Diagnostic clues of organizing pneumonia: a case presentation
Introduction
Cryptogenic organizing pneumonia (COP) is currently an established clinicopathologic entity, expression of reparative reactions to acute lung injury and the resulting inflammation within the alveoli and distal bronchioles. COP continues to be included in the classification of IIP (Idiopathic Interstitial Pneumonias) because of its idiopathic nature and to date in the last European Respiratory Society (ERS)/American Thoracic Society (ATS) update statement of IIP and according to acute or subacute presentation, is included in section “acute or subacute IIPs” (1). In 1983 Davison was the first to describe this disease, subsequently Epler called this entity “bronchiolitis obliterans with organizing pneumonia” (BOOP) (2,3). HRCT often demonstrates patchy migratory areas of consolidations predominantly with a subpleural, peribronchial distribution. Sometimes HRCT pattern is characterized by “band-like pattern commonly associated to ground glass opacities (GGO). Perilobular opacities and a typical HRCT sign called reverse halo sign or “atoll sign” may be extremely useful for clinic-radiological diagnosis. Slight pleural effusion is also described in literature in few cases (4). We describe a case of COP presenting as fever, productive cough and dyspnea persisting for 4 months, in which the role of CT-PET has been an element of considerable confounding in the therapeutic management and in which a radiological revaluation of the temporal behavior of the disease has allowed us to correctly achieve the diagnosis of organizing pneumonia (OP).
Case presentation
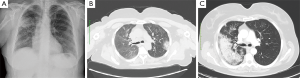
A 57-year-old female former “heavy” smoker (60 pack/years until 2 years ago) was referred to our Department, presenting cough with purulent sputum, low-grade fever (maximum temperature reached 37.5 °C), worsening of dyspnea for 4 months. Clinical history showed systemic hypertension and hypercholesterolemia being treated with drugs. Chest X-ray and chest CT were performed. Chest X-ray showed an extensive inhomogeneous opacity in upper and middle lung field of right hemithorax and another smaller opacity was visible to left in middle lung field (Figure 1A). Chest CT, performed 7 days after, showed a consolidation with irregular margins in both right and left upper lobe with mediastinal lymphadenopathy (Figure 1B,C). For this clinical condition the patient received the diagnosis of pneumonia. Antibiotic therapy with ceftriaxone (1 g/day) and ciprofloxacin (1 g/day) for 10 days showed no improvement. Physical examination showed fever (37.5 °C). Auscultation of lungs revealed only diffuse reduction of breath sounds. Blood tests showed an elevated erythrocyte sedimentation rate (ESR) (75 mm/h), while C- reactive protein was normal (2.8 mg/dL).
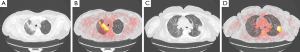
Hemogas analysis showed mild hypoxemia with respiratory alkalosis. Spirometric values were normal, and there was a moderate reduction of the alveolar capillary diffusion of the Carbon Dioxide (DLCO 60% predicted). Due to the absence of significant improvement of the symptomatology, patient was then submitted to bronchoscopy with bronchoalveolar lavage (BAL) which showed only the presence of inflammatory cells, the isolation of Haemophilus influenza (100,000 cfu/mL), an increase of neutrophilic and eosinophilic granulocyte and the inversion of the CD4/CD8 lymphocyte ratio. According to the antibiogram, the patient was treated for other 7 days with new antibiotic therapy: amoxicillin and clavulanic acid 875/125 mg (every 8 h), followed by a noteworthy clinical improvement. The clinical decision was to perform a CT-PET in order to characterize the previously identified irregular consolidations to rule out malignant disease suspicion. PET-CT indeed showed the almost complete disappearance of the extensive consolidation in the right upper lobe and appearance of new consolidation in apical segment of upper lobe next to mediastinal pleural line with increase of metabolic activity. Several other areas of high metabolic activity were visible in the context of the posterior segment of the right upper lobe, in the lingula and in the apical segment of the left lower lobe, as well as the basal posterior and lateral segment of the lower lobe (SUVmax 8.65 detected at the level of the posterior segment of the left lower lobe). The lesions were stable and without significant changes in size, with subpleural consolidation in the left upper lobe, characterized by high metabolic activity (Figure 2A-D).
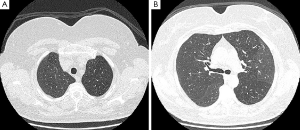
Following a detailed assessment of the clinical history and diagnostic exams performed for the patient, Radiologist suggested a diagnosis of OP, especially for a double typical sign of this disease: lack of response to antibiotic therapy and migration of pulmonary consolidations. The temporal migration of lesions, associated to the absence of response to antibiotic therapy, despite the high SUV levels at CT-PET, were considered by clinicians and radiologists an important factor to address the diagnosis to OP and finally start corticosteroid therapy, avoiding invasive procedures for histopathological confirmation. The patient started treatment with prednisone 0.50 mg/kg/day and azithromycin 500 mg/day for 3 days a week for 4 weeks. After a month of treatment, clinical symptoms regressed completely and pulmonary function tests showed a great improvement. The flow volume loop showed an increase in forced expiratory volume in 1 s (FEV1) (95% predicted), forced vital capacity (FVC) (105% predicted) and alveolar capillary diffusion of CO was completely normalized (20.96; 88% predicted). After 3 months of therapy, chest HRCT (gold standard for evaluation of IIPs) showed almost complete resolution of consolidations previously recognized, therefore the patient started a progressive dose reduction until complete suspension of the treatment, without signs of disease recurrence (Figure 3A,B).
