
Evaluating the efficacy of low-kV X-ray intraoperative radiotherapy in the treatment of locally advanced rectal cancer: a retrospective cohort study
Introduction
Rectal cancer is the third most common malignant tumor worldwide (1). At present, surgery is the primary treatment strategy for rectal cancer. However, the 5-year survival rate of stage II–III patients who receive surgical treatment alone is less than 70%, and local recurrence is a major concern (2). Various treatments, including advanced surgical strategies and chemoradiotherapy, have been shown to improve outcomes for patients with rectal cancer (3,4). Research indicates that combining surgery with radiotherapy could further reduce the risk of postoperative local recurrence and potentially improve the survival rate of rectal cancer patients (5,6). Therefore, the combination of surgery and chemoradiotherapy is widely used in the comprehensive treatment of rectal cancer. Neoadjuvant radiotherapy or postoperative radiotherapy has shown efficacy in treating patients with rectal cancer; however, the radiation dose is limited by the sensitivity of adjacent normal organs and tissues to radiation (7). If a lesion is adjacent to radiation-sensitive normal tissues, the radiation dose is reduced. Typically, the adjacent tissues, including the small intestine and pelvic nerve, are injured following radiation dose administration. Thus, it is critical to investigate effective comprehensive treatment strategies to improve the survival rate of patients with rectal cancer.
Intraoperative radiotherapy (IORT) refers to intraoperative high-dose irradiation, a technique that disrupts chromosomes using energy carried by radiation (8). This technique can directly and precisely deliver high-dose radiation to subclinical lesions, lymphatic drainage areas, or residual tumor tissues, thereby improving treatment efficacy while minimizing damage to surrounding normal tissue (9). The advantages of IORT include (10): (I) the direct and precise irradiation of recurrent tumor beds and tumor invasion area; (II) the use of high-dose irradiation to achieve a potent biological effect; (III) a reduced incidence of systemic side effects and myelosuppression; (IV) a shortened radiotherapy duration that effectively kills tumor cells with minimal harm to nearby normal tissues; and (V) no influence on subsequent external radiotherapy and chemotherapy.
At present, research on the efficacy of IORT in the treatment of locally advanced rectal cancer (LARC) is limited, and most studies have focused on intraoperative electron radiation therapy (IOERT) (11,12) and high-dose-rate IORT (HDR-IORT) (13,14). Compared to IOERT and HDR-IORT, low-kV X-ray IORT, which uses the INTRABEAM photon radiosurgery system (PRS) (Carl Zeiss, Oberkochen, Germany), can deliver a homogenous dose distribution to the spherical applicator surface with rapid dose attenuation from the applicator to the surface of the targeted site. This feature enables enhanced local control and reduced damage to adjacent tissues (15). Low-kV X-ray IORT has been used in the treatment of breast, bone, brain, and colorectal cancers (10). However, there is limited research on the long-term outcomes of low-kV X-ray IORT for patients with LARC. This study was the first to investigate the long-term feasibility and safety of radical surgical resection combined with low-kV X-ray IORT for patients with LARC. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-965/rc).
Methods
This retrospective cohort study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of The Second Hospital of Jilin University (No. 2015-230). Informed consent was obtained from all individual participants included in the study. We conducted a retrospective analysis of 69 patients with LARC [tumor3–4node0–2metastasis0 (T3–4N0–2M0)] who underwent curative surgical resection and low-kV X-ray IORT at our center from December 2015 to August 2023.
Consecutive patients were included in this study if they met the following inclusion criteria: (I) were aged 19 to 80 years; (II) had a diagnosis of rectal adenocarcinoma confirmed by pathological examination; (III) had undergone preoperative pelvic enhanced computed tomography (CT) or magnetic resonance imaging indicating a clinical stage of T3–4 with or without positive lymph nodes; and (IV) refused neoadjuvant therapy. Patients were excluded from the study if they met any of the following exclusion criteria: (I) required emergency surgery due to severe bleeding or intestinal obstruction; (II) had distant metastasis confirmed by preoperative examination; (III) had abnormal vital organ function; and/or (IV) had undergone neoadjuvant chemotherapy or radiotherapy. Figure 1 shows the study flowchart.
Based on factors such as the distance from the lower tumor edge to the anal verge, the relationship between the tumor and adjacent tissues, and the general condition of the patient, various surgical modalities were employed, including low anterior rectal resection (Dixon), intersphincteric resection (ISR), Miles surgery, and Hartmann surgery. After radical resection of the tumor, the appropriate IORT applicator was positioned in the tumor bed, mostly on the anterior part of the sacrum and the region of the promontory. The irradiation time (usually 30–40 min) and the dose were determined by the surgeon and radiologist. Wet gauze strips were used as a shield to protect the surrounding normal tissues and organs.
