
Percutaneous radiofrecuency thermal ablation for management of recurrent bone giant cell tumour
Introduction
Giant cell tumor of bones (GCTBs) are relatively rare, aggressive, and often benign neoplasms that predominantly affect the epiphyseal regions of long bones. These tumors are characterized by the presence of multinucleated giant cells and can cause significant morbidity due to their locally aggressive nature and potential to recur (1).
The gold standard treatments for GCTBs include surgical resection and curettage, often supplemented with adjuvant therapies. However, the recurrence of giant cell tumors remains a significant clinical challenge [GCTB overall recurrence occurs in 29% of cases as a recent meta-analysis has reported (2)], necessitating innovative and less invasive therapeutic approaches. Denosumab can be an alternative option in some cases when surgery is not possible or is associated with high morbidity (1). Denosumab is a monoclonal antibody that targets and inhibits RANKL (Receptor Activator of Nuclear Factor Kappa-B Ligand), a protein essential for the formation, function, and survival of osteoclasts. In the context of treating GCTB, denosumab has shown significant efficacy by reducing the number and activity of osteoclast-like giant cells, leading to tumor shrinkage and decreased bone destruction. However, the appropriate duration of treatment is still nuclear (3) and several authors have noted that long-term follow-up of denosumab treatment may developed high grade sarcomas in the same site of GCTB (4,5). Zolendronic acid (nitrogen containing bisphosphonate) also can be a good medical treatment due to its ability to induce apoptosis in the osteoclast related damage in GCTB (4,5).
One such promising technique is percutaneous radiofrecuency thermal ablation (RFTA). This minimally invasive procedure functions by inserting a specialized electrode or probe into the target tumor under imaging guidance, typically using computed tomography (CT) or ultrasound (US). Once positioned, the probe emits high-frequency alternating electrical currents, generating localized thermal energy that elevates the temperature within the tumor tissue to approximately 60–100 ℃. This heat induces coagulative necrosis, effectively destroying tumor cells by denaturing cellular proteins, disrupting cellular membranes, and causing irreversible damage to the tumor’s vascular supply (6,7). The thermal effect extends to the immediate peritumoral region, ensuring comprehensive ablation of malignant cells while sparing adjacent healthy tissue (8). The physiological response includes an initial inflammatory phase followed by fibrosis and scar tissue formation, which contributes to the overall reduction in tumor mass and symptomatic relief for the patient.
The primary purpose of this study is to describe our experience with percutaneous RFTA treatment for recurrent GCTBs, particularly in cases where additional surgery would have been excessively aggressive.
Case presentation
This study examines three patients who experienced recurrent GCTB and were treated with percutaneous RFTA at our institution between 2016 and 2024. All procedures performed in this study were in accordance with the ethical guidelines of the institutional and/or national research committees and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
The GCTB recurrences were located as follows:
- Case 1: medial intercondylar eminence of the right tibia;
- Case 2: lateral condyle of the right knee;
- Case 3: anterior intercondylar area tibial spine of the left tibia.
Each patient had a prior diagnosis of bone giant cell tumor confirmed through percutaneous CT-guided biopsy followed by surgical intervention. Initial treatments involved extended intralesional resection, curettage, high-speed burring, pulsatile lavage, phenol application, and reconstruction with bone allograft or cementoplasty.
Recurrence of the GCTB was confirmed in each case via percutaneous biopsy. Percutaneous RFTA was then performed under CT guidance. Before the procedure, informed consent was obtained, and patients were briefed on alternative treatment options. Procedures were conducted under general anesthesia or deep sedation in a CT suite.
The percutaneous RFTA technique followed established protocols for treating osteoid osteoma (6,7). Using CT guidance, an 11–13 gauge (G) introducer bone biopsy needle accessed the lesion. The stylet was replaced with a 17 G monopolar RF electrode (Cool-tip Covidien RFA System). CT imaging ensured accurate placement of the electrode’s active tip (7–10 mm) within the lesion. Radiofrequency activation ranged from 3 to 5 minutes, ensuring the core temperature reached 90 ℃ (Figure 1).
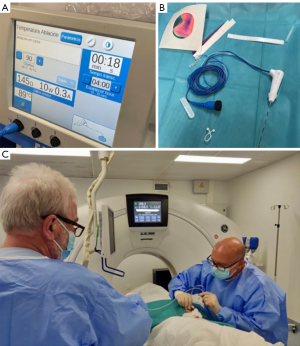
Post-treatment follow-up involved CT and magnetic resonance imaging (MRI) scans over periods ranging from 18 months to 5 years.