Discussion
OP, also called (in absence of specific cause) COP, is included in the classification of IIPs because of its idiopathic nature but many cases are surely secondary to different causes (for example: infections, drug toxicity, surgery, radiotherapy, connective tissue disease, systemic diseases included cancer). OP can be considered a non-specific response to lung injury and is characterized by patchy process involving bronchiolar walls, alveolar ducts and alveolar compartment, modulated by inflammatory cells and connective tissue matrix (4-6). Rare cases showed more marked inflammation in interstitial compartment, finding that is overlap with cellular non-specific interstitial pneumonia (NSIP). The term BOOP (bronchiolitis obliterans with organising pneumonia) is rarely used in order to avoid confusion with constrictive bronchiolitis, a strictly airway disease (7). The incidence of OP is not well established, even if some studies found a mean annual incidence of just under 2 per 100,000 and it has an equal sex distribution (8). COP typically presents with fever, malaise, cough and dyspnea, which may be severe. An history of prolonged antibiotic therapy (as in our patients) that does not show significant response, is common and diagnosis is often, for this reason, delayed. History of a previous viral-type illness can often be present and a seasonal (early spring) relapsing pattern of occurrence has been described (9). Inflammatory markers are elevated and there is often evidence of a peripheral neutrophilia. Pulmonary functional tests demonstrate a restrictive defect with a moderately reduced alveolar capillary DLCO (10). Arterial hypoxaemia is commonly seen. Bronchoscopic alveolar lavage (BAL) is not specific but usually there is a lymphocytosis with a decreased CD4:CD8 ratio (11). Characteristic clinical and imaging features can indicate the diagnosis of OP, but the cryptogenic form requires the exclusion of a possible cause. Extra-pulmonary findings are present in a minority of cases, including pleural effusions and mediastinal lymph node enlargement (12). HRCT is the gold standard for diagnosis and shows various patterns of presentation fully described in literature, such as changing multifocal peripheral consolidation (migratory consolidation), variable in location over a matter of weeks (as in our case) with possible spontaneous regressions (13). Others classic HRCT signs include “bronchocentric”, “peribronchial”, “crazy paving” and band-like” pattern. OP may also have solitary focal mass or nodule/multiple masses or progressive fibrotic presentations. Consolidation are often unilateral or bilateral with a predominantly peripheral distribution in lower and middle zone. The presence of reversed halo sign or “atoll sign” (described as crescentic and ring shaped opacities surrounding areas of ground glass attenuation) is considered suggestive of OP (14). In literature, the role of PET-CT is not yet well defined, because OP can produce false positive results, becoming an element of considerable confounding in the therapeutic management (15). However, patients with OP have a higher FDG accumulation which may reflect the degree of disease activity. Moreover the results may be used to guide invasive procedures that should be performed when there is a suspicion of malignancy (16). In most cases, CT and CT-PET cannot distinguish focal OP from lung cancer. Therefore, histopathological examination and surgical resection of the lesions are needed in the management of focal OP (17). Biopsy, if required in doubtful cases to confirm the diagnosis, may be performed by transbronchial, video-assisted thoracoscopy (VATS) and CT guided percutaneous approaches, depending on the distribution of disease. At a histopathological level, OP is characterized by intraluminal plugs of inflammatory debris predominantly within the alveolar ducts and surrounding alveoli, consisting of buds of granulation tissue, whorls of fibroblasts and myo-fibroblasts in a connective matrix (Masson bodies), but the presence of radiologic patterns associated with clinical history and PFT, makes OP diagnosis possible without histological confirmation (18). Glucocorticoids are traditionally a first-line agent for the treatment of OP (0.50–0.75 mg/kg daily) with, generally a good response. However, one study reported that 10–15% of patients were resistant to treatment and the disease progressed rapidly. A total of 13–58% of patients had recurrent disease during the reduction of glucocorticoids or following drug discontinuation. Macrolides, as regulators of immune responses, have been used in many disease and have also been reported to effectively treat mild COP, or as a steroid sparing medication (19,20).