
Cognition mediates the relationship between white matter hyperintensity and motor function in patients with cerebral small vessel disease: a cross-sectional study
Introduction
Cerebral small vessel disease (SVD) is a group of common heterogeneous disorders that affect the small vessels of the brain and is associated with aging (1). White matter hyperintensity (WMH), recognized as lesions on T2-weighted magnetic resonance imaging (MRI), is a common finding on brain MRI scans across a wide range of neurological disorders and in older adults without specific neurological diagnoses (2). Notably, the presence of WMH is the most prevalent neuroimaging marker of SVD (3,4). Cumulative evidence shows that the WMH burden is highly correlated with a decline in both cognitive and motor functions (5-7), which may be related to microstructural integrity, structure network efficiency, or cortical thickness (8-10).
Defining motor function is a very complex task. According to Hallemans et al. (11), it refers to “the ability to learn or to demonstrate the skillful and efficient assumption, maintenance, modification, and control of voluntary postures and movement patterns.” To simplify its assessment, gait and balance are considered two highly relevant and measurable (qualitatively and quantitative) aspects of motor function. Gait comprises postural and behavioral characteristics associated with walking, and walking speed is considered the sixth vital sign for older patients (12,13). Due to the growing number of patients with SVD in the aging global population, motor dysfunction has been recognized as an important public health concern (3). Motor dysfunction is significantly predictive of falls, disability, hospitalization, and all-cause mortality (14-17).
Additionally, cognitive impairments are associated with motor disturbances (18-20). For example, deficits in executive function and memory affect information processing and responses to the environment while walking, leading to the reduction of gait speed. Moreover, attention is important for the maintenance of postural balance and the regulation of gait. Hence, both cognitive impairment and motor dysfunction are inherently linked, and both contribute markedly to a patient’s overall degree of frailty.
However, to date, few studies have examined whether and how cognition mediates the relationship between WMH and motor function. Thus, our study aimed to (I) examine the relationship between WMH, cognition, and motor function, and (II) assess whether cognition mediates the association between WMH and motor function in patients with SVD. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1058/rc).
Methods
Design
A cross-sectional descriptive study was designed, and a convenience sampling method was used for this study.
Study participants
This report presents a cross-sectional analysis of the baseline data of the Guangdong SVD study in China, a prospective experimental study investigating frailty in SVD patients. Participants were recruited from the Department of Neurology of The Third Affiliated Hospital of Sun Yat-sen University in Guangzhou between July 2021 and December 2022. The study enrolled patients following a systematic and predefined procedure, which involved an initial screening for clinical symptoms and diagnosis based on the “Chinese Consensus on Diagnosis and Therapy of Cerebral Small Vessel Disease 2021” (21).
MRI is a critical diagnostic tool for identifying WMH and other SVD-related neuroimaging markers, and all the enrolled patients underwent MRI scanning unless they presented with contraindications. Following MRI, eligible patients underwent a comprehensive baseline evaluation, including clinical, neuropsychological, and physical examinations. Information regarding sociodemographic and disease-specific characteristics, cognition, and motor function was gathered in face-to-face interviews.
Among the participants, 230 with SVD who met the study criteria were enrolled in the current research. To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: be aged ≥18 years; and have sufficient vision, hearing, and communication skills to complete the assessment protocol. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had a walking inability; (II) had dementia other than vascular dementia (diagnosed according to the Vascular Behavioral and Cognitive Disorders criteria) (22), epilepsy, a brain tumor, Parkinson’s disease, or any other significant neurological disease; (III) recently (within 6 months) had a lacunar infarct, brain trauma, or infection; (IV) had a severe psychiatric disorder or intellectual disability; (V) had a non-SVD-related WMH such as multiple sclerosis; and/or (VI) had contraindications to MRI.
According to the requirements for pathological analysis, it was calculated that a minimum sample size of 200 patients was required for this study (23). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of The Third Affiliated Hospital of Sun Yat-sen University approved the study (No. [2021]02-285-01), and each participant provided informed consent.
Demographic and clinical data
A self-designed questionnaire was administered to the patients to collect their demographic information (e.g., age, gender, employment, education level, residence, marital status, household income, and smoking and alcohol consumption habits). The clinical data, including body mass index (BMI), waist-to-hip ratio, serologic data [i.e., C-reactive protein (CRP) and interleukin-6 levels], and a medical history of hypertension or diabetes mellitus, were extracted from medical records.