Case 1
Female, 33 years old with left Knee pain for the last year, accentuated in the last three months. CT and MRI showed an metaphyseal-epiphyseal osteolytic lesion in the right proximal tibia adjacent to the joint. Percutaneous CT guided biopsy showed histology of GCTB. Surgical treatment was performed without complications.
Follow-up eight years post-surgery single-photon emission computed tomography (SPECT-CT) showed a small recurrence in the intercondylar eminence of the tibia (Figure 2). CT guided percutaneous RFTA treatment was performed through a double approach to both the anterior and posterior lesions and then filled with cement (Figure 3).
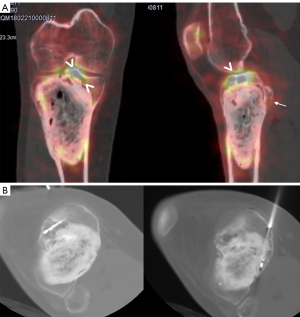

CT and MRI follow-up did not show residual nor recurrent tumor: no gadolinium enhancement until 5 years post-procedure. The patient is asymptomatic with complete recovery.
Case 2
Female, 19 years who notices a lump in the right knee of several months of evolution with pain after efforts and that in the last month it is continuous. Percutaneous US guided biopsy showed histology of GCTB. Surgery as described in methods and materials was performed with bone graft reconstruction.
Follow-up with MRI monitoring (one year later) showed a recurrence lesion in the surgical site with soft tissue extension on its posterior supracondylar margin, which was treated with another surgery but this time with cemment reconstruction instead. One year later MRI showed another recurrence/remaining tumor foci percutaneous RFTA was performed and the filled with cemment (Figure 4) without a complication. To date, the patient is asymptomatic with no evidence of recurrence or residual tumor after three years follow-up.

Case 3
Female, 18 years old with knee pain since 2018, diagnosed of GCTB and went under surgery in 2021 (intralesional curettage plus cementoplasty).
Two years later an X-ray showed small radiolucent lesion in the anterior intercondylar area of the left tibia adjacent to the cement filling. It was treated by percutaneous RFTA, two approaches were performed, one medial and one anterior with a 12 G Bonopty introducer needle and coaxial introduction of radiofrequency, all monitored with CT (Figure 5).

The patient is asymptomatic with clinical recovery. Due the procedure has been very recently we do not have controls yet.
Discussion
The traditional management of GCTB includes curettage with or without adjuvant therapies such as high-speed milling, pulsatile lavage, phenolization, and cementation or bone graft filling for the management of the condition (9).
Local tumor recurrence is a common challenge GCTB, occurring in 40–60% of cases, even after appropriate surgical interventions (1). This poses a significant therapeutic challenge due to the difficulties in achieving complete resection and the potential for considerable morbidity associated with repeat surgeries. This has led to the exploration of alternative, less invasive treatments such as percutaneous RFTA (9).
Several studies have reported favorable outcomes with percutaneous RFTA in terms of pain relief, local control of the disease, and preservation of function (10-12).
One of the primary advantages of percutaneous RFTA is its ability to provide effective local control with minimal damage to surrounding structures (8), making it particularly suitable for recurrent GCTBs located in anatomically challenging áreas such as ankle or foot or in cases with joint involvement. In addition, percutaneous RFTA can be performed on an outpatient basis, reducing the need for prolonged hospitalization and allowing for quicker recovery times compared to traditional surgical approaches.
Koo and Chung (8) conducted a study on RF ablation for bone tumors unsuitable for radical excision, with a sample of 43 patients, including one with a GCTB. Zhao et al. (11) described a case of a GCTB located in the and knee and treated with percutaneous RFTA guided by US fused with CT. This method provided real-time guidance without the use of radiation, offering a safer and more precise approach for the patient. More recently, Arrigoni et al. (12) described the management of surgical recurrences of GCTB using CT-guided radiofrequency ablation.
However, literature in this field is very scarce until today.
Our study shows successful percutaneous RFTA treatment of GCTB recurrence in 3 patients, who had been treated surgically previously. If we combine them with the 4 that we already published in our previous work (13), they make a total of 7. In this manner, the main difference between our studies and the Arrigoni et al. is the variability of the location of the recurrences, in our case they occur in 6 different locations (lateral cuneiform, distal and proximal tibia, proximal and distal femur and hip) while in them they occur mainly in the proximal tibia. In this way, we can talk about healing in three different joints while they limit themselves to one. The number of patients is somewhat greater, 7 versus 5, and we have greater long-term follow-up with the first patient, practically doubling the follow-up of theirs (15 versus 8 years). Similar to the approaches employed by Zhao et al. (11), Koo and Chung (8), and Arrigoni et al. (12), our procedure involves reaching the lesion under CT guidance, utilizing an introducer needle of 11–13 G and a length of 10 cm. In all instances, the radiofrequency (RF) needle utilized featured a length of 15 cm and a thickness of 17 G. In our study, the applied radiofrequency time is shorter than that used in the study by Arrigoni et al. (3–5 vs. 8 minutes). This is due to the shorter length of the active tip (7–10 mm), compared to that in Zhao et al. (11) (30 mm) among others, which in turn indicates a smaller size of the recurrent tumor tissue in our case series. Additionally, an in vivo experimental study conducted by our group (6) demonstrated sufficient medullary necrosis volume with shorter radiofrequency times.