Due to the diversity of surgical methods and intraoperative circumstances, the approach for implanting the IORT applicator can vary; for laparoscopic Dixon and Hartmann surgery, the IORT applicator can be applied through an auxiliary incision; for laparotomy Dixon and Hartmann surgery, the surgical incision can be selected; for Miles surgery, the perineal incision can be used; for ISR surgery, the anus approach is appropriate; for recurrent tumors with the combination of multiple surgery methods, multiple approaches can be used for IORT applicator implantation. In this study, the applicator was implanted in the tumor bed via the perineum, an auxiliary incision in abdominal laparoscopic surgery, the anus, or a traditional incision in laparotomy (Figure 2). A single dose ranging from 16 to 20 Gy was delivered for INTRABEAM IORT. The calibration and variability control of the radiation were determined by the radiologist. Generally, the prescribed IORT dose was converted by “TARGIT to V4.0” calibration factors provided by Zeiss to accurately estimate the dose to be delivered to the treatment targets as suggested by previous studies (16,17). The basic characteristics of patients, intraoperative and postoperative data, and early and late complications were recorded. Additionally, each patient was followed up periodically (Table 1).

Table 1
| Follow-up approach | Frequency |
|---|---|
| Anal digital examination | The first 2 years post-surgery: every 3 months; 3–5 years post-surgery: every 6 months |
| Routine blood examination | The same as that for the anal digital examination |
| Blood CEA level | The first 2 years post-surgery: every 3 months; 3–5 years post-surgery: every year |
| Pelvic CT | 4–6 weeks post-surgery; the first 3 years: every year; if abnormal, performed at any time |
| B-mode ultrasound | The first 2 years: every 3 months |
| Chest CT | The first 2 years: every 3 months |
| Colonoscopy | The first 2 years: every year; if the results obtained within the first 2 years were negative: every 3 years; if the intestinal polyps were positive: every year |
CEA, carcinoembryonic antigen; CT, computed tomography.
The Kolmogorov-Smirnov test was used to determine whether the data were normally distributed. The continuous variables were reported as the mean ± standard deviation if normally distributed, and the median and interquartile range if not normally distributed. The categorical data were expressed as the frequency (percentage). A Kaplan-Meier (K-M) survival analysis was used to assess both overall survival (OS) and disease-free survival (DFS). Log-rank tests were performed to further assess the association of various factors with OS and DFS. When the survival curves crossed, the two-stage test was used for further analysis. GraphPad Prism 10 (GraphPad Inc., San Diego, CA, USA) was used for all statistical analyses. A P value <0.05 was considered statistically significant, and the statistical tests were two-sided.
Results
A total of 69 patients were included in this study, of whom 48 were men and 21 were women. The ages of the patients ranged from 43 to 81 years. No patient received neoadjuvant chemotherapy or radiotherapy. The average distance from the lower tumor edge to the anal verge was 4.74±2.55 cm, and 39 patients had a distance of less than 5 cm, while 30 had a distance ranging from 5 to 10 cm. Additional basic patient characteristics, including the American Society of Anesthesiologists (ASA) scores, and preoperative imaging T and N stages of the patients, are listed in Table 2.
Table 2
| Characteristics | Data |
|---|---|
| Sex, n (%) | |
| Male | 48 (69.57) |
| Female | 21 (30.43) |
| Age (years) | |
| Mean ± SD | 62.94±9.61 |
| Median | 64 |
| Range | 43–81 |
| Obesity (BMI ≥30 kg/m2), n (%) | 4 (5.80) |
| Preoperative radiotherapy, n (%) | 0 |
| Preoperative chemotherapy, n (%) | 0 |
| ASA score, n (%) | |
| II | 53 (76.81) |
| III | 16 (23.19) |
| Tumor location (distance to anal verge) (cm) | |
| ≤5, n (%) | 39 (56.52) |
| >5 and ≤10, n (%) | 30 (43.48) |
| Mean ± SD | 4.74±2.55 |
| Preoperative imaging T stage, n (%) | |
| T3 | 62 (89.86) |
| T4 | 7 (10.14) |
| Preoperative imaging N stage, n (%) | |
| N0 | 10 (14.49) |
| N1 | 29 (42.03) |
| N2 | 30 (43.48) |
| Preoperative imaging M stage, n (%) | |
| M0 | 69 (100.00) |
| M1 | 0 |
SD, standard deviation; BMI, body mass index; ASA, American Society of Anesthesiologists; T, tumor; N, node; M, metastasis.
