Population reference range for developmental lumbar spinal canal size
Introduction
Spinal canal stenosis is the most common indication for lumbar surgery in patients older than 65 years (1). Factors governing spinal stenosis are the pre-existing developmental size of the spinal canal and the degree of acquired spinal canal narrowing (mainly from degenerative disease). Developmental size of the spinal canal is measured at the mid-pedicular level removed from any acquired narrowing that occurs at the discovertebral level (2). Anteroposterior spinal canal development is fully complete by 5 years of age while transverse spinal canal diameter increases until 15–17 years (3,4). Pre-natal (gestational age, placenta size, nutrition, birth weight) and maternal (age, parity, socioeconomic class, smoking) factors may all potentially influence spinal canal development (5-7). Once spinal canal development is complete by 17 years, further growth of the spinal canal will not occur. Other than uncommon spinal diseases, such as dural ectasia, intraspinal tumours or intradural cysts, no other disease or metabolic condition knowingly affects developmental size of the lumbar spinal canal (2).
Considerable variation in the developmental size of the normal lumbar spinal canal exists within and between populations such that each population needs to develop its own reference range (2). Little large scale population data on normal spinal canal dimensions exists, particularly with regard to cross-sectional area (CSA). The purpose of this study was to develop a population range for developmental lumbar spinal canal dimensions using a template that can be readily applied to all other populations. This was undertaken by measuring the spinal canal dimensions in patients undergoing abdominopelvic computed tomography (CT) examination. Although these patients were clearly not healthy at the time of the examination, it is nevertheless valid to use this population to determine developmental spinal canal size as spinal canal development is complete by adulthood and systemic diseases incurred thereafter do not knowingly affect spinal canal dimension at the mid-pedicular level.
Methods
Patients
Study was approved by the Clinical Research Ethics Committee of our institution (CRE-2013.058). Lumbar spinal canal dimensions were prospectively measured on ambulatory patients undergoing abdominopelvic CT examinations between Feb 2014 and Jan 2015. Over 99% of the patient population was ethnically Chinese. Patients with non-Chinese names were not included as were patients with (I) skeletal orders such as dwarfism or scoliosis; (II) prior lumbar surgery; and (III) childhood chronic inflammatory condition; (IV) major lumbar morphological abnormality such as vertebral fracture or dysraphism.
The height (centimeters) and weight (kg) of all patients was recorded prior to CT examination. One thousand and eighty patients aged between 21 and 80 years were studied, comprising 540 males (mean 50.53±16.98 years) and 540 females (mean 50.65±17.02 years). Patients were selected to yield 90 patients from each sex in each 10-year age group (20–29, 30–39 years etc.). Subgroup recruitment stopped once the required 90 patients were recruited.
CT examination and image analysis
CT examinations were performed on a 64-slice multidetector CT machine (LightSpeed VCT, GE Healthcare, Buckinghamshire, UK) with a reconstructive resolution of 0.6 mm. Spinal canal and vertebral body measurements were made on volumetric CT data reconstructed on bone windows in an axial plane though the mid-pedicles for each vertebral body from L1 to L5 inclusive (5,400 levels assessed). All analysis was performed by one operator. First, the volumetric image dataset for each lumbar vertebral level was adjusted to yield an image at right angles to the vertebral body. The mid-pedicle axial image was then automatically determined orthogonal to the mid-sagittal plane of the vertebral body.
Spinal canal CSA
Following rigid co-registration, a seed-growing image segmentation program was used to measure osseous spinal canal CSA. Thereafter, manual modification was performed to ensure correct demarcation of the spinal canal boundary (Figure 1).
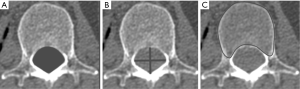
Spinal canal depth and width
Spinal canal depth was measured from the posterior margin of the vertebral body to the cortex of the neural arch at the base of the spinous process (Figure 1). Spinal canal width was measured from the inner margin of one pedicle to the inner margin of the contralateral pedicle (Figure 1).