Another thermal ablation technique is cryotherapy, an ablative technique that treats tumors by freezing the tissue, causing cell death through the formation of ice crystals and disruption of cellular structures. It is effective for targeting larger and irregularly shaped tumors due to the diffusion of cold. Papalexis et al. (14) have conducted the most comprehensive review in the literature on CT-guided cryoablation applied to musculoskeletal tumors, both bone and soft tissue. They argue that this technique allows for precise tumor targeting while preserving the surrounding healthy tissues, reducing post-interventional complications. CT guidance enables accurate needle placement and visualization of the ice ball, enhancing the safety and precision of the procedure. This makes it a viable option even for lesions located near critical structures, such as nerves.
Colangeli et al. (15) described cryotherapy as an adjunctive treatment that can lead to notably lower recurrence rates in locally aggressive tumors, including GCTBs. Based in a retrospective case series of 143 patients with musculoskeletal tumors who underwent cryotherapy as an adjuvant therapy or as percutaneous ablation treatment, many of which were GCTBs. Their findings indicated that cryotherapy is effective in reducing tumor size and minimizing recurrence rates. The study also highlighted significant pain relief and improved functional outcomes for patients. However carries a higher risk of complications like cryoshock, whereas percutaneous RFTA is associated with fewer systemic effects. Radiofrequency also offers shorter recovery times.
Another non-invasive technique described on the treatment of the GCTB is the high-intensity ultrasound ablation (HIFU) which works by focusing US waves on the tumor, causing rapid vibration of tissue molecules. This vibration generates heat, leading to coagulative necrosis and destruction of tumor cells. The technique also induces cavitation, where microbubbles form and collapse, further disrupting tumor cells. Chen et al. (16) conducted a study on 69 patients treated with HIFU, including a case of a bone GCT in a 40-year-old man at the distal femur, with favorable results after six years. The procedure, performed under US guidance, faces challenges in accuracy for bone lesions. The ablation volume is extensive, covering the lesion, 1–2 cm of soft tissue, and 3–5 cm of adjacent normal bone.
Finally, Zheng et al. (17) advocate that intraoperative microwave ablation is an optional surgical method for patients with recurrences of GCTB and chondroblastomas with clinical efficacy proved. Intraoperative microwave ablation involves the application of microwave energy to produce thermal coagulation and necrosis of tumor cells during surgery. The technique achieves precise tumor targeting by inducing dielectric heating, which causes rapid oscillation of water molecules and subsequent cell death. It effectively reduces tumor burden and enhances surgical margins while preserving surrounding healthy tissues.
The most important limitation of this study is the small number of patients, the short follow-up time of the patients in case 7 (6 months), as well as this study is not a randomized prospective study.
Another limitation is the potential for incomplete ablation of the tumor, particularly in cases of large or multilocular lesions. In such cases, percutaneous RFTA alone may not be sufficient, and a combination of treatments may be required to achieve optimal outcomes. Additionally, the use of percutaneous RFTA in the axial skeleton, particularly in the spine, poses technical challenges and carries a higher risk of complications, necessitating further research and refinement of techniques.
There are various treatment options available for GCTB. Although surgery is the standard treatment, percutaneous RFTA is a viable alternative in cases with high complication risks, due to its encouraging early results. Percutaneous RFTA offers several advantages over conventional surgical methods, including reduced recovery times, lower complication rates, and the ability to precisely target tumor tissues while sparing surrounding healthy structures.
While larger series need to be studied, percutaneous RFTA has proven to be an effective treatment for recurrent bone GCTB, showing no complications in all seven of our patients [including the other four that we performed in our previous work (13)].
