03-01). Informed consent was obtained from all the patients.
The cohort included 24 males and 6 females, with ages ranging from 29 to 71 years and an average age of 47.6±12.3 years. According to the eight edition of the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) staging criteria for NPC (24), 5 cases were T2 stage, 7 cases were T3 stage, and 18 cases were T4 stage. Inclusion criteria for patients were as follows: (I) pathological confirmation of NPC; (II) no treatment prior to TSE-IVIM and EPI-IVIM examinations; and (III) no contraindications to MRI.
Imaging protocol
All imaging was conducted using a 3-T MRI system with a 24-channel head and neck coil (Ingenia, Philips Healthcare, Andover, MA, USA). The TSE-IVIM and EPI-IVIM sequences were performed consecutively in each examination, with scanning parameters kept as consistent as possible between the two sequences. The specific scanning parameters are detailed in Table 1. For fitting the biexponential model, eight b-values (0, 20, 40, 80, 160, 300, 500, and 800 s/mm2, with averages of 1, 1, 1, 1, 1, 1, 1, and 2, respectively) were applied for both sequences.
Table 1
| Parameter | T2WI | TSE-IVIM | EPI-IVIM |
|---|---|---|---|
| TR/TE (ms) | 3,144/120 | 2,000/108 | 2,233/91 |
| FOV (mm2) | 220×225 | 230×230 | 230×230 |
| Matrix size | 252×225 | 92×92 | 92×92 |
| Reconstruction voxel size (mm) | 0.6×0.6 | 1.2×1.2 | 1.03×1.03 |
| TSE/EPI factor | – | 15 (TSE) | 61 (EPI) |
| SENSE factor | 2 | 2.5 | 2.5 |
| Slice thickness (mm) | 4 | 5 | 5 |
| Slice spacing | 1 | 1 | 1 |
| Flip angle (°) | 90 | 90 | |
| bandwidth (Hz/pixel) | 239.6 | 862.7 | 2,068.8 |
| Acquisition time | 1 min 24 s | 6 min 44 s | 56 s |
T2WI, T2-weighted imaging; TSE, turbo spin echo; DWI, diffusion-weighted imaging; EPI, echo-planar imaging; TR, repetition time; TE, echo time; FOV, field of view; SENSE, sensitivity encoding.
Image quality
Two experts with 10 years of experience in head-and-neck radiology who had no knowledge of the scan sequences or clinical information independently conducted the qualitative and quantitative evaluations of the IVIM images. The signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) of the TSE-IVIM and EPI-IVIM sequences at b=800 s/mm2 were recorded. SNR and CNR were calculated in several regions of the images. Specifically, circular regions of interest (ROIs) were placed over the nasopharyngeal lesion, nasal concha, spinal cord, and temporal pole. Given the significant heterogeneity of the target tissue, such as the nasal concha and nasopharyngeal lesions, the standard deviation (SD) of the target tissue can lead to overestimation of image noise. Therefore, the SD of the sinuses close to the target tissue was used to estimate image noise. The mean signal intensity (SI) and SD were recorded for each ROI, and SNR and CNR were calculated using the following formulae:
where SI(a) is the mean SI in the structures of interest, SD(sinus) is the SD of the SI in the sinuses, and SI(muscle) and SD(muscle) are the mean SI and SD of the SI in the masseter muscle, respectively (25).
We used a five-point scale to evaluate image quality, as has been employed in numerous studies (26,27). The evaluation parameters included susceptibility artifacts, geometric distortion, lesion conspicuity, and overall image quality. Susceptibility artifacts were rated as follows: (I) artifacts present in the lesion affecting diagnosis; (II) artifacts present in three or more areas but not affecting diagnosis; (III) artifacts present in three areas but not affecting diagnosis; (IV) artifacts present in fewer than three areas and not affecting diagnosis; and (V) almost no artifacts. Geometric distortion was rated as follows: (I) severe artifact with no diagnostic confidence; (II) obvious artifact with limited diagnostic confidence; (III) moderate artifact impairing diagnostic confidence; (IV) slight artifact not affecting diagnostic confidence; and 5, no geometric distortion with full diagnostic confidence. Lesion conspicuity was rated as follows: (I) unacceptable; (II) poor; (III) acceptable; (IV) good; and (V) excellent. Finally, overall image quality was rated as follows: (I) unacceptable; (II) poor; (III) acceptable; (IV) good; and (V) excellent.