Vertebral body CSA
Vertebral body CSA was measured using a semi-automatic approach. A threshold technique defined vertebral body CSA based on attenuation differences between the vertebral body and the surrounding soft tissues followed by manual modification of this CSA and demarcation of the pedicle base (8) (Figure 1).
Spinal canal CSA/vertebral body CSA
Spinal canal CSA/vertebral body CSA ratio was determined to assess how consistently one was related to the other.
Thresholds demarcating the smallest 25% of the population
Histograms were drawn separately for each measure and sex to ensure a normal distribution. A cut-off point demarcating the smallest 25% (quartile) of the population was arbitrarily used as an indicator of a developmentally small spinal canal CSA, depth or width.
Comparison of smallest and largest quartiles
To gauge variation within the population, the 2 patient quartiles (25%) with the largest and smallest spinal canal dimension at each level from L1 to L5 for both males and females were selected and compared. These subgroups were matched for patient height.
Reliability and concordance for computerized and manual measurements
For computerized measurements, one reader selected the appropriate image and measured spinal canal CSA, depth and width at five levels (L1–L5) on 20 randomly selected subjects (100 axial levels) on two separate occasions 1 week apart blinded to previous results. Another reader independently selected and manually measured the same parameters at five levels (L1–L5) on the same 20 subjects. Results of the second computerized readings were compared to the manual readings.
Statistical analysis
Statistical analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, Illinois, US). Intraclass correlation was used to test reliability and independent 2-samples t-test for gender and inter-quartile differences. Pearson correlation was used to determine associations between spinal canal dimensions and age, height, weight and BMI. A P value <0.05 was considered statistically significant.
Results
Gender and age-related differences
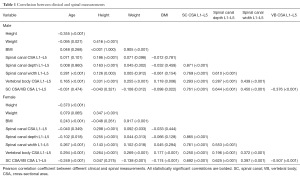
Males were taller (168.1±6.8 vs. 157.0±5.9 cm, P<0.0001), heavier (66.9±12.8 vs. 55.5±10.6 kg, P<0.0001) and had a higher BMI (23.7±4.1 vs. 22.5±4.0, P<0.0001) than females. With increasing age, both sexes had a slight reduction in mean height, males had a slight decrease in mean weight, while females had a slight increase in BMI (Table 1).
Full table
Spinal canal CSA
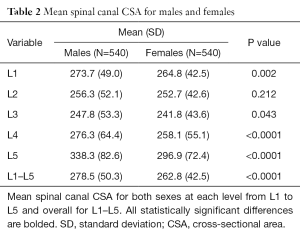
Developmental spinal canal CSA was smallest at L3 for both sexes increasing in size both cranially and caudally (Table 2). Average spinal canal CSA at L3 was about 9% smaller than at L1 and about 23% smaller than at L5 (Table 2).
Full table
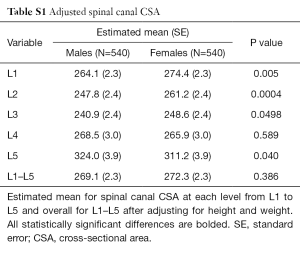
The spinal canal CSA was larger in males at all levels, other than L2 (Table 2). However, after adjustment for height and weight, spinal canal CSA was larger in females at L1, L2 and L3 and in males at L5 (Table S1).
Full table
For each gender, there was no detectable change in spinal canal CSA with age (Table 1). There was a weak but highly significant positive correlation between increasing height and increasing overall lumbar spine CSA (Table 1). For females only, there was a very slight increase in spinal CSA with increasing weight (Table 1).
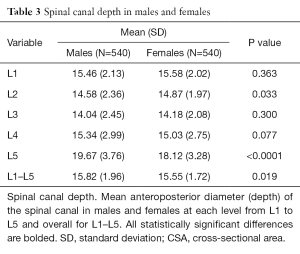
Spinal canal depth
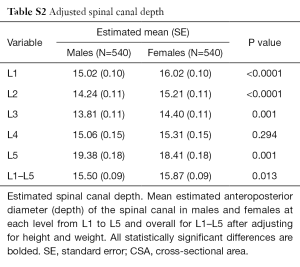
Developmental spinal canal depth was also smallest at L3, increasing cranially and caudally (Table 3). After height and weight adjustment, spinal canal depth was larger in females at L1, L2 and L3 and in males at L5 (Table S2). Overall, spinal change depth did not change with increasing age in males (Table 1) though did reduce slightly in females (Table 1). There was a weak but highly significant positive correlation between increasing height and increasing overall lumbar spine depth for both sexes (Table 1).