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, Behr J, Bouros D, Brown KK, Colby TV, Collard HR, Cordeiro CR, Cottin V, Crestani B, Drent M, Dudden RF, Egan J, Flaherty K, Hogaboam C, Inoue Y, Johkoh T, Kim DS, Kitaichi M, Loyd J, Martinez FJ, Myers J, Protzko S, Raghu G, Richeldi L, Sverzellati N, Swigris J, Valeyre D, ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Davison AG, Heard BE, McAllister WA, Turner-Warwick ME. Cryptogenic organizing pneumonitis. Q J Med 1983;52:382-94. [PubMed]
- Epler GR, Colby TV, McLoud TC, Carrington CB, Gaensler EA. Bronchiolitis obliterans organizing pneumonia. N Engl J Med 1985;312:152-8. [Crossref] [PubMed]
- Roberton BJ, Hansell DM. Organizing pneumonia: a kaleidoscope of concepts and morphologies. Eur Radiol 2011;21:2244-54. [Crossref] [PubMed]
- Zhao F, Yan SX, Wang GF, Wang J, Lu PX, Chen B, Yuan J, Zhang SZ, Wang YX. CT features of focal organizing pneumonia: an analysis of consecutive histopathologically confirmed 45 cases. Eur J Radiol 2014;83:73-8. [Crossref] [PubMed]
- Kligerman SJ, Franks TJ, Galvin JR. From the radiologic pathology archives: organization and fibrosis as a response to lung injury in diffuse alveolar damage, organizing pneumonia, and acute fibrinous and organizing pneumonia. Radiographics 2013;33:1951-75. [Crossref] [PubMed]
- Cordier JF. Organising pneumonia. Thorax 2000;55:318-28. [Crossref] [PubMed]
- Gudmundsson G, Sveinsson O, Isaksson HJ, Jonsson S, Frodadottir H, Aspelund T. Epidemiology of organising pneumonia in Iceland. Thorax 2006;61:805-8. [Crossref] [PubMed]
- Spiteri MA, Klenerman P, Sheppard MN, Padley S, Clark TJ, Newman-Taylor A. Seasonal cryptogenic organising pneumonia with biochemical cholestasis: a new clinical entity. Lancet 1992;340:281-4. [Crossref] [PubMed]
- Cordier JF, Loire R, Brune J. Idiopathic bronchiolitis obliterans organizing pneumonia. Definition of characteristic clinical profiles in a series of 16 patients. Chest 1989;96:999-1004. [Crossref] [PubMed]
- Nagai S, Handa T, Ito Y, Takeuchi M, Izumi T. Bronchoalveolar lavage in idiopathic interstitial lung diseases. Semin Respir Crit Care Med 2007;28:496-503. [Crossref] [PubMed]
- Souza CA, Müller NL, Lee KS, Johkoh T, Mitsuhiro H, Chong S. Idiopathic interstitial pneumonias: prevalence of mediastinal lymph node enlargement in 206 patients. AJR Am J Roentgenol 2006;186:995-9. [Crossref] [PubMed]
- Faria IM, Zanetti G, Barreto MM, Rodrigues RS, Araujo-Neto CA, Silva JL, Escuissato DL, Souza AS Jr, Irion KL, Mançano AD, Nobre LF, Hochhegger B, Marchiori E. Organizing pneumonia: chest HRCT findings. J Bras Pneumol 2015;41:231-7. [Crossref] [PubMed]
- Rea G, Lassandro F, Valente T. Exogenous lipoid pneumonia in laryngectomy patients: Is ground glass opacity/crazy paving pattern an organizing pneumonia reaction that can predict poor outcome? Arch Bronconeumol 2016;52:438-9. [PubMed]
- Erdoğan Y, Özyürek BA, Özmen Ö, Yılmaz Demirci N, Duyar SŞ. 1, Dadalı Y, Demirağ F, Karakaya J. The Evaluation of FDG PET/CT Scan Findings in Patients with Organizing Pneumonia Mimicking Lung Cancer. Mol Imaging Radionucl Ther 2015;24:60-5. [Crossref] [PubMed]
- Tateishi U, Hasegawa T, Seki K, Terauchi T, Moriyama N, Arai Y. Disease activity and 18F-FDG uptake in organising pneumonia: semi-quantitative evaluation using computed tomography and positron emission tomography. Eur J Nucl Med Mol Imaging 2006;33:906-12. [Crossref] [PubMed]
- Maldonado F, Daniels CE, Hoffman EA, Yi ES, Ryu JH. Focal organizing pneumonia on surgical lung biopsy: causes, clinicoradiologic features, and outcomes. Chest 2007;132:1579-83. [Crossref] [PubMed]
- Kim SJ, Lee KS, Ryu YH, Yoon YC, Choe KO, Kim TS, Sung KJ. Reversed halo sign on high-resolution CT of cryptogenic organizing pneumonia: diagnostic implications. AJR Am J Roentgenol 2003;180:1251-4. [Crossref] [PubMed]
- Cai M, Bonella F, Dai H, Sarria R, Guzman J, Costabel U. Macrolides inhibit cytokine production by alveolar macrophages in bronchiolitis obliterans organizing pneumonia. Immunobiology 2013;218:930-7. [Crossref] [PubMed]
- Rea G, Perna F, Calabrese G, Molino A, Valente T, Vatrella A. Exogenous lipoid pneumonia (ELP): when radiologist makes the difference. Transl Med UniSa 2016;14:64-8. [PubMed]