Neuroimaging
MRI was performed using a 3.0-Tesla scanner (Discovery MR750; GE Healthcare, Chicago, IL, USA); T1-weighted, T2-weighted, and fluid-attenuated inversion recovery (FLAIR) sequences were performed. The WMH burden of each patient was determined based on their MRI results, using the modified Fazekas scale (mild: Fazekas score = 0–1; moderate: Fazekas score = 2; and severe: Fazekas score = 3) (24,25). The Fazekas scale is the tool most frequently used to evaluate the WMH burden in clinical practice, and it is a convenient, quick, and reliable visual rating scale (26,27). The presence of other SVD markers, including microbleeds, lacunes, and enlarged perivascular spaces, was also documented as a categorical variable. The images were evaluated by neurologists who were blinded to the patient characteristics, and the radiologists’ reports were reviewed and compared with the interpretation of the neurologists for consistency and concurrency.
Cognitive measures
The Beijing version of the Montreal Cognitive Assessment (MoCA) (28) was used to evaluate the cognition of patients with SVD. This assessment evaluates seven cognitive domains (i.e., visuospatial and executive function, naming, attention, abstraction, language, delayed memory, and orientation). As the most widely used MoCA version in mainland China, this form of cognitive assessment ensured adequate internal consistency and validity (29). MoCA scores vary from 0 to 30, with higher scores indicating better cognitive function, and scores below 26 indicating cognitive impairment (28).
Motor function assessment
Motor function was assessed using the Tinetti Gait and Balance Scale (30) and the Short Physical Performance Battery (SPPB) (31). The Tinetti Gait and Balance Scale comprises 17 items, and has a maximum score of 28. On the scale, nine items relate to body balance, including balance while sitting, standing, turning around, and with eyes closed (scores 0–16), and eight items relate to gait, including stride length, height, symmetry, and continuity (scores 0–12). The SPPB was used to evaluate balance, gait, strength, and endurance. The scale comprises the following three subtests: the standing balance test (which tests the ability of the patient to stand with their feet together in side-by-side, semi-tandem, and tandem positions); the 4-meter walking speed test (on which a time to walk 4 meters of >8.70 seconds is scored 1; a time of 6.21–8.70 seconds is scored 2; a time of 4.82–6.20 seconds is scored 3; and a time of <4.82 seconds is scored 4); and the repeated chair-stands test (a time to rise from a chair and return to the seated position five times of >60 seconds is scored 0; a time of ≥16.70 seconds is scored 1; a time of 13.70–16.69 seconds is scored 2; a time of 3.62–4.65 seconds is scored 3; and a time of ≤11.19 seconds is scored 4). The total score ranges from 0 to 12. For both scales, higher scores indicate better motor performance.
Statistical analysis
The data were analyzed using SPSS 25.0 (IBM Corp., Armonk, NY, USA). The statistical tests were two-sided, and P values <0.05 were considered statistically significant. Initially, all the participants were categorized into three groups based on their WMH burden (mild, moderate, or severe). The between-group differences were assessed using a univariate analysis of variance followed by least significant difference post-hoc tests for continuous variables, and chi-square tests with Z-tests for categorical variables for multiple comparisons. Next, the t-test was used to compare the Tinetti and SPPB scores of the cognitively unimpaired versus impaired patients in the three WMH groups, and a correlation analysis of cognition and motor function was performed using Pearson’s correlation coefficient (r). Finally, the mediation analyses were carried out with model 4 of PROCESS macro 3.5 (32) to test whether cognition (as measured by the continuous MoCA scores) mediated the relationship between WMH and motor function (as measured by the continuous Tinetti scale/SPPB score). The 95% confidence intervals (CIs) of the coefficients for the direct, indirect, and total effects were derived using bias-corrected bootstrapping with 5,000 resamples, which are significant if they do not contain zero. Age and presence of other neuroimaging markers (recent small subcortical infarcts, enlargement of perivascular spaces, cerebral microbleeds, and brain atrophy) were controlled for as covariates.
Results
Patient characteristics
For this study, 230 patients were enrolled, and nine were excluded because their data had more than 20% missing values. Thus, a total of 221 patients were included in the final analysis. All the patients were divided into three groups based on their WMH burden: 30.3% had mild WMH, 37.6% had moderate WMH, and 32.1% had severe WMH.
Table 1 sets out the demographic and clinical characteristics of all patients and of the three WMH groups. There were significant differences between the groups in terms of age, employment, hypertension, BMI, waist-to-hip ratio, and CRP values.