Full table
Full table
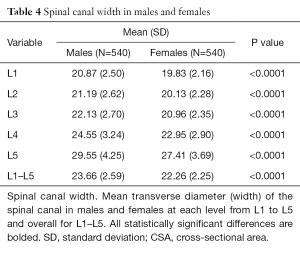
Spinal canal width
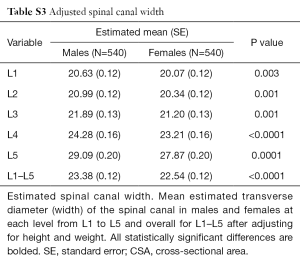
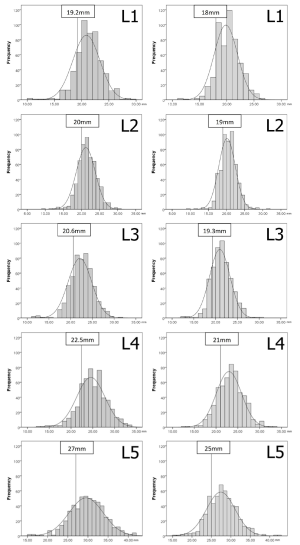
As opposed to spinal canal CSA and depth, which were smallest at L3, developmental spinal canal width gradually increased from L1 to L5 (Table 4). Spinal canal width was larger in males at all levels (Table 4), even after adjustment for height and weight (Table S3).
Full table
Full table
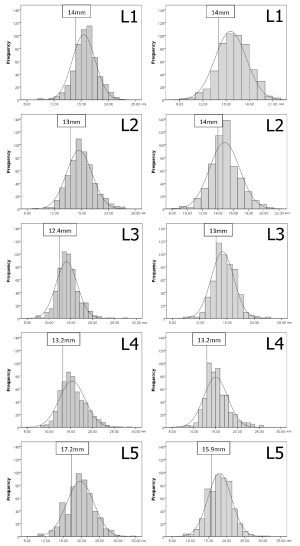
Thresholds demarcating the smallest 25% of the population for developmental spinal canal CSA, depth or width
The values demarcating the smallest 25% of the population for developmental spinal canal CSA, depth or width for each level and gender are shown in Figures S1-S3.
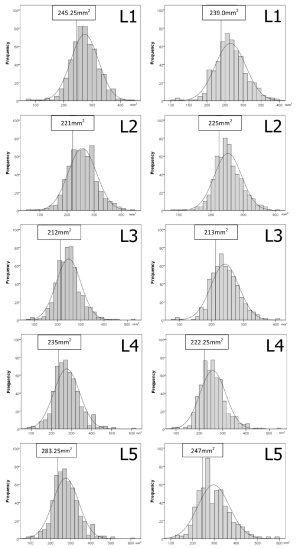
Vertebral body CSA
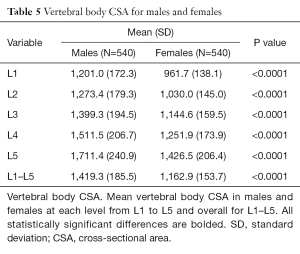
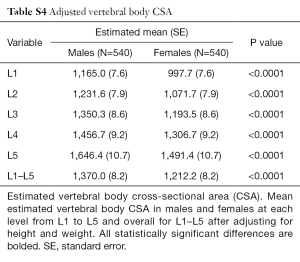
Vertebral body CSA was significantly larger in males at all levels (Table 5). This difference remained true even after adjustment for height and weight (Tables S4,S5). Vertebral body CSA increased with age in both sexes with the rate of increase being almost twice as great in females (Table 1). For both sexes, there was a weak but highly significant positive correlation between increasing vertebral body CSA and increasing height, weight and BMI (Table 1).