Various surgical methods were employed in this study. Specifically, 38 patients underwent the Dixon procedure, 21 underwent ISR, 7 underwent the Miles procedure, and 3 underwent the Hartmann procedure. The circumferential resection margin (CRM) was negative in all 69 patients, and the CRM completion rate was 100% in the surgical specimens. Detailed information regarding the approach for implanting the IORT applicator is provided in Table 3. Postoperative histological analysis confirmed adenocarcinoma in 61 patients and mucinous adenocarcinoma in 8 patients. The XELOX chemotherapy regimen (oxaliplatin and capecitabine) was recommended for 51 patients based on their postoperative pathology, of whom 45 received the treatment. The duration of hospitalization ranged from 9 to 43 days (median, 21 days). The number of examined lymph nodes was 17.52±2.55. The median follow-up time was 47.5 months (range, 6.5–98.5 months). At the time of analysis, 15 patients had died (21.74%), 6 (8.70%) had been lost to follow-up, and 48 (69.57%) were alive. The local recurrence rate and distant metastasis rate were 26.09% and 23.19%, respectively. Other intraoperative and postoperative data, including operation time, intraoperative blood loss, length of the resected specimen, tumor size, pathologic T and N stages, and number of positive lymph nodes are listed in Table 3.
Table 3
| Characteristics | Data |
|---|---|
| Type of surgery, n (%) | |
| Dixon | 38 (55.07) |
| ISR | 21 (30.43) |
| Miles | 7 (10.14) |
| Hartmann | 3 (4.35) |
| Applicator approach, n (%) | |
| Abdomen | 34 (49.28) |
| Anus | 28 (40.58) |
| Perineum | 7 (10.14) |
| Operation time (min), mean ± SD | 140.65±28.03 |
| Intraoperative blood loss (mL), mean ± SD | 48.19±22.85 |
| Length of resected specimen (cm), mean ± SD | 16.35±7.83 |
| Tumor size (largest diameter) (mm), mean ± SD | 8.58±4.86 |
| Histology, n (%) | |
| Adenocarcinoma | 61 (88.41) |
| Mucinous adenocarcinoma | 8 (11.59) |
| Pathologic T stage, n (%) | |
| T3 | 62 (89.86) |
| T4 | 7 (10.14) |
| Pathologic N stage, n (%) | |
| N0 | 30 (43.48) |
| N1 | 23 (33.33) |
| N2 | 16 (23.19) |
| Pathologic M stage, n (%) | |
| M0 | 69 (100.00) |
| M1 | 0 |
| Number of lymph nodes examined | |
| Median | 17 |
| Range | 12–24 |
| Mean ± SD | 17.52±2.55 |
| Number of lymph nodes positive | |
| Median | 1 |
| Range | 0-9 |
| Hospital stay (days) | |
| Median | 21 |
| Range | 9–43 |
| Chemotherapy after surgery, n (%) | |
| Yes | 45 (65.22) |
| No | 24 (34.78) |
| Follow-up time (months) | |
| Median | 47.5 |
| Range | 6.5–98.5 |
| Local recurrence, n (%) | 18 (26.09) |
| Distant metastasis, n (%) | 16 (23.19) |
ISR, intersphincteric resection; SD, standard deviation; T, tumor; N, node; M, metastasis.
Table 4 presents both the early and late complications observed in the study. There was no mortality within 30 days post-surgery. Anastomotic leak was observed in five patients, all of whom had a tumor height of less than 3 cm, and all of whom underwent ileostomy. Urinary retention and diarrhea were reported in four and seven patients, respectively. Bowel obstruction occurred in four patients and was relieved using conservative treatment methods. Wound infection or breakdown was observed in three patients who underwent laparotomy, and one patient underwent reoperation for wound suture. In relation to late complications occurring more than 30 days post-surgery, ureteral obstruction was observed in three patients, while bladder dysfunction was reported in two patients. Sexual dysfunction occurred in five patients with low LARC. Additionally, six patients presented with bowel obstruction due to intestinal adhesion or tumor local recurrence, and two patients underwent intestinal adhesion-release surgery. The percentage of re-operations was 4.35% in this study.
Table 4
| Complications | Data |
|---|---|
| Early (≤30 days), n (%) | |
| Mortality | 0 |
| Anastomotic leak | 5 (7.25) |
| Urinary retention | 4 (5.80) |
| Diarrhea | 7 (10.14) |
| Bowel obstruction | 4 (5.80) |
| Wound infection or breakdown | 3 (4.35) |
| Late (>30 days), n (%) | |
| Pelvic or abdominal abscess | 0 |
| Fistula with abscess | 0 |
| Ureteral obstruction | 3 (4.35) |
| Bladder dysfunction | 2 (2.90) |
| Sexual dysfunction | 5 (7.24) |
| Bowel obstruction | 6 (8.70) |
| Others | 2 (2.90) |
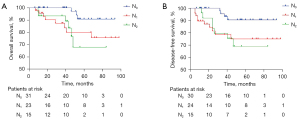
The 3-year K-M estimates for OS and DFS were 89.42% and 71.47%, respectively (Figure 3). Similarly, the 5-year K-M estimates for OS and DFS were 68.59% and 66.91%, respectively. All the patients were followed-up for the first 3 years post-surgery. After which, two patients were lost to follow-up at 37 months, and 4 other patients were lost to follow-up at 38, 41, 42, and 48 months, respectively. As a result, the high loss to follow-up rate (8.70%) might have affected the 5-year OS and DFS rates. Further analyses of the association between various factors and OS or DFS revealed that sex, ASA score, tumor location, type of surgery, IORT applicator implantation approach, histological type, pathologic T stage, and adjuvant chemotherapy were not associated with either OS or DFS. The association between the T stage and OS and DFS is illustrated in Figure 4. The T stage of tumors was not predictive of either OS or DFS. Tables 5,6 present the 3- and 5-year OS and DFS rates for patients with T3 and T4 stages, respectively. Conversely, different N stages and nerve/vascular invasion were positively correlated with OS and DFS (P<0.05) (Figures 5,6). Nerve/vascular invasion indicates that tumor cells invade nerve sheath or blood vessels outside the musculi propria of the rectum wall. The 3- and 5-year OS and DFS of patients with different pathologic lymphatic stages are presented in Tables 5,6, respectively. Table 5 also presents the 3-year OS and DFS of patients with nerve/vascular invasion.