Table 1
| Variable | WMH burden | Total sample (n=221) | Group comparisons (P) | ||
|---|---|---|---|---|---|
| Mild (n=67) | Moderate (n=83) | Severe (n=71) | |||
| Age, years | 62.84±10.85 | 67.90±10.16* | 70.69±9.05* | 67.26±10.48 | <0.001 |
| Sex (male) | 35 (52.2) | 47 (56.6) | 47 (66.2) | 129 (58.4) | 0.231 |
| Employment | 0.011 | ||||
| Employed | 16 (23.9) | 13 (15.7) | 4 (5.6) | 33 (14.9) | |
| Retired/unemployed | 51 (76.1) | 70 (84.3)* | 67 (94.4)† | 188 (85.1) | |
| Education level | 0.207 | ||||
| Primary school or below | 14 (20.9) | 16 (19.3) | 22 (31.0) | 52 (23.5) | |
| Secondary school | 27 (40.3) | 39 (47.0) | 21 (29.6) | 87 (39.4) | |
| Higher education | 26 (38.8) | 28 (33.7) | 28 (39.4) | 82 (37.1) | |
| Residence (urban) | 55 (82.1) | 67 (80.7) | 65 (91.5) | 187 (84.6) | 0.141 |
| Marital status (married) | 63 (94.0) | 76 (91.6) | 64 (90.1) | 203 (91.9) | 0.701 |
| Family per capita monthly income, yuan | 0.955 | ||||
| <1,000 | 9 (13.4) | 8 (9.6) | 8 (11.3) | 25 (11.3) | |
| 1,000–4,999 | 33 (49.3) | 44 (53.0) | 35 (49.3) | 112 (50.7) | |
| ≥5,000 | 25 (37.3) | 31 (37.3) | 28 (39.4) | 84 (38.0) | |
| Smoking | 15 (22.4) | 11 (13.3) | 17 (23.9) | 43 (19.5) | 0.190 |
| Drinking | 3 (4.5) | 1 (1.2) | 1 (1.4) | 5 (2.3) | 0.343 |
| BMI, kg/m2 | 22.96±2.84 | 24.23±3.18* | 23.98±2.81* | 23.76±3.01 | 0.027 |
| Waist-to-hip ratio | 0.90±0.07 | 0.93±0.07* | 0.94±0.06* | 0.92±0.07 | <0.001 |
| CRP, mg/dL | 1.58±1.48 | 2.17±2.24 | 4.18±8.48*,† | 2.63±5.16 | 0.007 |
| Hypertension | 35 (52.2) | 62 (74.7)* | 52 (73.2)* | 149 (67.4) | 0.006 |
| Diabetes | 15 (22.4) | 21 (25.3) | 20 (28.2) | 56 (25.3) | 0.737 |
| Other imaging markers of SVD | 29 (43.3) | 28 (33.7) | 33 (46.5) | 90 (40.7) | 0.242 |
Values are presented as the mean ± standard deviation or number (percentage). *, P<0.05 vs. mild WMH; †, P<0.05 vs. moderate WMH. Bolded P values indicate statistically significant group comparisons. WMH, white matter hyperintensity; BMI, body mass index; CRP, C-reactive protein; SVD, small vessel disease; other imaging markers of SVD, the presence of microbleeds, lacunes, or enlarged perivascular spaces on magnetic resonance imaging.
WMH, cognition, and motor function
Table 2 sets out the cognition and motor function results. The results showed that increasing WMH burdens were significantly associated with lower MoCA, Tinetti scale, and SPPB scores. As Table 3 shows, across the three WMH groups, the average Tinetti and SPPB scores were generally lower in patients with cognitive impairment.