ADC and IVIM-derived parameters
Digital Imaging and Communications in Medicine (DICOM) images from the IVIM acquisition were postprocessed using a vendor-provided advanced diffusion analysis (ADA) tool (IntelliSpace Portal 10.1, Philips Healthcare). The biexponential model was used to calculate true diffusion coefficient (D), reflecting the Brownian motion of water molecules; the perfusion fraction (f), representing the proportion of overall signal attenuation; and the pseudodiffusion coefficient (D*), which is related to tissue perfusion (28). When b is between 0 and 200 s/mm2, the signal attenuation primarily reflects microvascular perfusion information and quantifies D*. When b>200 s/mm2, it reflects the main diffusion situation and quantifies D. Values of b below 200 s/mm2 were excluded from the fit for the simplified IVIM model to avoid bias due to the perfusion effect. ADC maps were calculated using the MR diffusion tool (IntelliSpace Portal 10.1), incorporating multiple b-values with a monoexponential model. Two radiologists delineated the ROIs of the nasopharyngeal lesions, nasal concha, spinal cord, and temporal pole on the DWI images, respectively. Each ROI was drawn three times, with mucus and necrosis being avoided as much as possible. Finally, the ADC, D, f, and D* values were calculated corresponding to the ROIs defined on the relevant images.
Statistical analysis
All statistical analyses were conducted using SPSS 26 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA), with P<0.05 indicating a statistically significant difference. The subjective evaluation of the TSE-IVIM and EPI-IVIM sequences was performed using the Wilcoxon signed rank test. The Wilcoxon signed rank test for two paired samples was used to compare the SNR, CNR, ADC, and IVIM-derived parameters between the two sequences. Additionally, Bland-Altman plots with 95% limits of agreement (LoAs) were used to analyze the consistency of the ADC and IVIM derived-parameters for TSE-IVIM and EPI-IVIM. The coefficient of variation (CV) was used to measure the degree of variation in the data.
Results
Comparison of subjective evaluation
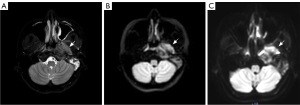
The subjective evaluation results of TSE-IVIM and EPI-IVIM are shown in Table 2. Representative images of TSE, EPI, and T2-weighted imaging (T2WI) Dixon are presented in Figure 1. In the comparison of susceptibility artifacts, geometric distortion, lesion conspicuity, and overall image quality, there was a statistical difference between the subjective evaluation results of the TSE-IVIM and EPI-IVIM sequences for nasopharyngeal lesion, nasal concha, and the temporal pole, with the TSE-IVIM sequence being significantly better than the EPI-IVIM sequence. However, for the spinal cord, there was no statistically significant difference between the two sequences in terms of susceptibility artifacts, geometric distortion, lesion conspicuity, or overall image quality, and the TSE-IVIM sequence did not demonstrate superiority.