Table 5
| Variables | OS (%) | DFS (%) |
|---|---|---|
| Total | 89.42 | 71.47 |
| Different T stage | ||
| T3 | 92.12 | 73.47 |
| T4 | 64.29 | 75.00 |
| Different N stage | ||
| N0 | 100.00 | 90.90 |
| N1 | 74.64 | 57.09 |
| N2 | 86.67 | 58.72 |
| Nerve/vascular invasion | ||
| Yes | 75.16 | 28.25 |
| No | 97.06 | 97.73 |
OS, overall survival; DFS, disease-free survival; T, tumor; N, node.
Table 6
| Variables | OS (%) | DFS (%) |
|---|---|---|
| Total | 68.59 | 66.91 |
| Different T stage | ||
| T3 | 68.85 | 65.62 |
| T4 | 64.29 | 75.00 |
| Different N stage | ||
| N0 | 81.57 | 80.96 |
| N1 | 59.71 | 49.96 |
| N2 | 35.66 | 37.76 |
OS, overall survival; DFS, disease-free survival; T, tumor; N, node.

Discussion
Total mesorectal excision (TME) is recommended for the surgical treatment of LARC (18). However, the narrow confines of the pelvic cavity present challenges in achieving the complete removal of subclinical lesions and lymph nodes surrounding the rectum (lymph nodes smaller than 5 mm are easily missed by the operator, resulting in incomplete radical resection). Further, the difficult of radical treatment increases when tumors invade large blood vessels or adjacent fixed tissues and organs. Radiotherapy plays a crucial role in the comprehensive treatment of LARC, reducing recurrence rates and improving survival outcomes. Early studies primarily investigated the efficacy of postoperative radiotherapy (19,20). Generally, in vitro radiation doses of 60–70 Gy are required to kill tumor cells. However, due to the adverse reactions of pelvic tissues to irradiation, doses generally cannot exceed 50 Gy (21). IORT can achieve similar or even better therapeutic effects than external irradiation while minimizing radiation doses to pelvic tissues. At present, research has shown that a single IORT dose of 15–20 Gy exerts the same effect as external irradiation with 50 Gy (22). The present study is the first to report the long-term outcomes of INTRABEAM IORT for LARC in a sample size of 69 patients. Notably, our study employed multiple surgical methods (the Dixon method, ISR, the Miles method, and the Hartmann method) and IORT applicator implantation approaches (via the perineum, an auxiliary incision in abdominal laparoscopic surgery, via the anus, and a traditional incision in laparotomy).
Currently, research on the use of low-kV X-ray IORT to treat rectal cancer, particularly LARC, is limited. In a retrospective review of experiences at Cleveland Clinic, researchers reviewed the data of 42 patients with locally advanced or recurrent rectal cancer treated with radical resection and IORT (23). INTRABEAM PRS® (Carl Zeiss) was used in that study, and the surface dose of the IORT applicator ranged from 13.4 to 23.1 Gy. No intraoperative complications were observed, and hydronephrosis after IORT only occurred in 10 patients (24%). The study showed the safety of IORT based on INTRABEAM PRS® in the treatment of rectal cancer. Ma et al. also reported encouraging results for patients with locally advanced colon cancer treated with low-kV X-ray IORT, with radiation doses ranging from 10 to 18 Gy, and also reported no increase in short- or long-term complications (24). In our previous study, 16–18 Gy was chosen for INTRABEAM IORT in patients with LARC who underwent ISR, and no acute radiation injuries or symptoms were observed (15). In the current study, the final dose of IORT was determined through collaboration with a surgeon and a radiologist.
