
Characteristics, treatment, and outcomes of spontaneous renal artery dissection: a 10-year retrospective single-center experience
Introduction
Spontaneous renal artery dissection (SRAD), first reported in 1944 by Bumpus et al. (1), is a rare cause of renovascular hypertension and renal failure. Typically characterized by abdominal pain, lower back pain, and elevated blood pressure (BP), its diagnosis is often delayed, and its treatment varies across different centers. For several decades after the first reported case, the literature describing this rare disease was dominated by case reports or small case series. With the technical advances made in artery transluminal angioplasty technology, medical treatment and stent placement, as opposed to surgical intervention, are generally the preferred treatment of choice for SRAD. This is due to the less aggressive nature of these approaches, which also allows rapid recovery and contributes to a more favorable and benign prognosis (2-7). We retrospectively evaluated the characteristics, treatment, and outcomes of SRAD in 21 patients in Peking University First Hospital over 10 years. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-994/rc).
Methods
Patients
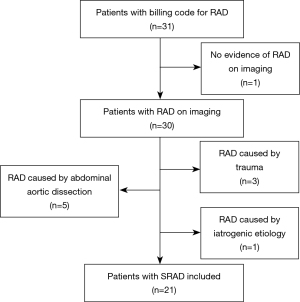
From December 2013 to December 2023, 21 consecutive patients presenting with SRAD at the Department of Interventional Radiology and Vascular Surgery, Peking University First Hospital, were retrospectively evaluated. The diagnosis for SRAD, based on luminal irregularities associated with aneurysmal dilatation, secular dissection with segmental stenosis, extension of dissection distal to the first renal artery bifurcation, and a “cuffing-shape” sleeve at branch points (8), was confirmed in all patients through digital subtraction angiography (DSA), computed tomography angiography (CTA), or a combination of both. These diagnostic images were thoroughly examined by two experienced radiologists to validate the diagnoses and assess the length of the intimal flap, the presence of concomitant renal artery stenosis (RAS), renal artery dissecting aneurysm, renal infarction, and renal artery thrombosis in the affected kidney (9). SRAD location was characterized as follows: ostial (involving the renal ostium and within 5 mm of the aortic origin), truncal (confined to the main renal artery), and branch (extending into the divisional or segmental branches). RAS was diagnosed when artery stenosis >60% on DSA (10). Renal artery dissecting aneurysm was defined as artery dilatation exceeding the normal diameter by 1.5 times on DSA (11). Renal infarction was characterized as low enhancement or malperfusion compared with contralateral or peripheral normal renal parenchyma on CTA or DSA. Discovery of renal artery thrombosis was based on low-density areas in the renal artery on a CTA scan (12). Diagnosis of Takayasu arteritis (TA) was based on typical angiographic abnormalities and serum biochemical parameters, including a thickened arterial wall and an elevated erythrocyte sedimentation rate (20 mm/h) and C-reactive protein levels (13). A patient with a “string of beads” angiographic appearance in ipsilateral or bilateral renal arteries and no additional genetic or vasculitis syndromes was presumed to have fibromuscular dysplasia (FMD) (14).
The inclusion criteria in this retrospective study were an age older than 18 years and SRAD found in the unilateral or bilateral renal artery. Patients with renal artery dissection (RAD) secondary to medical intervention, aortic dissection, and traumatic injury were excluded. Patients diagnosed with renal carcinoma who exhibited evidence of direct invasion by the tumor into the ipsilateral renal artery structure were not included in the study. The medical records of each patient were reviewed for extraction of demographic data, comorbid conditions, and other relevant clinical information. All patients underwent CTA of the aorta and intracranial arteries in order to exclude additional vascular abnormalities, such as dissection or aneurysm. Estimated glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI) (15). At the time of presentation and at the last follow-up, BP, serum creatinine, and eGFR were measured.