Table 2
| Comparison | Susceptibility artifacts | Geometric distortion | Lesion conspicuity | Overall image quality |
|---|---|---|---|---|
| Nasopharyngeal lesion | ||||
| TSE-IVIM | 5 [5, 5] | 4 [5, 4] | 5 [5, 4] | 5 [5, 4] |
| EPI-IVIM | 3 [4, 1] | 3 [4, 1] | 3 [4, 1] | 3 [4, 1] |
| Z | −4.878 | −4.710 | −4.712 | −4.796 |
| P | <0.001 | <0.001 | <0.001 | <0.001 |
| Nasal concha | ||||
| TSE-IVIM | 5 [5, 4] | 4 [4, 3] | 4 [5, 3] | 4 [5, 4] |
| EPI-IVIM | 2 [3, 1] | 2 [3, 1] | 2 [4, 1] | 2 [3, 1] |
| Z | −4.871 | −4.966 | −4.815 | −4.901 |
| P | <0.001 | <0.001 | <0.001 | <0.001 |
| Spinal cord | ||||
| TSE-IVIM | 5 [5, 5] | 4 [5, 4] | 5 [5, 4] | 4 [5, 4] |
| EPI-IVIM | 5 [5, 5] | 4 [4, 4] | 5 [5, 4] | 4 [5, 4] |
| Z | 0 | −1.00 | −0.277 | −1.00 |
| P | 1 | 0.317 | 0.782 | 0.317 |
| Temporal pole | ||||
| TSE-IVIM | 5 [5, 4] | 4 [5, 4] | 4 [5, 4] | 4 [5, 4] |
| EPI-IVIM | 3 [4, 2] | 3 [4, 2] | 4 [4, 2] | 3 [4, 2] |
| Z | −5.106 | −4.730 | −3.753 | −4.702 |
| P | <0.001 | <0.001 | <0.001 | <0.001 |
Data are presented as median [maximum, minimum]. Wilcoxon signed rank test. TSE, turbo spin echo; IVIM, intravoxel incoherent motion; EPI, echo-planar imaging.
Comparison of SNR and CNR
As shown in Table 3, there was a statistically significant difference in CNR between the TSE-IVIM and EPI-IVIM sequences for the nasopharyngeal lesion and nasal concha, while the SNR in TSE-IVIM had no statistical difference compared to that of the EPI-IVIM sequence. The spinal cord and temporal pole exhibited significantly higher SNR for EPI-IVIM than for TSE-IVIM, but there was no significant difference in CNR.
Table 3
| Anatomical structure | SNR | CNR | |||||
|---|---|---|---|---|---|---|---|
| TSE-IVIM | EPI-IVIM | P | TSE-IVIM | EPI-IVIM | P | ||
| Nasopharyngeal lesion | 74.6±28.3 | 72.7±28.4 | 0.926 | 56.8±26.9 | 41.4±23.9 | 0.003* | |
| Nasal concha | 50.0±15.1 | 47.7±24.4 | 0.428 | 34.8±19.0 | 25.5±16.9 | 0.01* | |
| Spinal cord | 73.4±24.2 | 84.7±19.2 | 0.027* | 53.9±22.6 | 49.7±20.2 | 0.245 | |
| Temporal pole | 82.2±21.5 | 98.6±23.7 | 0.026* | 61.9±23.0 | 57.6±24.4 | 0.299 | |
Data are presented as the mean ± standard deviation. Wilcoxon signed rank test. *, P<0.05. SNR, signal-to-noise ratio; CNR, contrast-to-noise ratio; TSE, turbo spin echo; IVIM, intravoxel incoherent motion; EPI, echo-planar imaging.
Comparison of ADC and IVIM-derived parameters
The ADC and IVIM-derived parameters for TSE-IVIM and EPI-IVIM sequences are presented in Table 4. Representative images of the D, f, and D* maps for TSE and EPI are shown in Figure 2. Analysis revealed no statistically significant difference in D between the TSE-IVIM and EPI-IVIM sequences for the nasopharyngeal lesions, nasal concha, spinal cord, or temporal pole. However, for the spinal cord, the ADC and D* values for the TSE-IVIM sequence were significantly higher than those for EPI-IVIM. Additionally, for the nasopharyngeal region, the f value for the TSE-IVIM sequence was lower than that for the EPI-IVIM sequence.