In the present study, the median follow-up time was 47.5 months, and 15 patients died due to local recurrence or distant metastasis. Local recurrence includes local recurrence, lateral lymph node metastasis, and the dissemination of tumor cells. Six patients were lost to follow-up. The local recurrence rate was 26.09% and the 5-year local control, OS, and DFS rates were 78.90%, 68.59%, and 66.91%, respectively. Zhang et al. examined the efficacy of IORT followed by adjuvant chemotherapy in 45 patients diagnosed with pT3N0M0 well-differentiated and moderately differentiated rectal adenocarcinoma (25). Compared to our findings, they reported slightly higher 5-year local control, OS, and DFS rates of 84%, 84%, and 71%, respectively. This disparity could be attributed to the inclusion criteria, as our study focused solely on LARC cases (T3–4N0–2M0), with 7 cases (10.14%) in the T4 stage and 39 cases (56.52%) in the N1–2 stage. Moreover, not all patients in our study (45, 65.22%) received postoperative chemotherapy. Compared to our study, the earlier tumor stage and higher rate of postoperative chemotherapy might have improved the prognosis of patients with rectal cancer in Zhang et al.’s study (25).
A review conducted at the Cleveland Clinic of 42 patients, of whom 33 had recurrent rectal cancer and 8 had LARC, treated with definitive resection and INTRABEAM PRS® IORT (23), reported a 3-year OS rate of 43% for recurrent rectal cancer and 65% for LARC. In our study, the 3-year OS rate was 89.42%, which is higher than that reported in the study at the Cleveland Clinic. This discrepancy could be attributed to the achievement of margin negative (R0) resection in all our patients; only 52.4% of patients achieved R0 resection in the Cleveland Clinic study. A total of 18 patients had tumors fixed to the sidewall, but no detailed R0 resection data were found for either the recurrent or non-recurrent patients.
Cai et al. performed a systematic review and meta-analysis, which included three randomized controlled studies and 12 observational studies of 1,460 patients, to evaluate the efficacy of IORT in treating patients with rectal cancer (26). A total of 9 studies reported a 5-year OS rate ranging from 19% to 84%. The study with the lowest 5-year OS rate (19%) was conducted at the Mayo Clinic in 1995. In that study, 42 patients with LARC underwent palliative surgical resection followed by IOERT, rather than INTRABEAM PRS® IORT (27). The highest 5-year OS rate (84%) was reported in Zhang et al.’s study (25). Another study investigating the efficacy of IORT for resectable advanced lower rectal cancer in 38 patients reported a 5-year OS of 71.5% (28). Notably, this study included patients with T1/T2 stages, which might have contributed to the increased 5-year OS rate compared to that reported in our study. In relation to the 5-year local control rates, 14 studies reported rates ranging from 44% to 98%. The study with the highest 5-year local control rate (98%) involved patients with T3–4NxM0 rectal cancer treated with preoperative radio/chemoradiotherapy, radical surgery, and IORT (29). Additionally, that study reported 5-year OS and DFS rates of 79% and 71%, respectively, surpassing the outcomes observed in our study. Notably, the patients included in our study did not receive neoadjuvant radio/chemotherapy, and the inclusion of a large sample size might have influenced these outcomes.
In another study involving 19 patients with advanced lower rectal cancer who underwent curative resection with TME combined with IORT, a notable higher 5-year local control rate of 95% was achieved (30). Compared to our study, this study included patients with T1–2 rectal cancer and excluded those with T4 rectal cancer, which could account for the differences in the observed outcomes. However, the 5-year OS and DFS rates (64% and 60%, respectively) were slightly lower than those observed in our study (68.59% and 66.91%, respectively). The studies reported in Liu et al.’s systematic review and meta-analysis (26) did not report notably higher 5-year OS, DFS, and local control rates compared to our study.
The low-kV X-ray IORT system is small in size, light weight, and transportable. It can produce a low penetration depth and relatively mild low-energy X-rays (30–50 kV) that act on tumor tissue and the tumor bed (31). Compared with other forms of IORT, the low-kV X-ray IORT showed promising results in the treatment of LARC in our study. In a previous study, researchers evaluated the long-term results of IOERT in combination with TME and chemoradiation in patients with LARC (32). The 5-year OS and DFS rates (69% and 66%, respectively) did not differ to those reported in our study. However, more than 50% of the patients received preoperative chemotherapy in that study, which takes more time and has higher treatment costs than those in our study.
In another study, the long-term survival of patients after HDR-IORT for LARC was investigated (33). The 5-year OS and DFS rates (56% and 48%, respectively) were obviously lower than those reported in our study. Further, compared with neoadjuvant therapy for LARC, IORT also shows satisfactory results. In a clinical investigation, neoadjuvant chemoradiotherapy, TME, and adjuvant chemotherapy were administered to patients with LARC (34). The 3-year OS and DFS rates were 88% and 68%, which were slightly lower than those reported in our study (89.42% and 71.47%, respectively). IORT not only reduces the treatment time, but also shows promising treatment efficacy for LARC.