Management
A multidisciplinary team comprising rheumatoid immunologists, urologists, and interventional radiologists with at least 15 years of clinical experience, made management decisions for patients with SRAD. All patients underwent pain management, received optimal medicine for hypertension, and were administered systemic antithrombotic therapy. All patients received life-long aspirin (100 mg qd). In addition to stenting, dual-antiplatelet therapy of aspirin (100 mg qd) and clopidogrel (75 mg qd) were prescribed for 6 months, followed by lifelong aspirin therapy.
Indications for SRAD revascularization had two main goals: renal blood flow preservation and treatment of renovascular hypertension (2,16). Systemic heparinization was performed for percutaneous transluminal renal angioplasty (PTRA) procedures. The stents were Sterling (Boston Scientific, Marlborough, MA, USA) or NC Trek (Abbott, Chicago, IL, USA), and their number and size were decided up at the surgeons’ discretion. Technical success was characterized as successful deployment of the stent with reopening of the true arterial lumen, a residual stenosis <30% on angiography, and the absence of additional infarction of the affected kidney on the final angiogram (10). The time interval between the initial and final angiograms was more than half hour but less than two hours. For patients with SRAD with ipsilateral renal carcinoma, nephrectomy was chosen according to the opinion of urologists.
Follow-up
A follow-up visit was scheduled for patients with SRAD at 1, 3, 6, and 12 months after discharge, which continued every 6 to 12 months thereafter. The follow-up included BP measurement, antihypertensive drugs, and ultrasound assessment of renal artery patency. Renal function change in all patients with SRAD was classified as follows: improvement, the absolute value of eGFR increased by at least 30% after treatment; stabilization, the absolute value of the eGFR remaining within 10% of the pretreatment value; and failure, deterioration in eGFR by at least 30% after treatment (2). Restenosis in the stented artery was defined as stenosis ≥60% on DSA at follow-up. Hypertension change was classified as follows: cure, hypertension eliminated without antihypertensive medications; improvement, hypertension controlled by the same or a reduced number of defined daily doses; and failure, no modification in hypertension found at last follow-up (2). All patients were followed up by outpatient visits or telephone, and no patients were lost to follow-up.
Ethical statement
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Peking University First Hospital Institutional Review Board (No. 2024-130-001). Informed consent forms were obtained from the involved patients.
Statistical analysis
Statistical analysis was conducted using SPPS 26 (IBM Corp., Armonk, NY, USA). The data are presented as medians, ranges, means, standard deviations (SD), and proportions. BP, serum creatinine, and eGFR were compared using a paired t-test. An independent samples t-test was used to analyze baseline BP and BP change in different treatment groups. A P value <0.05 was considered to indicate statistical significance.
Results
As seen in Table 1, the mean age, weight, and height of the 21 patients with SRAD enrolled (16 men and 5 women) were 49.2±13.0 (range, 18–69) years, 69.0±9.7 (range, 50–80) kg, and 1.7±0.1 (range, 1.6–1.8) m, respectively (Figure 1). Two patients (9.5%) were asymptomatic, with SRAD and ipsilateral renal carcinoma found incidentally upon abdominal imaging. Abdominal pain, which is the main manifestation in patients with SRAD, was found in 15 (71.4%) patients. New-onset hypertension was found 8 (38.1%) patients, and there were 8 (38.1%) smokers in this case group. TA and renal FMD was diagnosed in 2 (9.5%) patients and 1 (4.8%) patient, respectively. The comorbidities in patients with SRAD predominantly included chronic kidney disease (CKD), diabetes, coronary heart disease (CHD), vertebral artery stenosis, contralateral RAS, renal carcinoma, and superior mesenteric artery dissection, which were found in a minority of patients. Notably, with the exception of the two asymptomatic patients, the diagnosis of SRAD was confirmed 8.2±8.6 (range, 1–30) days after the symptoms appeared for the first time. No instances of dissection or aneurysms in other arteries were detected among the patients.