Table 4
| Anatomical structure | ADC (10−3 mm2/s) | D (10−3 mm2/s) | f | D* (10−3 mm2/s) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSE-IVIM | EPI-IVIM | P | TSE-IVIM | EPI-IVIM | P | TSE-IVIM | EPI-IVIM | P | TSE-IVIM | EPI-IVIM | P | ||||
| Nasopharyngeal lesion | 0.76±0.14 | 0.77±0.18 | 0.366 | 0.67±0.14 | 0.67±0.18 | 0.914 | 0.10±0.05 | 0.14±0.07 | 0.004* | 23.99±21.23 | 23.99±21.23 | 0.644 | |||
| Nasal concha | 1.75±0.22 | 1.68±0.29 | 0.658 | 1.56±0.23 | 1.46±0.24 | 0.086 | 0.20±0.11 | 0.22±0.12 | 0.321 | 19.59±11.50 | 22.04±20.46a | 0.82 | |||
| Spinal cord | 1.03±0.11 | 0.91±0.11 | 0.000* | 0.89±0.11 | 0.86±0.12 | 0.124 | 0.08±0.12 | 0.06±0.06 | 0.808 | 9.11±2.83 | 6.59±2.73 | 0.001* | |||
| Temporal pole | 0.88±0.07 | 0.87±0.07 | 0.537 | 0.77±0.05 | 0.79±0.08 | 0.247 | 0.09±0.04 | 0.08±0.05 | 0.970 | 5.47±1.32 | 10.09±14.98 | 0.637 | |||
Data are presented as the mean ± standard deviation. Wilcoxon signed rank test. *, P<0.05; a, n=29, one participant was excluded due to having abnormally high values. ADC, apparent diffusion coefficient; IVIM, intravoxel incoherent motion; TSE, turbo spin echo; EPI, echo-planar imaging; D, true diffusion coefficients; f, perfusion fraction; D*, pseudodiffusion coefficient.
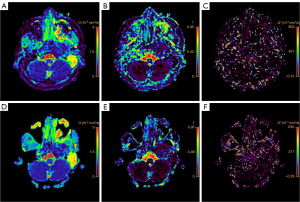
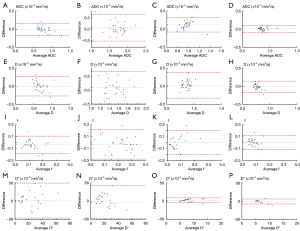
Table 5 shows the LoAs and CV for the ADC and IVIM-derived parameters between TSE-IVIM and EPI-IVIM. The 95% LoAs for the nasopharyngeal lesion were −22.5% to 21.2% for ADC, −39.9% to 43.2% for D, −163.6% to 104.2% for f, and −160.8% to 174.5% for D*. For the CV, the ADC and D values were relatively stable in the spinal cord and temporal pole. The Bland-Altman plots in Figure 3 illustrate the consistency of ADC and IVIM-derived parameters between the two sequences.
Table 5
| Anatomical structure | LoA (%) | CV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ADC | D | f | D* | ADC | D | f | D* | ||
| Nasopharyngeal lesion | −22.5 to 21.2 | −39.9 to 43.2 | −163.6 to 104.2 | −160.8 to 174.5 | 18.3 vs. 23.5 | 20.4 vs. 27.5 | 50.5 vs. 67.4 | 90.1 vs. 100.6 | |
| Nasal concha | −35.5 to 44.9 | −33.2 to 47.9 | −195.1 to 186.5 | −161.3 to 173.8 | 12.8 vs. 17.2 | 14.5 vs. 16.7 | 54.0 vs. 51.8 | 57.4 vs. 300.7 | |
| Spinal cord | −7.1 to 31.4 | −34.0 to 39.2 | −345.5 to 270.5 | −40.72 to 106.6 | 10.7 vs. 11.8 | 12.0 vs. 14.8 | 149.7 vs. 96.9 | 31.0 vs. 41.4 | |
| Temporal pole | −10.0 to 11.1 | −21.3 to 17.6 | −127.7 to 144.9 | −82.8 to 67.1 | 8.4 vs. 8.6 | 6.00 vs. 10.00 | 43.6 vs. 54.6 | 24.1 vs. 148.4 | |
Data of the LoAs are the 95% LoAs. Data for the CV are TSE-IVIM versus EPI-IVIM. LoA, limit of agreement; CV, coefficient of variance; ADC, apparent diffusion coefficient; IVIM, intravoxel incoherent motion; TSE, turbo spin echo; EPI, echo-planar imaging; D, true diffusion coefficient; f, perfusion fraction; D*, pseudodiffusion coefficient.