Table 2
| Scale/subscale (scores) | WMH burden | Total sample (n=221) |
Group comparisons (P) |
||
|---|---|---|---|---|---|
| Mild (n=67) | Moderate (n=83) | Severe (n=71) | |||
| MoCA total | 25.54±4.11 | 23.29±5.21* | 18.44±7.04*,† | 22.41±6.27 | <0.001 |
| Visuospatial and executive function | 3.97±1.24 | 3.12±1.51* | 2.89±1.82* | 3.28±1.61 | 0.006 |
| Naming | 2.68±0.67 | 2.55±0.79 | 2.59±0.76 | 2.60±0.74 | 0.742 |
| Attention | 5.41±0.99 | 5.10±1.28 | 4.14±1.79*,† | 4.86±1.49 | <0.001 |
| Abstraction | 1.46±0.73 | 1.31±0.85 | 0.95±0.86*,† | 1.23±0.84 | 0.018 |
| Language | 2.19±0.88 | 1.96±1.08 | 1.86±1.32 | 1.99±1.12 | 0.415 |
| Delayed memory | 2.11±1.81 | 1.94±1.77 | 1.39±1.62 | 1.80±1.75 | 0.140 |
| Orientation | 5.41±1.26 | 5.08±1.75 | 4.36±2.25* | 4.93±1.86 | 0.032 |
| Tinetti total | 26.88±1.49 | 25.82±3.25 | 23.30±4.58*,† | 25.33±3.67 | <0.001 |
| Tinetti balance | 15.45±1.00 | 14.92±1.92 | 13.56±3.06*,† | 14.64±2.29 | <0.001 |
| Tinetti gait | 11.43±0.78 | 10.94±1.63 | 9.73±1.95*,† | 10.70±1.70 | <0.001 |
| SPPB total | 8.01±2.22 | 7.05±2.32* | 5.85±2.04*,† | 6.95±2.35 | <0.001 |
| Standing balance | 2.86±0.73 | 2.66±0.80 | 2.28±0.71*,† | 2.60±0.78 | <0.001 |
| 4-m walking speed | 2.89±1.02 | 2.54±1.07* | 2.06±1.04* | 2.49±1.09 | <0.001 |
| Chair-stands | 2.34±1.06 | 1.88±1.06* | 1.54±0.95* | 1.91±1.07 | <0.001 |
Values are presented as the mean ± standard deviation. *, P<0.05 vs. mild WMH; †, P<0.05 vs. moderate WMH. Bolded P values indicate statistically significant group comparisons. WMH, white matter hyperintensity; MoCA, Montreal Cognitive Assessment; Tinetti, Tinetti Balance and Gait Scale; SPPB, Short Physical Performance Battery.
Table 3
| WMH burden | Motor function | Cognitive unimpairment | Cognitive impairment | Group comparisons (P) |
|---|---|---|---|---|
| Mild (n=67) | Tinetti scores | 27.38±0.92 | 26.27±1.82 | 0.021 |
| SPPB scores | 8.32±2.04 | 7.63±2.40 | 0.337 | |
| Moderate (n=83) | Tinetti scores | 27.50±0.72 | 24.76±3.75 | <0.001 |
| SPPB scores | 7.63±1.91 | 6.69±2.49 | 0.151 | |
| Severe (n=71) | Tinetti scores | 26.31±1.97 | 22.62±4.73 | 0.014 |
| SPPB scores | 7.08±1.89 | 5.57±1.98 | 0.689 |
Values are presented as the mean ± standard deviations. Bolded P values indicate statistically significant group comparisons. MoCA scores below 26 denote cognitive impairment, scores of 26 or higher indicate cognitive unimpairment. WMH, white matter hyperintensity; Tinetti, Tinetti Balance and Gait Scale; SPPB, Short Physical Performance Battery; MoCA, Montreal Cognitive Assessment.
The relationship between cognition and motor function
Table 4 summarizes the Pearson’s correlations between cognition and motor function. Notably, lower MoCA scores were significantly correlated with decreased Tinetti scale and SPPB scores. Among the cognitive domains, visuospatial and executive function, attention, language, delayed memory, and orientation were primarily associated with motor function.
Table 4
| Scale/subscale | Tinetti total | Tinetti balance | Tinetti gait | SPPB total | Standing balance | 4-m walking speed | Chair-stands |
|---|---|---|---|---|---|---|---|
| MoCA total | |||||||
| r | 0.545 | 0.486 | 0.521 | 0.365 | 0.380 | 0.304 | 0.188 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.006 |
| Visuospatial and executive function | |||||||
| r | 0.259 | 0.206 | 0.273 | 0.258 | 0.384 | 0.186 | 0.119 |
| P | 0.003 | 0.019 | 0.002 | 0.003 | <0.001 | 0.039 | 0.187 |
| Naming | |||||||
| r | 0.126 | 0.098 | 0.138 | 0.084 | 0.167 | −0.014 | 0.023 |
| P | 0.152 | 0.267 | 0.116 | 0.342 | 0.063 | 0.882 | 0.797 |
| Attention | |||||||
| r | 0.338 | 0.243 | 0.372 | 0.290 | 0.415 | 0.198 | 0.160 |
| P | <0.001 | 0.005 | <0.001 | 0.001 | <0.001 | 0.028 | 0.075 |
| Abstraction | |||||||
| r | 0.093 | 0.063 | 0.106 | 0.142 | 0.126 | 0.148 | 0.081 |
| P | 0.291 | 0.479 | 0.232 | 0.108 | 0.161 | 0.102 | 0.369 |
| Language | |||||||
| r | 0.237 | 0.248 | 0.173 | 0.251 | 0.267 | 0.237 | 0.165 |
| P | 0.007 | 0.004 | 0.049 | 0.004 | 0.003 | 0.008 | 0.066 |
| Delayed memory | |||||||
| r | 0.345 | 0.330 | 0.297 | 0.174 | 0.267 | 0.142 | 0.078 |
| P | <0.001 | <0.001 | 0.001 | 0.048 | 0.003 | 0.117 | 0.386 |
| Orientation | |||||||
| r | 0.137 | 0.110 | 0.135 | 0.178 | 0.205 | 0.214 | 0.040 |
| P | 0.120 | 0.214 | 0.125 | 0.043 | 0.022 | 0.017 | 0.662 |
Bolded r (P) values indicate statistical significance. MoCA, Montreal Cognitive Assessment; Tinetti, Tinetti Balance and Gait Scale; SPPB, Short Physical Performance Battery.