Full table
Full table
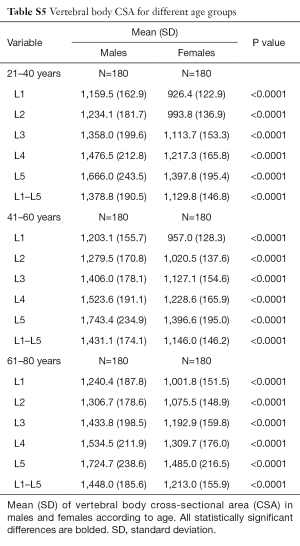
Full table
Spinal canal CSA/vertebral body CSA
Developmental spinal canal CSA was approximately one-fifth that of vertebral body CSA (Table 6). Although spinal canal CSA (Table 2) and vertebral body CSA (Table 5) were both larger in males, the spinal canal CSA/vertebral body CSA ratio was consistently larger in females (Table 6) indicating that, relative to the CSA of the vertebral body, the CSA of the spinal canal is actually larger in females than males, even after adjusted for height and weight (Table S6).
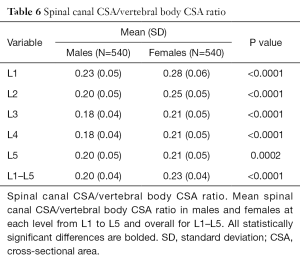
Full table
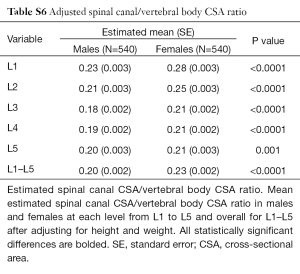
Full table
The spinal canal CSA/vertebral body CSA ratio decreased with age only in females as female vertebral body CSA tended to increase more with age than males (Table 1). The spinal canal CSA/vertebral body CSA ratio did decrease slightly with increasing weight and BMI (Table 1).
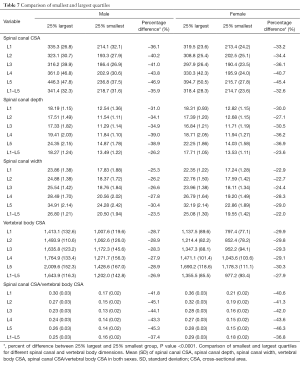
Comparison of smallest and largest quartiles
Patients in the smallest quartile had a spinal canal CSA about 34% smaller than those in the largest quartile (Table 7). This variation was ~24% for spinal canal depth, and ~22% for spinal canal width (Table 7), after adjustment for height and weight (P<0.0001).
Full table
Reliability and concordance for computerized and manual measurements
Intra-operator reliability of computerized measurements was 0.85, 0.87, 0.91, 0.85, and 0.96 respectively for each level from L1–L5. Reliability between computerized and manual readings was 0.80, 0.89, 0.88, 0.83 and 0.95 respectively.
Discussion
Symptomatic lumbar spinal canal stenosis is a function of both the developmental size of the spinal canal (i.e., how large the spinal canal is to begin with) and the degree of superimposed (i.e., acquired) bony and soft tissue spinal canal encroachment, usually from degenerative disease. Developmental size of the spinal canal has considerable bearing on the likelihood of nerve root compression (9), spinal canal stenosis (9), or the need for decompressive surgery (10). The larger the developmental size of the spinal canal, the lower the risk of neurological compromise (2).
Geographic, racial and gender differences in developmental size of the spinal canal do exist such that each region, race and gender should have its own reference range (2). Such normative reference data will become routine with the automated availability of quantitative spinal canal size data during routine MR spine examination (11-14). This study utilized abdominopelvic CT data to establish a population reference range for developmental lumbar spinal canal size. This provides a good template for comparative studies from other populations given that over 100 million abdominopelvic CTs are performed yearly around the world (15). This is by far the largest study of developmental spinal canal size undertaken to date.