Discussion
This study compared the qualitative and quantitative indicators between TSE-IVIM and EPI-IVIM in patients with NPC. We found that TSE-IVIM achieved better image quality with higher subjective scores and did not show a significantly lower SNR, while exhibiting a better CNR. TSE-IVIM provided more stable quantitative indicators for nasopharyngeal lesions and nasal concha, with reduced magnetic sensitivity artifacts.
As a common MR scanning sequence, EPI can be rapidly acquired by obtaining spatially encoded information after a single-shot excitation (29), while TSE uses radiofrequency refocusing pulses to reduce image distortion and improve quantitative parameter calculations (30). In our study, subjective scores for nasopharyngeal lesions, nasal concha, and the temporal pole were higher with the TSE sequence compared to with the EPI sequence, except for the spinal cord, where no significant difference was observed. It has been reported that EPI has two main disadvantages: low bandwidth and eddy currents (31). When the diffusion gradient pulse is switched on and off, eddy currents are generated. Additionally, although the acquisition time of EPI is short and the acquisition bandwidth large, the bandwidth in the phase encoding direction is relatively low, leading to local frequency offsets. This results in severe geometric deformation and magnetic sensitivity artifacts in EPI. In contrast, the TSE sequence employs refocused pulses to mitigate the effects of magnetic field nonuniformity and reduce phase error accumulation. Despite these advantages, we found no statistically significant difference in subjective scores for the spinal cord between the two sequences. The distortion and SNR of the spinal cord are relatively better compared to those of the nasopharyngeal lesion in EPI-DWI. This may be the reason why there was no difference in subjective scores of the spinal cord between the two sequences.
In this study, although there was no statistically significant difference in SNR between the two sequences in the nasopharyngeal lesions and nasal concha, EPI-IVIM exhibited a higher SNR than did TSE-IVIM in the temporal pole and spinal cord, which is consistent with other reports (32). However, the CNR for the TSE sequence was higher than that for EPI in the nasopharyngeal lesions and nasal concha. This discrepancy may be attributed to the use of spectral presaturation with inversion recovery lipid compression in both TSE and EPI sequences, which requires high uniformity of the B0 field (33). Due to the influence of the air–bone and air–soft tissue interfaces, the B0 field in the nasopharynx is highly uneven, leading to severe degradation in images obtained with EPI. IVIM, as an imaging technique for describing microscopic motion, uses multiple b-values to provide information on the diffusion of pure water molecules (D), pseudo diffusion coefficient (D*), and perfusion fraction (f) for tissue perfusion. We observed significant differences in the ADC and D* values between TSE-IVIM and EPI-IVIM in the spinal cord. This may be due to the proximity of spinal cord to the skull base and the complex anatomical features affected by air, bone, muscle, and blood vessels. In IVIM theory, f represents the volume fraction of blood in vessels and is related to angiogenesis and microvessel density (34). We found a statistically significant difference in the f values of lesions between the two sequences. The uneven B0 field in the nasopharynx due to air-bone and air-soft tissue interfaces leads to severe deformation in images obtained under EPI. Consequently, many studies have highlighted the difficulty in measurement and poor reliability of IVIM-derived parameters in EPI-IVIM for small NPCs (17-19). In addition, the TSE sequence employs refocusing pulses, which have a longer T2 relaxation time than does the EPI sequence, resulting in an underestimation of the f value (35-37).
Additionally, we analyzed the LoAs and CV for the ADC and IVIM-derived parameters. The results indicated that the 95% LoAs for nasopharyngeal lesions and nasal concha were wider and exhibited larger CV than did those for the spinal cord and temporal pole, suggesting that the LoAs for lesions and nasal concha between the two sequences were unacceptable. The ADC values for nasopharyngeal lesions and nasal concha, as well as the derived parameters from these sequences, were not interchangeable. In addition, the TSE sequence yielded superior subjective and objective evaluation indicators; therefore, TSE-IVIM should be prioritized for evaluating nasopharyngeal lesions and nasal concha. Additionally, the two sequences have stronger substitutability for the spinal cord and temporal pole. However, considering the short scanning time and high SNR of spinal cord, using EPI for IVIM measurement in this area is recommended. For the temporal pole, although EPI had a high SNR, the overall image quality was poor and the image distortion more severe. Therefore, substituting EPI-IVIM for TSE-IVIM in the temporal pole is not recommended.