Mediation
Multi-categorical mediation models were run to examine whether cognition (as measured by the MoCA scores) mediated the effects of the WMH burden (mild, moderate, severe) on motor function (as measured by the Tinetti scale/SPPB score) after adjusting for the covariates, using the mild WMH group as the reference group.
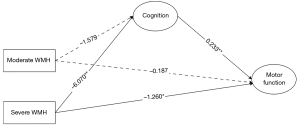
Two points can be made regarding motor function assessed through the Tinetti scale (Figure 1). First, the indirect path “moderate WMH → cognition → motor function” was significant (B=−0.368, 95% CI: −0.751 to −0.031), while the direct effect was not significant. Therefore, the total effect of moderate WMH versus mild WMH on motor function was −0.368. Second, the indirect path “severe WMH → cognition → motor function” was significant (B=−1.414, 95% CI: −2.105 to −0.830), as was the direct path “severe WMH → motor function” (B=−1.260, 95% CI: −2.375 to −0.146). Thus, the total effect of severe WMH versus mild WMH on motor function was −2.674, and the mediating effects accounted for 52.88%.
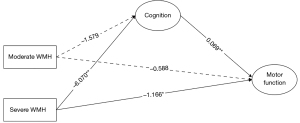
Equally, two points can be made in relation to motor function, which was assessed using the SPPB (Figure 2). First, the indirect path “moderate WMH → cognition → motor function” was significant (B=−0.109, 95% CI: −0.257 to −0.003), while the direct path “moderate WMH → motor function” was not significant. Therefore, the total effect of moderate WMH versus mild WMH on motor function was –0.109. Second, the indirect path “severe WMH → cognition → motor function” and the direct path “severe WMH → motor function” were both significant (indirect effect B=−0.419, 95% CI: −0.745 to −0.112; direct effect B=−1.166, 95% CI: −1.937 to −0.395). Thus, the total effect of severe WMH versus mild WMH on motor function was −1.585, and the mediating effects accounted for 26.44%.
Discussion
This study revealed that the WMH burden is associated with cognition and motor function in patients with SVD. A mediation analysis further suggested that WMH contributes to decreased motor function through cognitive processes.
In this study, the differences in age and hypertension were significantly associated with WMH burden. This is not surprising, as they have both been shown to be risk factors for WMH in previous studies (33,34). The observed relationship between WMH and BMI, waist-to-hip ratio, and CRP levels were also consistent with the findings reported by Lampe et al. (35), who proposed a mediation mechanism relating visceral obesity to WMH through increases in levels of proinflammatory cytokines. In relation to the possible pathological mechanism, Hilal et al. (36) proposed that elevated CRP levels were signs of atherosclerosis involving vascular occlusion, altered cerebral autoregulation, and increased vascular permeability, leading to WMH. However, published studies on this relationship have yielded conflicting results (37-39), highlighting the need for further scrutiny in the obese population.
Consistent with the results of a previous study (40), we found that global cognition significantly decreased as the WMH burden increased. Notably, of the seven cognitive domains evaluated by the MoCA, the WMH groups differed mainly in visuospatial and executive function, attention, abstraction, and orientation. Oosterman et al. (41) showed that WMH caused deficits in executive function, probably by reducing the functional connectivity of the prefrontal cortex with other (sub-)cortical regions. Meanwhile, Yamanaka et al. (42) suggested that the presence of WMH is anatomically connected to medial temporal atrophy, which in turn is associated with the manifestation of executive dysfunction, potentially due to the disruption of neural networks critical for executive processing. Additionally, various studies have reported associations between WMH and other cognitive domains (43-45).