This study confirms previous findings that nearly all developmental spinal canal dimensions are smaller in females (16-19). Spinal canal CSA and depth were consistently smallest at L3 from where they enlarged cranially and caudally. Spinal canal width gradually increased from L1 to L5. Spinal canal depth rather than width is the primary contributor to developmental CSA (3,20).
Considerable variation in developmental spinal canal dimensions that exist within a single population with a 34% difference in spinal canal CSA and a 24% difference in depth between the largest and smallest quartiles. No accepted definition as to what defines normal and abnormal spinal canal development exists (21). Anteroposterior diameters from <10 to <14 mm have been quoted as measures of mid-vertebral spinal canal ‘stenosis’ (21-23) with a value of <11 mm at L2 and L3 being the most widely accepted (23). The current study uses an arbitrary cut-off of the lowest quartile to represent a developmentally narrow canal. For the study population, an anteroposterior value of <13 mm in males and <14 mm in females at L2 indicates the lowest 25% of the population range with different values for other levels. From a US-based study, Peter F. Ullrich Jr et al. suggested <145 mm2 as a measure of ‘developmental stenosis’ at L3. This value is too low for the current population where a mid-vertebral spinal canal CSA of <212 mm2 in males and <213 mm2 in females indicates a patient in the smallest 25% of the population.
Taller patients were slightly more inclined to developmentally have a spinal canal with a larger CSA and depth. No change in developmental spinal canal CSA or depth was apparent with increasing age. Vertebral body CSA did, however, increase slightly in old age, particularly in females. This is not unexpected as reduced bone mineral density has a recognized association with increased lumbar vertebral CSA, probably as an inherent compensatory mechanism for reduced bone strength (24,25). Although males have a larger spinal canal CSA and vertebral body CSA than females, relative to vertebral body CSA, the spinal canal CSA is actually larger in females. This relative difference lessened in older females as overall vertebral body CSA increased.
One similar smaller scale study performed in Lausanne, Switzerland (2) showed a similar trend with males having larger spinal canal dimensions and the smallest lumbar CSA being at L3. The CSA of the lumbar spinal canal was generally 8–16% larger in Swiss subjects than in the current population. In contrast, the current study found no increase in lumbar canal CSA with age.
In conclusion, a population reference range for normal developmental size of the spinal canal was developed using data from abdominopelvic CT examinations from a large patient cohort. The reference range developed will be useful for gauging individual spinal canal development and ultimately adopting a more quantitative approach to the assessment of developmental spinal canal narrowing. The template used in this study is suited to cross-comparison with CT databases from other populations. An accurate relevant population reference range is critical to defining what constitutes a developmentally narrow canal, its clinical significance and to exploring, in great detail, the factors governing the aetiology of developmental spinal canal stensosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Joint Clinical Research Ethics Committee of The Chinese University of Hong Kong (CRE-2013.058) and written informed consent was obtained from all patients.
References
- Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med 2008;358:818-25. [Crossref] [PubMed]
- Schizas C, Schmit A, Schizas A, Becce F, Kulik G, Pierzchała K. Secular changes of spinal canal dimensions in Western Switzerland: a narrowing epidemic? Spine (Phila Pa 1976) 2014;39:1339-44. [Crossref] [PubMed]
- Papp T, Porter RW, Aspden RM. The growth of the lumbar vertebral canal. Spine (Phila Pa 1976) 1994;19:2770-3. [Crossref] [PubMed]
- Watts R. Lumbar vertebral canal size in adults and children: observations from a skeletal sample from London, England. Homo 2013;64:120-8. [Crossref] [PubMed]
- Jeffrey JE, Campbell DM, Golden MH, Smith FW, Porter RW. Antenatal factors in the development of the lumbar vertebral canal: a magnetic resonance imaging study. Spine (Phila Pa 1976) 2003;28:1418-23. [Crossref] [PubMed]
- Papp T, Porter RW, Craig CE, Aspden RM, Campbell DM. Significant antenatal factors in the development of lumbar spinal stenosis. Spine (Phila Pa 1976) 1997;22:1805-10. [Crossref] [PubMed]
- Clark GA, Panjabi MM, Wetzel FT. Can infant malnutrition cause adult vertebral stenosis? Spine (Phila Pa 1976) 1985;10:165-70. [Crossref] [PubMed]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006;31:1116-28. [Crossref] [PubMed]
- Dullerud R, Amundsen T, Lie H, Juel NG, Magnaes B. CT-diskography, diskomanometry and MR imaging as predictors of the outcome of lumbar percutaneous automated nucleotomy. Acta Radiol 1995;36:613-9. [Crossref] [PubMed]
- Schizas C, Theumann N, Burn A, Tansey R, Wardlaw D, Smith FW, Kulik G. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine (Phila Pa 1976) 2010;35:1919-24. [Crossref] [PubMed]
- Koh J, Kim T, Chaudhary V, Dhillon G. Automatic segmentation of the spinal cord and the dural sac in lumbar MR images using gradient vector flow field. Conf Proc IEEE Eng Med Biol Soc 2010;2010:3117-20.