Table 1
| Sociodemographic and clinical characteristics | N (%)/mean ± SD |
|---|---|
| Sex | |
| Male | 16 (76.2) |
| Female | 5 (23.8) |
| Age (years) | 49.2±13.0 |
| Height (m) | 1.7±0.1 |
| Weight (kg) | 69.0±9.7 |
| BMI (kg/m2) | 23.8±2.5 |
| Symptom | |
| Asymptomatic | 2 (9.5) |
| Abdominal pain | 15 (71.4) |
| Low back pain | 5 (23.8) |
| Headache | 1 (4.8) |
| Hematuria | 1 (4.8) |
| Nausea | 2 (9.5) |
| Hypertension | 15 (71.4) |
| Original hypertension | 9 (42.9) |
| New-onset hypertension | 8 (38.1) |
| Smoker | 8 (38.1) |
| Comorbidity | |
| Atherosclerosis | 6 (28.6) |
| TA | 2 (9.5) |
| Renal FMD | 1 (4.8) |
| CKD | 1 (4.8) |
| Diabetes | 2 (9.5) |
| CHD | 1 (4.8) |
| Vertebral artery stenosis | 1 (4.8) |
| Contralateral renal artery stenosis | 1 (4.8) |
| Renal carcinoma | 2 (9.5) |
| Superior mesenteric artery dissection | 1 (4.8) |
| Dyslipidemia | 9 (42.9) |
| Peripheral arterial disease | 2 (9.5) |
| Follow-up period (months) | 18.1±4.5 |
| Treatment option | |
| Medical treatment alone | 15 (71.4) |
| Stent implantation with medical treatment | 4 (19.0) |
| Nephrectomy with medical treatment | 2 (9.5) |
SD, standard deviation; BMI, body mass index; TA, Takayasu arteritis; FMD, fibromuscular dysplasia; CKD, chronic kidney disease; CHD, coronary heart disease.
Ostial lesions, truncal lesions, and branch lesions were diagnosed in 2 (9.5%), 18 (85.7%), and 1 (4.8%) patient, respectively. The lengths of the intimal flap were 19.2±8.2 (range, 10.3–42.8) mm. Renal infarction was found in nine (42.9%) patients, with four (19.0%) of these patients exhibiting renal artery thrombosis among them. Renal artery dissecting aneurysm and RAS were found in 1 (4.8%) and 4 (19.0%) patients (Table 2), respectively. No instances of disease recurrence were observed among the patients with SRAD throughout the follow-up period.
Table 2
| Lesion characteristics | N (%) |
|---|---|
| Lesion side | |
| Left | 10 (47.6) |
| Right | 11 (52.4) |
| Bilateral case | 0 (0) |
| Lesion location | |
| Ostial | 2 (9.5) |
| Truncal | 18 (85.7) |
| Branch | 1 (4.8) |
| Comorbidity | |
| Renal artery thrombosis | 4 (19.0) |
| Renal infarction | 9 (42.9) |
| Renal artery stenosis | 4 (19.0) |
| Renal artery aneurysm | 1 (4.8) |
SRAD, spontaneous renal artery dissection.
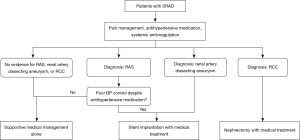
Proposed treatment algorithm was made to systematize the clinical approach to SRAD (Figure 2). For treatment, 15 patients with SRAD received supportive medical management alone, including analgesics, antihypertensive medications, and anti-thrombotic therapy, with a median follow-up of 16.7 (range, 12–32) months. Thirteen (86.7%) patients had resolution of their symptoms at their last follow-up of 16.0 (range, 12–32) months. In 11 patients with SRAD diagnosed with hypertension, the systolic and diastolic BP decreased from 157.2±24.7 and 93.7±19.6 mmHg to 124.2±8.0 (P<0.001) and 75.7±5.7 (P=0.004) mmHg, respectively. Renal function was classified as stabilized in all patients. No significant changes in serum creatinine (from 104.4±38.1 to 101.0±40.8 µmol/L; P=0.890) or eGFR (from 74.0±21.4 to 73.5±23.0 mL/min/1.73 m2; P=0.770) were observed over time.