This study involved several limitations which should be noted. First, the sample size was relatively small. Second, while this study focused on comparing the image quality of the two sequences, it did not address the diagnostic efficacy, and the clinical significance of the findings may require further evaluation. Third, although we aimed to maintain consistency in the location and size of the ROI outlines, minor mismatches were inevitable.
Conclusions
TSE-IVIM demonstrated superior image quality and more stable quantitative indicators in nasopharyngeal lesions and the nasal concha, with better subjective image scores. Given the differences in f values and the wider LoAs between the two sequences for nasopharyngeal lesions, we recommend using TSE-IVIM for follow-up in patients with nasopharyngeal cancer. TSE-IVIM addresses the limitations present in the assessment of small lesions and may be significant for the long-term evaluation of radiochemotherapy outcomes in patients with NPC in the future.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1021/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1021/coif). P.S. is an employee of Philips Healthcare (MSC Clinical & Technical Solutions, Philips Healthcare, Beijing, China). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of Union Hospital of Tongji Medical College, Huazhong University of Science and Technology (No. UHCT-IEC-SOP-016-03-01). Informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen Y, Lin T, Tang L, He L, He Y. MiRNA signatures in nasopharyngeal carcinoma: molecular mechanisms and therapeutic perspectives. Am J Cancer Res 2023;13:5805-24.
- Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet 2019;394:64-80. [Crossref] [PubMed]
- Wu W, Xia J, Li B, Liu W, Ge Z, Tan Z, Bu Q, Chen W, Li Y. Feasibility evaluation of intravoxel incoherent motion diffusion-weighted imaging in the diagnosis of skull-base invasion in nasopharyngeal carcinoma. J Cancer 2023;14:290-8. [Crossref] [PubMed]
- Xiao Y, Qing J, Li B, Chen L, Nong S, Yang W, Tang X, Chen Z. TIM-3 Participates in the Invasion and Metastasis of Nasopharyngeal Carcinoma via SMAD7/SMAD2/SNAIL1 Axis-Mediated Epithelial-Mesenchymal Transition. Onco Targets Ther 2020;13:1993-2006. [Crossref] [PubMed]
- Jiang M, Jin S, Han J, Li T, Shi J, Zhong Q, Li W, Tang W, Huang Q, Zong H. Detection and clinical significance of circulating tumor cells in colorectal cancer. Biomark Res 2021;9:85. [Crossref] [PubMed]
- Huang Y, Feng M, Yang X, Zhou J, Li L, Xu K, Xu G, Lang J. DW-MRI-Guided Dose Escalation Improves Local Control of Locally Advanced Nasopharyngeal Carcinoma Treated with Chemoradiotherapy. Cancer Manag Res 2020;12:3107-16. [Crossref] [PubMed]
- Chen WS, Li JJ, Hong L, Xing ZB, Wang F, Li CQ. Comparison of MRI, CT and 18F-FDG PET/CT in the diagnosis of local and metastatic of nasopharyngeal carcinomas: an updated meta analysis of clinical studies. Am J Transl Res 2016;8:4532-47.
- Li Q, Yu Q, Gong B, Ning Y, Chen X, Gu J, Lv F, Peng J, Luo T. The Effect of Magnetic Resonance Imaging Based Radiomics Models in Discriminating stage I-II and III-IVa Nasopharyngeal Carcinoma. Diagnostics (Basel) 2023.