- Koh J, Chaudhary V, Dhillon G. Automated boundary extraction of the spinal canal in MRI based on dynamic programming. Conf Proc IEEE Eng Med Biol Soc 2012;2012:6559-62.
- Koh J, Chaudhary V, Jeon EK, Dhillon G. Automatic spinal canal detection in lumbar MR images in the sagittal view using dynamic programming. Comput Med Imaging Graph 2014;38:569-79. [Crossref] [PubMed]
- Ghosh S, Chaudhary V. Supervised methods for detection and segmentation of tissues in clinical lumbar MRI. Comput Med Imaging Graph 2014;38:639-49. [Crossref] [PubMed]
- Berrington de González A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, Land C. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med 2009;169:2071-7. [Crossref] [PubMed]
- Zhou SH, McCarthy ID, McGregor AH, Coombs RR, Hughes SP. Geometrical dimensions of the lower lumbar vertebrae--analysis of data from digitised CT images. Eur Spine J 2000;9:242-8. [Crossref] [PubMed]
- Lee HM, Kim NH, Kim HJ, Chung IH. Morphometric study of the lumbar spinal canal in the Korean population. Spine (Phila Pa 1976) 1995;20:1679-84. [Crossref] [PubMed]
- Amonoo-Kuofi HS. The sagittal diameter of the lumbar vertebral canal in normal adult Nigerians. J Anat 1985;140:69-78. [PubMed]
- Eisenstein S. The morphometry and pathological anatomy of the lumbar spine in South African negroes and caucasoids with specific reference to spinal stenosis. J Bone Joint Surg Br 1977;59:173-80. [PubMed]
- Verbiest H. Further experiences on the pathological influence of a developmental narrowness of the bony lumbar vertebral canal. J Bone Joint Surg Br 1955;37-B:576-83. [PubMed]
- Steurer J, Roner S, Gnannt R, Hodler J. LumbSten Research Collaboration. Quantitative radiologic criteria for the diagnosis of lumbar spinal stenosis: a systematic literature review. BMC Musculoskelet Disord 2011;12:175. [Crossref] [PubMed]
- Kalichman L, Guermazi A, Li L, Hunter DJ. Association between age, sex, BMI and CT-evaluated spinal degeneration features. J Back Musculoskelet Rehabil 2009;22:189-95. [Crossref] [PubMed]
- Mamisch N, Brumann M, Hodler J, Held U, Brunner F, Steurer J. Lumbar Spinal Stenosis Outcome Study Working Group Zurich. Radiologic criteria for the diagnosis of spinal stenosis: results of a Delphi survey. Radiology 2012;264:174-9. [Crossref] [PubMed]
- Link TM, Dören M, Lewing G, Meier N, Heinecke A, Rummeny E. Cross-sectional area of lumbar vertebrae in peri- and postmenopausal patients with and without osteoporosis. Osteoporos Int 2000;11:304-9. [Crossref] [PubMed]
- Junno JA, Paananen M, Karppinen J, Niinimäki J, Niskanen M, Maijanen H, Väre T, Järvelin MR, Nieminen MT, Tuukkanen J, Ruff C. Age-related trends in vertebral dimensions. J Anat 2015;226:434-9. [Crossref] [PubMed]