It is essential to assess the safety of IORT combined with surgery for the treatment of LARC. Detailed early and late complications were recorded in our study. Notably, no mortality occurred within 30 days post-surgery. Urinary retention, diarrhea, and bowel obstruction were observed in four, seven, and four patients, respectively, but all these patients were cured by conservative treatment. Anastomotic leak occurred in 5 patients (7.24%), but was effectively managed with ileostomy without significant discomfort. As for late complications, no abdominal abscess or fistula with abscess was observed. A small number of patients presented with ureteral obstruction (three patients), bladder dysfunction (two patients), and bowel obstruction (six patients). These late complications may be related to tumor recurrence or abdominal adhesion. Sexual dysfunction occurred in five patients, all of whom underwent Miles surgery. There was no evidence that the early and late complications observed in this study were related to the application of low-kV X-ray IORT. All these complications are common in the surgery of LARC. Enhanced surgical operations, early detection, and proper treatment remain effective methods for treating these complications. Overall, our findings suggest that radical resection plus IORT does not induce severe early and late complications and may be safe for LARC treatment.
K-M estimates for OS and DFS did not reveal any differences in the patients stratified by T stage in this study. The number of patients in the T3 and T4 stages was 62 and 7, respectively. However, two of the seven patients with T4 stage were lost to follow-up. As a result, the question of whether the T stage can influence OS and DFS following IORT requires further verification. K-M estimates for OS and DFS showed statistical differences among patients stratified by lymph node status and nerve/vascular invasion. Specifically, the later the regional lymph node stage of the tumor, the lower the long-term OS and DFS. Further, positive nerve/vascular invasion often indicates that the local stage of the tumor is late, which may result in lower OS and DFS than that with negative nerve/vascular invasion. This discrepancy may be attributed to the effects of regional lymph node status and nerve/vascular invasion on tumor stage and prognosis (35,36).
This study had some limitations. First, the retrospective nature of the study and the limited sample size might affect the generalizability of the results. Second, the follow-up time was insufficient, and some patients were lost to follow-up. Third, the scarcity of data on low-kV X-ray IORT for LARC hampers direct comparisons with our study, highlighting the need for further research in this area.
Conclusions
This study showed that combining radical resection with low-kV X-ray IORT is a promising treatment approach for LARC, resulting in improved survival rates and manageable toxicity.
Acknowledgments
Funding: This research was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-965/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-965/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The Second Hospital of Jilin University (No. 2015-230). Informed consent was obtained from all individual participants included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Henley SJ, Ward EM, Scott S, Ma J, Anderson RN, Firth AU, Thomas CC, Islami F, Weir HK, Lewis DR, Sherman RL, Wu M, Benard VB, Richardson LC, Jemal A, Cronin K, Kohler BA. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer 2020;126:2225-49. [Crossref] [PubMed]
- Brouwer NPM, Bos ACRK, Lemmens VEPP, Tanis PJ, Hugen N, Nagtegaal ID, de Wilt JHW, Verhoeven RHA. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer 2018;143:2758-66. [Crossref] [PubMed]
- Bando H, Ohtsu A, Yoshino T. Therapeutic landscape and future direction of metastatic colorectal cancer. Nat Rev Gastroenterol Hepatol 2023;20:306-22. [Crossref] [PubMed]
- Kasi A, Abbasi S, Handa S, Al-Rajabi R, Saeed A, Baranda J, Sun W. Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open 2020;3:e2030097. [Crossref] [PubMed]
- Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet 2019;394:1467-80. [Crossref] [PubMed]