- Huang Y, Zhu Y, Yang Q, Luo Y, Zhang P, Yang X, Ren J, Ren Y, Lang J, Xu G. Automatic tumor segmentation and metachronous single-organ metastasis prediction of nasopharyngeal carcinoma patients based on multi-sequence magnetic resonance imaging. Front Oncol 2023;13:953893. [Crossref] [PubMed]
- Zong D, Jiang N, Kong C, Wen J, Wang LJ, Guo YS, Zhang LF, He X, Chen ZZ, Huang SF. Distribution pattern of medial group retropharyngeal lymph nodes and its implication in optimizing clinical target volume in nasopharyngeal carcinoma. Front Oncol 2023;13:1228994. [Crossref] [PubMed]
- Guo Y, Dai G, Xiong X, Wang X, Chen H, Zhou X, Huang W, Chen F. Intravoxel incoherent motion radiomics nomogram for predicting tumor treatment responses in nasopharyngeal carcinoma. Transl Oncol 2023;31:101648. [Crossref] [PubMed]
- Gullo RL, Partridge SC, Shin HJ, Thakur SB, Pinker K. Update on DWI for Breast Cancer Diagnosis and Treatment Monitoring. AJR Am J Roentgenol 2024;222:e2329933. [Crossref] [PubMed]
- Payabvash S. Quantitative diffusion magnetic resonance imaging in head and neck tumors. Quant Imaging Med Surg 2018;8:1052-65. [Crossref] [PubMed]
- Wang Q, Yu G, Qiu J, Lu W. Application of Intravoxel Incoherent Motion in Clinical Liver Imaging: A Literature Review. J Magn Reson Imaging 2024;60:417-40. [Crossref] [PubMed]
- Mori N, Mugikura S, Miyashita M, Mori Y, Maekawa Y, Nagasaka T, Takase K. Turbo Spin-echo Diffusion-weighted Imaging Compared with Single-shot Echo-planar Diffusion-weighted Imaging: Image Quality and Diagnostic Performance When Differentiating between Ductal Carcinoma in situ and Invasive Ductal Carcinoma. Magn Reson Med Sci 2021;20:60-8. [Crossref] [PubMed]
- Verhappen MH, Pouwels PJ, Ljumanovic R, van der Putten L, Knol DL, De Bree R, Castelijns JA. Diffusion-weighted MR imaging in head and neck cancer: comparison between half-fourier acquired single-shot turbo spin-echo and EPI techniques. AJNR Am J Neuroradiol 2012;33:1239-46. [Crossref] [PubMed]
- Zhang SX, Jia QJ, Zhang ZP, Liang CH, Chen WB, Qiu QH, Li H. Intravoxel incoherent motion MRI: emerging applications for nasopharyngeal carcinoma at the primary site. Eur Radiol 2014;24:1998-2004. [Crossref] [PubMed]
- Lai V, Li X, Lee VH, Lam KO, Fong DY, Huang B, Chan Q, Khong PL. Nasopharyngeal carcinoma: comparison of diffusion and perfusion characteristics between different tumour stages using intravoxel incoherent motion MR imaging. Eur Radiol 2014;24:176-83. [Crossref] [PubMed]
- Qamar S, King AD, Ai QH, So TY, Mo FKF, Chen W, Poon DMC, Tong M, Ma BB, Hui EP, Yeung DK, Wang YX, Yuan J. Pre-treatment intravoxel incoherent motion diffusion-weighted imaging predicts treatment outcome in nasopharyngeal carcinoma. Eur J Radiol 2020;129:109127. [Crossref] [PubMed]
- Yang L, Wu X, Wang Y, Shi G, Hu H, Duan X. Comparison of image quality and quantitative parameters in intravoxel incoherent motion imaging at 3-T based on turbo spin-echo and echo-planar imaging in patients with oral cancer. Diagn Interv Radiol 2023;29:786-93. [Crossref] [PubMed]
- Wan Q, Lei Q, Wang P, Hu J, Zhang T, Yu D, Li X, Liang C. Intravoxel Incoherent Motion Diffusion-Weighted Imaging of Lung Cancer: Comparison Between Turbo Spin-Echo and Echo-Planar Imaging. J Comput Assist Tomogr 2020;44:334-40. [Crossref] [PubMed]
- Kamimura K, Nakajo M, Yoneyama T, Fukukura Y, Fujio S, Goto Y, Iwanaga T, Akamine Y, Yoshiura T. Assessment of microvessel perfusion of pituitary adenomas: a feasibility study using turbo spin-echo-based intravoxel incoherent motion imaging. Eur Radiol 2020;30:1908-17. [Crossref] [PubMed]
- Yang L, Liu Y, Kong X, Guo X, Liu X, Qi Q, Wang J. Diffusion tensor magnetic resonance imaging of the postoperative spine with metallic implants. NMR Biomed 2020;33:e4321. [Crossref] [PubMed]
- Pan JJ, Ng WT, Zong JF, Chan LL, O'Sullivan B, Lin SJ, Sze HC, Chen YB, Choi HC, Guo QJ, Kan WK, Xiao YP, Wei X, Le QT, Glastonbury CM, Colevas AD, Weber RS, Shah JP, Lee AW. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer 2016;122:546-58.