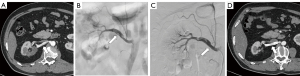
Four patients with new-onset hypertension were treated with stent implantation and medical management (Figure 3). Three of these patients experienced poor BP control despite antihypertensive medications. One of these patients with SRAD who was diagnosed with RAS and renal artery dissecting aneurysm received additional aneurysm embolization treatment. An interval of 7–30 days (mean 16.8±10.4 days) separated the onset of symptoms from stent implantation. The technical success rate was 100% (4/4). There were no interventional procedure-related complications. No restenosis was found after a median follow-up of 19.3 (range, 14–24) months. The symptoms resolved completely in all patients. Hypertension was cured in one patient and improved in three patients. The systolic and diastolic BP decreased from 150.5±16.8 and 97.3±10.0 mmHg to 127.3±8.9 (P=0.045) and 76.5±10.1 (P=0.021) mmHg, respectively. Renal function was classified as stabilized in all patients. Serum creatinine (from 78.6±21.5 to 80.7±28.9 µmol/L; P=0.764) and eGFR (from 87.7±10.3 to 85.0±14.6 mL/min/1.73 m2; P=0.638) remained stable. No significant changes in baseline BP and BP change were observed between the medical and endovascular treatment groups (Table S1).
According to the opinion of urologists, nephrectomy was selected for two patients with SRAD and ipsilateral renal carcinoma. In both cases, hypertension resolved after nephrectomy, with antihypertensive medications no longer being required. The serum creatinine changed from 82.0 and 84.2 to 109.0 and 99.5 µmol/L, respectively. The eGFR changed from 67.7 and 73.5 to 48.0 and 54.9 mL/min/1.73 m2, respectively.
Discussion
This retrospective study represents a large collection of cases in which medical therapy and endovascular therapy were predominant and extensively detailed, demonstrating encouraging outcomes in terms of antihypertensive and renoprotective effects.
The exact etiology of SRAD remains unknown. Despite some cases being associated with atherosclerosis, TA, and FMD, the majority of RAD cases are classified as idiopathic (6). In a study comparing the characteristics of patients with SRAD with those of matched controls from the general population, hypertension, cancer, and connective tissue disorders were more likely to be present in association with SRAD after adjustment were made for other comorbidities (16). The rate of hypertension in patients with SRAD was 76.2% in our series, which was comparable to that in previous studies (2,6,16). In our study, 2 cases of renal cell carcinoma were diagnosed among 21 patients, and nephrectomy was chosen ad hoc. However, no connective tissue disorders were diagnosed after meticulous review of the patients in our series, although some previous studies have revealed the connection between the connective tissue diseases and SRAD (4,16). The possible association between cancer, connective tissue disorders, and SRAD has not been clarified.
It has reported that uncontrolled hypertension and sudden onset flank pain ipsilateral to the dissection site are the most common presentation features for patients with SRAD (17). The development of arterial dissection may be facilitated by sustained elevated BP, which can worsen arteriosclerosis and medial degeneration (18). Moreover, SRAD frequently leads to renovascular hypertension via renal ischemia and activation of the renin–angiotensin system. In our series, nine patients had a history of hypertension, while eight patients were diagnosed with new-onset hypertension, suggesting a close interaction between SRAD and hypertension.
There is a general agreement that the definite diagnosis of SRAD is commonly achieved via DSA (16). Multidetector CTA, being a relatively more efficient, safe, and inexpensive procedure, offers a reliable assessment of SRAD location and intimal flap characteristics (19,20). In our series, the bulk of SRAD lesions were found at a truncal location, with the average intimal flap length being approximately 20 mm. Although aortic and intracranial CTA examinations were conducted at our facility to exclude the presence of FMD, differentiating dissecting aneurysms with SRAD from aneurysms with FMD poses a challenge.
With frequent misdiagnosis of acute renal colic or acute pyelonephritis, SRAD is often associated with treatment delay. Intervals of 2–120 and 14–180 days between the onset of symptoms and stent implantation were reported by Pellerin et al. and Rooden et al., respectively (2,21). Delays of 1–30 and 7–30 days for diagnosis and angioplasty were found in our study.