In terms of motor function, similar to a previous study (46), we observed a general decline in motor performance in SVD patients with WMH. Further, motor deterioration was more pronounced at severe WMH stages. However, the patients obtained relatively higher scores on the Tinetti scale than the SPPB, indicating that the former scale was less likely to detect motor decline in patients. Additionally, based on the insignificant differences in the Tinetti scale scores between the moderate WMH and mild WMH groups, it appears that the Tinetti scale might be less effective at capturing the motor deficits that occur at earlier stages of WMH. Thus, further clinical studies should be conducted to assess the sensitivity and accuracy of these scales in patients with SVD.
Overall, our results confirmed our research hypothesis that a correlation exists between cognition and motor function in SVD patients, which is also consistent with previous research findings (46). Notably, our findings also indicated that the cognitive domains of visuospatial and executive function, attention, language, delayed memory, and orientation were mostly linked to motor function. A study of patients with Parkinson’s disease (43) showed that WMH in the left temporal area was significantly associated with falls, potentially because of deficits in memory, navigation, orientation, and so on. Therefore, the relationship between cognitive domains and motor function, and consequently falls, should be examined in further SVD studies.
In relation to the mediation analysis, unlike Bolandzadeh et al.’s (47) and Zheng et al.’s (48) models of community adults, we included the overall evaluation of gait and balance as the motor outcome variable and proposed global cognition as the mediator in patients with SVD, not only for walking speed or parts of cognitive domains. To investigate the indirect effects in two models, we took the mild WMH group as the reference group and found that patients with moderate and severe WMH had worse cognitive function and, concomitantly, impaired motor control. First, the disruption of interconnected neuronal pathways due to WMH in cerebral regions critical for both cognition and motor function may contribute to the observed relationship; for example, the decreased activity in the frontal and cingulate cortex areas, triggered by WMH, may impair the frontostriatal circuits and other essential neural pathways, thereby negatively impacting both cognitive and motor function (49).
Further, it should be noted that the total effect of moderate WMH on motor function became insignificant after adding cognition to the models. This finding suggests that cognitive impairment, rather than the severity of WMH alone, is the primary driver of motor dysfunction in this context. Cai et al.’s (8) study supported this notion, highlighting the essential mediating role of cognition between the brain’s structural network efficiency and gait in SVD patients.
Several possible mechanisms may link cognitive decline to motor dysfunction; for example, reduced attentional resources due to cognitive decline may negatively affect an individual’s motor coordination and ability to perform tasks requiring divided attention (9). Additionally, impaired executive function linked to WMH may disrupt the planning and initiation of complex motor tasks, as these cognitive processes are integral to motor control (20,38). Li et al. (20) suggested that visuospatial dysfunction, impairing the ability to perceive and navigate the environment, can lead to gait disturbances. Moreover, cognitive impairments may also affect the capacity for motor recovery and adaptation following neurological injury or disease, as cognitive resources are necessary for rehabilitation processes (50). Therefore, when investigating motor disturbances in patients with SVD, the underlying cognitive pathway that links WMH and motor function must be considered. Additionally, consideration should be given to whether training focusing on executive function, attention, language, delayed memory, and orientation mitigates motor dysfunction.
Limitation of the study
This study had several limitations. First, while its single-center and cross-sectional design increased its internal validity, they also limited the representativeness of the sample, which is subject to cross-lag effects over time. Therefore, further research should include multicenter longitudinal studies. Further, given the potential for non-causal associations and bidirectionality in the relationship between cognitive and motor functions, further research should delve into their intricate interplay in this context. Second, the Fazekas scale is a simple and reliable method of WMH classification commonly used in clinical practice; however, this method may still be interpreted as conservative when compared to quantitative volumetric measures. Therefore, studies using alternative quantitative measures for WMH should be conducted to corroborate the findings of this investigation. Additionally, the regional distribution of WMH and its potential differential effects on cognitive and motor functions require further investigation. Finally, Alzheimer’s disease (AD) patients were excluded from this study to concentrate on vascular cognitive impairment; however, given the potential pathophysiological overlap, the absence of any assessment of AD biomarkers represents a limitation of this study. Future studies should seek to assess AD biomarkers to gain a more nuanced understanding of the underlying mechanisms.
Conclusions
This study showed that WMH is associated with motor dysfunction, and this association is mediated by cognitive function in patients with SVD. These findings highlight the importance of considering early cognitive function in the assessment and management of motor dysfunction in patients with SVD. Further, our results appear to suggest that therapies targeting cognitive function to reduce motor dysfunction risk may be effective in improving outcomes for these patients.