- Mikayama R, Yabuuchi H, Sonoda S, Kobayashi K, Nagatomo K, Kimura M, Kawanami S, Kamitani T, Kumazawa S, Honda H. Comparison of intravoxel incoherent motion diffusion-weighted imaging between turbo spin-echo and echo-planar imaging of the head and neck. Eur Radiol 2018;28:316-24. [Crossref] [PubMed]
- Kaltenbach B, Roman A, Polkowski C, Gruber-Rouh T, Bauer RW, Hammerstingl R, Vogl TJ, Zangos S. Free-breathing dynamic liver examination using a radial 3D T1-weighted gradient echo sequence with moderate undersampling for patients with limited breath-holding capacity. Eur J Radiol 2017;86:26-32. [Crossref] [PubMed]
- Kim JR, Yoon HM, Cho YA, Lee JS, Jung AY. Free-breathing contrast-enhanced upper abdominal MRI in children: comparison between Cartesian acquisition and stack-of-stars acquisition with two different fat-suppression techniques. Acta Radiol 2021;62:541-50. [Crossref] [PubMed]
- Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986;161:401-7. [Crossref] [PubMed]
- DeLaPaz RL. Echo-planar imaging. Radiographics 1994;14:1045-58. [Crossref] [PubMed]
- Yoshizako T, Yoshida R, Asou H, Nakamura M, Kitagaki H. Comparison between turbo spin-echo and echo planar diffusion-weighted imaging of the female pelvis with 3T MRI. Acta Radiol Open 2021;10:2058460121994737. [Crossref] [PubMed]
- Le Bihan D, Poupon C, Amadon A, Lethimonnier F. Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging 2006;24:478-88. [Crossref] [PubMed]
- Dudau C, Draper A, Gkagkanasiou M, Charles-Edwards G, Pai I, Connor S. Cholesteatoma: multishot echo-planar vs non echo-planar diffusion-weighted MRI for the prediction of middle ear and mastoid cholesteatoma. BJR Open 2019;1:20180015. [Crossref] [PubMed]
- Dong Y, Riedel M, Koolstra K, van Osch MJP, Börnert P. Water/fat separation for self-navigated diffusion-weighted multishot echo-planar imaging. NMR Biomed 2023;36:e4822. [Crossref] [PubMed]
- Chen R, Ye H, Wu Z, Zhou Y, Lin H, Xu Y, He L, Liang C, Liu Z, Wang G. Using the non-distortion IVIM to reduce the need for contrast agents in nasopharyngeal MRI. Magn Reson Imaging 2023;104:115-20. [Crossref] [PubMed]
- Ma FZ, Wáng YXJ T. (2) relaxation time elongation of hepatocellular carcinoma relative to native liver tissue leads to an underestimation of perfusion fraction measured by standard intravoxel incoherent motion magnetic resonance imaging. Quant Imaging Med Surg 2024;14:1316-22. [Crossref] [PubMed]
- Gaddamanugu S, Shafaat O, Sotoudeh H, Sarrami AH, Rezaei A, Saadatpour Z, Singhal A. Clinical applications of diffusion-weighted sequence in brain imaging: beyond stroke. Neuroradiology 2022;64:15-30. [Crossref] [PubMed]
- Wáng YXJ. Observed paradoxical perfusion fraction elevation in steatotic liver: An example of intravoxel incoherent motion modeling of the perfusion component constrained by the diffusion component. NMR Biomed 2021;34:e4488. [Crossref] [PubMed]