- Schrag D, Shi Q, Weiser MR, Gollub MJ, Saltz LB, Musher BL, et al. Preoperative Treatment of Locally Advanced Rectal Cancer. N Engl J Med 2023;389:322-34. [Crossref] [PubMed]
- Amarnath SR. The Role of Intraoperative Radiotherapy Treatment of Locally Advanced Rectal Cancer. Clin Colon Rectal Surg 2024;37:239-47. [Crossref] [PubMed]
- Palavani LB, de Barros Oliveira L, Reis PA, Batista S, Santana LS, de Freitas Martins LP, Rabelo NN, Bertani R, Welling LC, Figueiredo EG, Paiva WS, Neville IS. Efficacy and Safety of Intraoperative Radiotherapy for High-Grade Gliomas: A Systematic Review and Meta-Analysis. Neurosurg Rev 2024;47:47. [Crossref] [PubMed]
- Yang Y, Hou X, Kong S, Zha Z, Huang M, Li C, Li N, Ge F, Chen W. Intraoperative radiotherapy in breast cancer: Alterations to the tumor microenvironment and subsequent biological outcomes Mol Med Rep 2023;28:231. (Review). [Crossref] [PubMed]
- Sethi A, Gros S, Brodin P, Ghavidel B, Chai X, Popovic M, Tomé WA, Trichter S, Yang X, Zhang H, Uhl V. Intraoperative radiation therapy with 50 kV x-rays: A multi-institutional review. J Appl Clin Med Phys 2024;25:e14272. [Crossref] [PubMed]
- Calvo Manuel FÁ, Serrano J, Solé C, Cambeiro M, Palma J, Aristu J, Garcia-Sabrido JL, Cuesta MA, Del Valle E, Lapuente F, Miñana B, Morcillo MÁ, Asencio JM, Pascau J. Clinical feasibility of combining intraoperative electron radiation therapy with minimally invasive surgery: a potential for electron-FLASH clinical development. Clin Transl Oncol 2023;25:429-39. [Crossref] [PubMed]
- Roeder F, Fastner G, Fussl C, Sedlmayer F, Stana M, Berchtold J, Jäger T, Presl J, Schredl P, Emmanuel K, Colleselli D, Kotolacsi G, Scherer P, Steininger P, Gaisberger C. First clinical application of image-guided intraoperative electron radiation therapy with real time intraoperative dose calculation in recurrent rectal cancer: technical procedure. Radiat Oncol 2023;18:186. [Crossref] [PubMed]
- Agas RAF, Tan J, Xie J, Van Dyk S. C H Kong J, Heriot A, Ngan SY. Intensification of Local Therapy With High Dose Rate, Intraoperative Radiation Therapy (HDR-IORT) and Extended Resection for Locally Advanced and Recurrent Colorectal Cancer. Clin Colorectal Cancer 2023;22:257-66. [Crossref] [PubMed]
- Voogt ELK, van Rees JM, Hagemans JAW, Rothbarth J, Nieuwenhuijzen GAP, Cnossen JS, Peulen HMU, Dries WJF, Nuyttens J, Kolkman-Deurloo IK, Verhoef C, Rutten HJT, Burger JWA. Intraoperative Electron Beam Radiation Therapy (IOERT) Versus High-Dose-Rate Intraoperative Brachytherapy (HDR-IORT) in Patients With an R1 Resection for Locally Advanced or Locally Recurrent Rectal Cancer. Int J Radiat Oncol Biol Phys 2021;110:1032-43. [Crossref] [PubMed]
- Xue W, Wang S, Zhao Z, Li Y, Shang A, Li D, Yang J, Wang T, Wang M. Short-term outcomes of laparoscopic intersphincteric resection with intraoperative radiotherapy using low-energy X-rays for primary locally advanced low rectal cancer: a single center experience. World J Surg Oncol 2020;18:26. [Crossref] [PubMed]
- Watson PGF, Popovic M, Liang L, Tomic N, Devic S, Seuntjens J. Clinical Implication of Dosimetry Formalisms for Electronic Low-Energy Photon Intraoperative Radiation Therapy. Pract Radiat Oncol 2021;11:e114-21. [Crossref] [PubMed]
- Chiodo C, Gros S, Emami B, Lee B, Block A, Sethi A, Small W Jr, Refaat T. Intraoperative radiation therapy for locally advanced and recurrent head and neck cancer. Mol Clin Oncol 2022;17:158. [Crossref] [PubMed]
- Rondelli F, Sanguinetti A, Polistena A, Avenia S, Marcacci C, Ceccarelli G, Bugiantella W, De Rosa M. Robotic Transanal Total Mesorectal Excision (RTaTME): State of the Art. J Pers Med 2021;11:584. [Crossref] [PubMed]
- Li Q, Li Y, Dai W, Wang S, Xu Y, Li X, Cai S. Adjuvant radiotherapy improves cause specific survival in stage II, not stage III mucinous carcinoma of the rectum. BMC Cancer 2017;17:80. [Crossref] [PubMed]
- Stender MT, Larsen TB, Lundbye-Christensen S, Yilmaz MK, Thorlacius-Ussing O. Haemostatis activity in rectal cancer patients exposed to preoperative radiotherapy: a clinical prospective cohort study. Blood Coagul Fibrinolysis 2009;20:276-82. [Crossref] [PubMed]
- Lybeert ML, Martijn H, de Neve W, Crommelin MA, Ribot JG. Radiotherapy for locoregional relapses of rectal carcinoma after initial radical surgery: definite but limited influence on relapse-free survival and survival. Int J Radiat Oncol Biol Phys 1992;24:241-6. [Crossref] [PubMed]