Acknowledgments
The authors would like to thank the medical staff and all the participants. Mr. M.G.’s contribution to this article was made in the context of the Oxford Global Neurosurgery Initiative.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1058/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1058/coif). M.G. serves as an unpaid editorial board member of Quantitative Imaging in Medicine and Surgery. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of The Third Affiliated Hospital of Sun Yat-sen University approved the study (No. [2021]02-285-01), and each participant provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chojdak-Łukasiewicz J, Dziadkowiak E, Zimny A, Paradowski B. Cerebral small vessel disease: A review. Adv Clin Exp Med 2021;30:349-56. [Crossref] [PubMed]
- Wardlaw JM, Valdés Hernández MC, Muñoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc. 2015;4:001140. [Crossref] [PubMed]
- Markus HS, de Leeuw FE. Cerebral small vessel disease: Recent advances and future directions. Int J Stroke 2023;18:4-14. [Crossref] [PubMed]
- Duering M, Biessels GJ, Brodtmann A, Chen C, Cordonnier C, de Leeuw FE, et al. Neuroimaging standards for research into small vessel disease-advances since 2013. Lancet Neurol 2023;22:602-18. [Crossref] [PubMed]
- Pinter D, Ritchie SJ, Gattringer T, Bastin ME, Hernández MDCV, Corley J, Maniega SM, Pattie A, Dickie DA, Gow AJ, Starr JM, Deary IJ, Enzinger C, Fazekas F, Wardlaw J. Predictors of gait speed and its change over three years in community-dwelling older people. Aging (Albany NY) 2018;10:144-53. [Crossref] [PubMed]
- Su C, Yang X, Wei S, Zhao R. Periventricular white matter hyperintensities are associated with gait and balance in patients with minor stroke. Front Neurol 2022;13:941668. [Crossref] [PubMed]
- Gronewold J, Jokisch M, Schramm S, Himpfen H, Ginster T, Tenhagen I, Doeppner TR, Jockwitz C, Miller T, Lehmann N, Moebus S, Jöckel KH, Erbel R, Caspers S, Hermann DMHeinz Nixdorf Recall Study Investigative Group. Association of regional white matter hyperintensities with hypertension and cognition in the population-based 1000BRAINS study. Eur J Neurol 2023;30:1174-90. [Crossref] [PubMed]
- Cai M, Jacob MA, Norris DG, Duering M, de Leeuw FE, Tuladhar AM. Cognition mediates the relation between structural network efficiency and gait in small vessel disease. Neuroimage Clin 2021;30:102667. [Crossref] [PubMed]
- Ghanavati T, Smitt MS, Lord SR, Sachdev P, Wen W, Kochan NA, Brodaty H, Delbaere K. Deep white matter hyperintensities, microstructural integrity and dual task walking in older people. Brain Imaging Behav 2018;12:1488-96. [Crossref] [PubMed]
- Rizvi B, Narkhede A, Last BS, Budge M, Tosto G, Manly JJ, Schupf N, Mayeux R, Brickman AM. The effect of white matter hyperintensities on cognition is mediated by cortical atrophy. Neurobiol Aging 2018;64:25-32. [Crossref] [PubMed]
- Hallemans A, Verbeque E, Van de Walle P. Motor functions. Handbook of Clinical Neurology 2020;173:157-70. [Crossref] [PubMed]
- Dapp U, Vinyard D, Golgert S, Krumpoch S, Freiberger E. Reference values of gait characteristics in community-dwelling older persons with different physical functional levels. BMC Geriatr 2022;22:713. [Crossref] [PubMed]
- Fritz S, Lusardi M. White paper: "walking speed: the sixth vital sign". J Geriatr Phys Ther 2009;32:46-9.