Treatment options for SRAD include supportive medical management, endovascular procedures, revascularization surgery, and nephrectomy (Table S2). Medical management, considered as the basis of all treatment, includes pain management, hypertension control, antithrombotic therapy, and coexistent symptom management (22-24). In Dicks et al.’s study, more than 90% of patients with SRAD were treated with medications alone (7). Despite half of the patients having persistent RAD on imaging, stable renal function was found in over 80 patients who were followed up.
Surgical procedures involve drawbacks such as large trauma, prolonged hospital stays, and a greater number of complications (17). High rates of nephrectomy (8–27%), acute thrombosis of the renal artery (6–12%), and late anastomotic restenosis (15%) were reported in cases treated with surgical operations (21,25,26). With the advancement of endovascular technology, a greater number SRAD cases treated with angioplasty have been reported (27,28). In Pellerin et al.’ study, known as the largest series of SRAD treated with stent placement, the technical success and clinical success rate for the treatment of hypertension in a total of 16 patients was 100% (2). Nearly half of the patients were cured and remained normotensive without taking any antihypertensive medications. No evidence of renal failure, restenosis, or occlusion were found in any of patients in more than 8 years of follow-up. Endovascular therapy has shown promising hypertension control for patients with SRAD and renovascular hypertension. In our study, four SRAD patients with RAS and new-onset hypertension attained satisfactory hypertension control after stent placement. In summary, interventional management is an effective approach for controlling renovascular hypertension secondary to RAS in patients with SRAD.
The kidney is conventionally considered capable of tolerating a lack of blood flow for approximately 60–90 minutes, but after this period, irreversible loss of kidney function occurs (29). However, in Pellerin et al.’s study, serum creatinine decreased from 142±42.5 to 96±12 µmol/L and renal function improved in all 16 stented patients after 8.6 years of follow-up (2). In contrast, in our study, renal function remained stable whether supportive medical management alone was applied or combined with endovascular treatment. We consider this to be mainly due to the normal baseline serum creatinine levels in all patients in our series. Yasuhiro et al. reported a stent treatment for a patient with SRAD and severe RAS (3). Surprisingly, the left renal function recovered from the renal failure pattern despite a 2-week angioplasty delay, demonstrating that ischemia-damaged renal parenchyma could still be salvaged by revascularization. Vitiello et al. reported a case of SRAD with severe stenosis in the left renal artery that was managed appropriately with therapeutic anticoagulation, yet progressed dissection and new acute renal infarction occurred 2 weeks later, which suggests that hemodynamic instability may be a hidden risk precipitating additional renal parenchymal injury (30). Consequently, it is our belief that patients with SRAD who exhibit renal hemodynamic instability, such as severe RAS and dissecting aneurysm, may be more likely to benefit from additional interventional management, which can protect and potentially restore renal function.
Currently, endovascular therapy is the predominant surgical intervention for patients with SRAD. Nevertheless, the majority of patients with SRAD do not require endovascular therapy. Previous studies have demonstrated a tendency toward overmedicalization through the indiscriminate use of stents in all patients with SRAD (6,7,16). For the majority of patients with SRAD, medical management alone is adequate. However, it is crucial to identify those patients with hemodynamic instability or renal vascular hypertension, as stenting represents the optimal treatment modality for this subset of individuals. For the first time, we have outlined the indications for medical treatment alone and for stent therapy, which may contribute to more favorable therapeutic outcomes.
There are inherent limitations and biases associated with this present study’s retrospective, single-center design, which call for caution in the interpretation and generalization of findings. As a result of the low incidence of SRAD, the sample size of our study was small. Moreover, DSA was not performed in all patients for confirming the definite diagnosis of SRAD. There was a small number of endovascular procedures for patients with SRAD in our series due to strict angioplastic indications. Finally, determination of glomerular filtration rate via diethylenetriaminepentaacetic acid scan was not performed in our series, but this may be able to better assess split renal function.