Conclusions
In conclusion, percutaneous RFTA represents an innovative and minimally invasive option for managing recurrent GCTB. Its ability to achieve local tumor control, relieve pain, and preserve functional bone structures holds great potential in the management of these challenging cases. Further research and clinical experience are needed to establish standardized protocols and refine its role in the multidisciplinary approach to GCTB.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: With the arrangement by the Guest Editors and the editorial office, this article has been reviewed by external peers.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1453/coif). The special issue “Advances in Diagnostic Musculoskeletal Imaging and Image-guided Therapy” was commissioned by the editorial office without any funding or sponsorship. J.M.V. served as the unpaid Guest Editor of the issue. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical guidelines of the institutional and/or national research committees and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pitsilos C, Givissis P, Papadopoulos P, Chalidis B. Treatment of Recurrent Giant Cell Tumor of Bones: A Systematic Review. Cancers (Basel) 2023;15:3287. [Crossref] [PubMed]
- Lin X, Liu J, Xu M. The prognosis of giant cell tumor of bone and the vital risk factors that affect its postoperative recurrence: a meta-analysis. Transl Cancer Res 2021;10:1712-22. [Crossref] [PubMed]
- Matcuk GR Jr, Patel DB, Schein AJ, White EA, Menendez LR. Giant cell tumor: rapid recurrence after cessation of long-term denosumab therapy. Skeletal Radiol 2015;44:1027-31. [Crossref] [PubMed]
- Montgomery C, Couch C, Emory CL, Nicholas R. Giant Cell Tumor of Bone: Review of Current Literature, Evaluation, and Treatment Options. J Knee Surg 2019;32:331-6. [Crossref] [PubMed]
- van der Heijden L, Lipplaa A, van Langevelde K, Bovée JVMG, van de Sande MAJ, Gelderblom H. Updated concepts in treatment of giant cell tumor of bone. Curr Opin Oncol 2022;34:371-8. [Crossref] [PubMed]
- Martel J, Bueno A, Ortiz E. Percutaneous radiofrequency treatment of osteoid osteoma using cool-tip electrodes. Eur J Radiol 2005;56:403-8. [Crossref] [PubMed]
- Martel Villagrán J, Bueno Horcajadas A, Ortiz Cruz EJ. Percutaneous radiofrequency ablation of benign bone tumors: osteoid osteoma, osteoblastoma, and chondroblastoma. Radiologia 2009;51:549-58. [Crossref] [PubMed]
- Koo JS, Chung SH. The Efficacy of Radiofrequency Ablation for Bone Tumors Unsuitable for Radical Excision. Clin Orthop Surg 2021;13:278-85. [Crossref] [PubMed]
- Gibbs CP, Lewis VO, Peabody T. Beyond bone grafting: techniques in the surgical management of benign bone tumors. Instr Course Lect 2005;54:497-503.
- Martel J, Ortiz E, Bueno Á, Dhimes P. Tratamiento percutáneo mediante radiofrecuencia del osteoma osteoide. Radiología 2001;43:337-40.
- Zhao Q, Wang L, Chen F, Jiang TA. Percutaneous radiofrequency ablation for treatment of giant cell tumor of bone guided by real-time US fused with CT. J Med Ultrason (2001) 2014;41:223-7. [Crossref] [PubMed]
- Arrigoni F, Zoccali C, Evangelista L, Giuliani L, Daffinà J, Zugaro L, Masciocchi C. CT-Guided RFA for Management of Surgical Relapses of Giant Cell Tumour of Bone. Cardiovasc Intervent Radiol 2023;46:508-11. [Crossref] [PubMed]
- Franco IL, Horcajadas AB, Martel J, Ortiz E. Percutaneous Radiofrequency Thermal Ablation Treatment of Recurrent Bone Giant Cell Tumor. J Orth Rhe Sp Med 2016;2:114.
- Papalexis N, Savarese LG, Peta G, Errani C, Tuzzato G, Spinnato P, Ponti F, Miceli M, Facchini G. The New Ice Age of Musculoskeletal Intervention: Role of Percutaneous Cryoablation in Bone and Soft Tissue Tumors. Curr Oncol 2023;30:6744-70. [Crossref] [PubMed]
- Colangeli S, Parchi P, Andreani L, Beltrami G, Scoccianti G, Sacchetti F, Ceccoli M, Totti F, Campanacci DA, Capanna R. Cryotherapy efficacy and safety as local therapy in surgical treatment of musculoskeletal tumours. A retrospective case series of 143 patients. J Biol Regul Homeost Agents 2018;32:65-70.
- Chen W, Zhu H, Zhang L, Li K, Su H, Jin C, Zhou K, Bai J, Wu F, Wang Z. Primary bone malignancy: effective treatment with high-intensity focused ultrasound ablation. Radiology 2010;255:967-78. [Crossref] [PubMed]
- Zheng K, Yu XC, Xu M, Wang JM. Conservative surgery with microwave ablation for recurrent bone tumor in the extremities: a single-center study. BMC Cancer 2022;22:1122. [Crossref] [PubMed]