- Kienle P, Abend F, Dueck M, Abel U, Treiber M, Riedl S. Influence of intraoperative and postoperative radiotherapy on functional outcome in patients undergoing standard and deep anterior resection for rectal cancer. Dis Colon Rectum 2006;49:557-67. [Crossref] [PubMed]
- Guo S, Reddy CA, Kolar M, Woody N, Mahadevan A, Deibel FC, Dietz DW, Remzi FH, Suh JH. Intraoperative radiation therapy with the photon radiosurgery system in locally advanced and recurrent rectal cancer: retrospective review of the Cleveland clinic experience. Radiat Oncol 2012;7:110. [Crossref] [PubMed]
- Ma L, Qiang J, Yin H, Lin L, Jiao Y, Ma C, Li X, Dong L, Cui J, Wei D, Sharma AM, Schwartz DL, Gu W, Chen H. Low-kilovolt x-ray intraoperative radiotherapy for pT3 locally advanced colon cancer: a single-institution retrospective analysis. World J Surg Oncol 2020;18:132. [Crossref] [PubMed]
- Zhang Q, Tey J, Yang Z, Li P, Peng L, Jiang R, Xiong F, Fu S, Lu JJ. Intraoperative radiotherapy in the combination of adjuvant chemotherapy for the treatment of pT3N0M0 rectal cancer after radical surgery. Am J Clin Oncol 2014;37:8-12. [Crossref] [PubMed]
- Liu B, Ge L, Wang J, Chen YQ, Ma SX, Ma PL, Zhang YQ, Yang KH, Cai H. Efficacy and safety of intraoperative radiotherapy in rectal cancer: A systematic review and meta-analysis. World J Gastrointest Oncol 2021;13:69-86. [Crossref] [PubMed]
- Suzuki K, Gunderson LL, Devine RM, Weaver AL, Dozois RR, Ilstrup DM, Martenson JA, O'Connell MJ. Intraoperative irradiation after palliative surgery for locally recurrent rectal cancer. Cancer 1995;75:939-52. [Crossref] [PubMed]
- Masaki T, Matsuoka H, Kishiki T, Kojima K, Aso N, Beniya A, Tonari A, Takayama M, Abe N, Sunami E. Intraoperative radiotherapy for resectable advanced lower rectal cancer-final results of a randomized controlled trial (UMIN000021353). Langenbecks Arch Surg 2020;405:247-54. [Crossref] [PubMed]
- Sadahiro S, Suzuki T, Ishikawa K, Fukasawa M, Saguchi T, Yasuda S, Makuuchi H, Murayama C, Ohizumi Y. Preoperative radio/chemo-radiotherapy in combination with intraoperative radiotherapy for T3-4Nx rectal cancer. Eur J Surg Oncol 2004;30:750-8. [Crossref] [PubMed]
- Masaki T, Takayama M, Matsuoka H, Abe N, Ueki H, Sugiyama M, Tonari A, Kusuda J, Mizumoto S, Atomi Y. Intraoperative radiotherapy for oncological and function-preserving surgery in patients with advanced lower rectal cancer. Langenbecks Arch Surg 2008;393:173-80. [Crossref] [PubMed]
- Sethi A, Emami B, Small W Jr, Thomas TO. Intraoperative Radiotherapy With INTRABEAM: Technical and Dosimetric Considerations. Front Oncol 2018;8:74. [Crossref] [PubMed]
- Krempien R, Roeder F, Oertel S, Roebel M, Weitz J, Hensley FW, Timke C, Funk A, Bischof M, Zabel-Du Bois A, Niethammer AG, Eble MJ, Buchler MW, Treiber M, Debus J. Long-term results of intraoperative presacral electron boost radiotherapy (IOERT) in combination with total mesorectal excision (TME) and chemoradiation in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2006;66:1143-51. [Crossref] [PubMed]
- Terezakis S, Morikawa L, Wu A, Zhang Z, Shi W, Weiser MR, Paty PB, Guillem J, Temple L, Nash GM, Zelefsky MJ, Goodman KA. Long-Term Survival After High-Dose-Rate Brachytherapy for Locally Advanced or Recurrent Colorectal Adenocarcinoma. Ann Surg Oncol 2015;22:2168-78. [Crossref] [PubMed]
- Markovina S, Youssef F, Roy A, Aggarwal S, Khwaja S, DeWees T, Tan B, Hunt S, Myerson RJ, Chang DT, Parikh PJ, Olsen JR. Improved Metastasis- and Disease-Free Survival With Preoperative Sequential Short-Course Radiation Therapy and FOLFOX Chemotherapy for Rectal Cancer Compared With Neoadjuvant Long-Course Chemoradiotherapy: Results of a Matched Pair Analysis. Int J Radiat Oncol Biol Phys 2017;99:417-26. [Crossref] [PubMed]
- Quere P, Facy O, Manfredi S, Jooste V, Faivre J, Lepage C, Bouvier AM. Epidemiology, Management, and Survival of Peritoneal Carcinomatosis from Colorectal Cancer: A Population-Based Study. Dis Colon Rectum 2015;58:743-52. [Crossref] [PubMed]
- Huh JW, Lee JH, Kim HR, Kim YJ. Prognostic significance of lymphovascular or perineural invasion in patients with locally advanced colorectal cancer. Am J Surg 2013;206:758-63. [Crossref] [PubMed]