Conclusions
SRAD is a rare cause of renovascular hypertension and renal failure and is associated with frequent diagnostic and treatment delay. Standard medical treatment and endovascular therapy are commonly accepted methods for patients with SRAD. Medical management is a reasonable choice in most patients with SRAD. The utilization of interventional management has been shown to be an efficacious strategy for the management of renovascular hypertension and the preservation of renal function.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-994/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-994/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Peking University First Hospital Institutional Review Board (No. 2024-130-001). Informed consent was signed by all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bumpus HC Jr. A case of renal hypertension. Trans Am Assoc Genitourin Surg 1945;37:135-40.
- Pellerin O, Garçon P, Beyssen B, Raynaud A, Rossignol P, Jacquot C, Plouin PF, Sapoval M. Spontaneous renal artery dissection: long-term outcomes after endovascular stent placement. J Vasc Interv Radiol 2009;20:1024-30. [Crossref] [PubMed]
- Aoki Y, Sakai Y, Kimura T, Yamaoka T, Maekawa S, Maekawa J, Sano M, Matsuno K, Ishibashi I. Renal Artery Stenting Recovered Renal Function after Spontaneous Renal Artery Dissection. Intern Med 2019;58:2191-4. [Crossref] [PubMed]
- Conway R, Bergin D, Coughlan RJ, Carey JJ. Renal infarction due to spontaneous renal artery dissection in Ehlers-Danlos syndrome type IV. J Rheumatol 2012;39:199-200. [Crossref] [PubMed]
- Gao F, Chen Z, Gao F, Ren D, Huang X. Spontaneous renal artery dissection complicated by renal infarction: description of two cases. Quant Imaging Med Surg 2022;12:4972-8. [Crossref] [PubMed]
- Jha A, Afari M, Koulouridis I, Bhat T, Garcia L. Isolated Renal Artery Dissection: A Systematic Review of Case Reports. Cureus 2020;12:e6960. [Crossref] [PubMed]
- Dicks AB, Elgendy IY, Thondapu V, Ghoshhajra B, Waller HD, Rubio M, Schainfeld RM, Weinberg I. Clinical characteristics, treatment and outcomes of patients with spontaneous renal artery dissections. J Nephrol 2023;36:377-84. [Crossref] [PubMed]
- Hare WS, Kincaid-Smith P. Dissecting aneurysm of the renal artery. Radiology 1970;97:255-63. [Crossref] [PubMed]
- Jiang Q, Du J, Lei Y, Gu C, Hong L, Hu S. The relationship between false-lumen area ratio and renal replacement therapy after acute aortic dissection repair on bilateral artery cannulation: a cross-sectional study. Quant Imaging Med Surg 2023;13:3104-14. [Crossref] [PubMed]
- Martin LG, Rundback JH, Wallace MJ, Cardella JF, Angle JF, Kundu S, Miller DL, Wojak JCSociety of Interventional Radiology (SIR). Quality improvement guidelines for angiography, angioplasty, and stent placement for the diagnosis and treatment of renal artery stenosis in adults. J Vasc Interv Radiol 2010;21:421-30; quiz 230. [Crossref] [PubMed]
- Chaer RA, Abularrage CJ, Coleman DM, Eslami MH, Kashyap VS, Rockman C, Murad MH. The Society for Vascular Surgery clinical practice guidelines on the management of visceral aneurysms. J Vasc Surg 2020;72:3S-39S. [Crossref] [PubMed]
- Yu YT, Ren XS, An YQ, Yin WH, Zhang J, Wang X, Lu B. Changes in the renal artery and renal volume and predictors of renal atrophy in patients with complicated type B aortic dissection after thoracic endovascular aortic repair. Quant Imaging Med Surg 2022;12:5198-208. [Crossref] [PubMed]
- Grayson PC, Ponte C, Suppiah R, Robson JC, Gribbons KB, Judge A, Craven A, Khalid S, Hutchings A, Danda D, Luqmani RA, Watts RA, Merkel PADCVAS Study Group. 2022 American College of Rheumatology/EULAR classification criteria for Takayasu arteritis. Ann Rheum Dis 2022;81:1654-60. [Crossref] [PubMed]
- Gornik HL, Persu A, Adlam D, Aparicio LS, Azizi M, Boulanger M, et al. First International Consensus on the diagnosis and management of fibromuscular dysplasia. Vasc Med 2019;24:164-89. [Crossref] [PubMed]
- Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 2010;55:622-7. [Crossref] [PubMed]
- Afshinnia F, Sundaram B, Rao P, Stanley J, Bitzer M. Evaluation of characteristics, associations and clinical course of isolated spontaneous renal artery dissection. Nephrol Dial Transplant 2013;28:2089-98. [Crossref] [PubMed]
- Jain A, Tracci MC, Coleman DM, Cherry KJ, Upchurch GR Jr. Renal malperfusion: spontaneous renal artery dissection and with aortic dissection. Semin Vasc Surg 2013;26:178-88. [Crossref] [PubMed]
- Tanaka Y, Yoshimuta T, Kimura K, Iino K, Tamura Y, Sakata K, Hayashi K, Takemura H, Yamagishi M, Kawashiri MA. Clinical characteristics of spontaneous isolated visceral artery dissection. J Vasc Surg 2018;67:1127-33. [Crossref] [PubMed]
- Dobrilovic N, Bennett S, Smith C, Edwards J, Luchette FA. Traumatic renal artery dissection identified with dynamic helical computed tomography. J Vasc Surg 2001;34:562-4. [Crossref] [PubMed]
- Muroya T, Koga S, Maemura K. Chronic renal artery dissection with aneurysm formation treated by stent implantation with coil embolization with detailed intravascular ultrasound evaluation. Catheter Cardiovasc Interv 2013;81:574-7. [Crossref] [PubMed]
- van Rooden CJ, van Baalen JM, van Bockel JH. Spontaneous dissection of renal artery: long-term results of extracorporeal reconstruction and autotransplantation1. J Vasc Surg 2003;38:116-22. [Crossref] [PubMed]
- Edwards BS, Stanson AW, Holley KE, Sheps SG. Isolated renal artery dissection, presentation, evaluation, management, and pathology. Mayo Clin Proc 1982;57:564-71.
- Stawicki SP, Rosenfeld JC, Weger N, Fields EL, Balshi JD. Spontaneous renal artery dissection: three cases and clinical algorithms. J Hum Hypertens 2006;20:710-8. [Crossref] [PubMed]
- Ramamoorthy SL, Vasquez JC, Taft PM, McGinn RF, Hye RJ. Nonoperative management of acute spontaneous renal artery dissection. Ann Vasc Surg 2002;16:157-62. [Crossref] [PubMed]
- Lacombe M. Isolated spontaneous dissection of the renal artery. J Vasc Surg 2001;33:385-91. [Crossref] [PubMed]
- Müller BT, Reiher L, Pfeiffer T, Müller W, Hort W, Voiculescu A, Grabensee B, Fürst G, Sandmann W. Surgical treatment of renal artery dissection in 25 patients: indications and results. J Vasc Surg 2003;37:761-8. [Crossref] [PubMed]
- Bilge AK, Nisanci Y, Yilmaz E, Umman B, Hunerel D, Ozsaruhan O. Renovascular hypertension secondary to spontaneous renal artery dissection and treatment with stenting. Int J Clin Pract 2003;57:435-6.
- Lee SH, Lee HC, Oh SJ, Park MC, Park KJ, Moon YS, Min JW, Hwang EJ, Baek JE, Jo ES, Jang GJ. Percutaneous intervention of spontaneous renal artery dissection complicated with renal infarction: a case report and literature review. Catheter Cardiovasc Interv 2003;60:335-8. [Crossref] [PubMed]
- Komolafe B, Dishmon D, Sultan W, Khouzam RN. Successful aspiration and rheolytic thrombectomy of a renal artery infarct and review of the current literature. Can J Cardiol 2012;28:760.e1-3. [Crossref] [PubMed]
- Vitiello GA, Blumberg SN, Sadek M. Endovascular Treatment of Spontaneous Renal Artery Dissection After Failure of Medical Management. Vasc Endovascular Surg 2017;51:509-12. [Crossref] [PubMed]