- Argyridou S, Zaccardi F, Davies MJ, Khunti K, Yates T. Walking pace improves all-cause and cardiovascular mortality risk prediction: A UK Biobank prognostic study. Eur J Prev Cardiol 2020;27:1036-44. [Crossref] [PubMed]
- Charles A, Buckinx F, Locquet M, Reginster JY, Petermans J, Gruslin B, Bruyère O. Prediction of Adverse Outcomes in Nursing Home Residents According to Intrinsic Capacity Proposed by the World Health Organization. J Gerontol A Biol Sci Med Sci 2020;75:1594-9. [Crossref] [PubMed]
- Wang DXM, Yao J, Zirek Y, Reijnierse EM, Maier AB. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle 2020;11:3-25. [Crossref] [PubMed]
- Zucchelli A, Vetrano DL, Grande G, Calderón-Larrañaga A, Fratiglioni L, Marengoni A, Rizzuto D. Comparing the prognostic value of geriatric health indicators: a population-based study. BMC Med 2019;17:185. [Crossref] [PubMed]
- Han F, Kong X, Lv W, Li S, Sun Y, Wu Y. Association of diabetes mellitus with gait and falls in community-dwelling older adults: Serial mediation of vision and cognition. Arch Gerontol Geriatr 2023;104:104827. [Crossref] [PubMed]
- Ogawa EF, Shi L, Bean JF, Hausdorff JM, Dong Z, Manor B, McLean RR, Leveille SG. Chronic Pain Characteristics and Gait in Older Adults: The MOBILIZE Boston Study II. Arch Phys Med Rehabil 2020;101:418-25. [Crossref] [PubMed]
- Li H, Zhang J, Zou X, Jia X, Zheng D, Guo X, Xie W, Yang Q. The Bidirectional Association Between Cognitive Function and Gait Speed in Chinese Older Adults: Longitudinal Observational Study. JMIR Public Health Surveill 2023;9:e44274. [Crossref] [PubMed]
- Hu W, Yang L, Li X, Huang H. Chinese Consensus on Diagnosis and Therapy of Cerebral Small Vessel Disease 2021. Chinese Journal of Stroke 2021;16:716-726.
- Sachdev P, Kalaria R, O'Brien J, Skoog I, Alladi S, Black SE, Blacker D, Blazer DG, Chen C, Chui H, Ganguli M, Jellinger K, Jeste DV, Pasquier F, Paulsen J, Prins N, Rockwood K, Roman G, Scheltens PInternationlal Society for Vascular Behavioral and Cognitive Disorders. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 2014;28:206-18. [Crossref] [PubMed]
- Kline RB, Little TD. Principles and Practice of Structural Equation Modeling. Fourth Edition. New York: The Guilford Press; 2015.
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 1987;149:351-6. [Crossref] [PubMed]
- Pantoni L, Basile AM, Pracucci G, Asplund K, Bogousslavsky J, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Hennerici M, O'brien J, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Inzitari D. Impact of age-related cerebral white matter changes on the transition to disability -- the LADIS study: rationale, design and methodology. Neuroepidemiology 2005;24:51-62. [Crossref] [PubMed]
- Valdés Hernández Mdel C, Morris Z, Dickie DA, Royle NA, Muñoz Maniega S, Aribisala BS, Bastin ME, Deary IJ, Wardlaw JM. Close correlation between quantitative and qualitative assessments of white matter lesions. Neuroepidemiology 2013;40:13-22. [Crossref] [PubMed]
- Kynast J, Lampe L, Luck T, Frisch S, Arelin K, Hoffmann KT, Loeffler M, Riedel-Heller SG, Villringer A, Schroeter ML. White matter hyperintensities associated with small vessel disease impair social cognition beside attention and memory. J Cereb Blood Flow Metab 2018;38:996-1009. [Crossref] [PubMed]
- Yu J, Li J, Huang X. The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry 2012;12:156. [Crossref] [PubMed]
- Sun Y, An C, He W, Zhu Y. A preliminary study of the application of Montreal Cognitive Assessment Beijing version in community dwelling older adults residing in Shenyang. Chinese Journal of Behavioral Medicine and Brain Science 2012;21:948-50.
- de Laat KF, van Norden AG, Gons RA, van Oudheusden LJ, van Uden IW, Bloem BR, Zwiers MP, de Leeuw FE. Gait in elderly with cerebral small vessel disease. Stroke 2010;41:1652-8. [Crossref] [PubMed]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85-94. [Crossref] [PubMed]
- Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. Br J Math Stat Psychol 2014;67:451-70. [Crossref] [PubMed]
- Muñoz Maniega S, Chappell FM, Valdés Hernández MC, Armitage PA, Makin SD, Heye AK, Thrippleton MJ, Sakka E, Shuler K, Dennis MS, Wardlaw JM. Integrity of normal-appearing white matter: Influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J Cereb Blood Flow Metab 2017;37:644-56. [Crossref] [PubMed]
- Solé-Guardia G, Custers E, de Lange A, Clijncke E, Geenen B, Gutierrez J, Küsters B, Claassen JAHR, de Leeuw FE, Wiesmann M, Kiliaan AJ. Association between hypertension and neurovascular inflammation in both normal-appearing white matter and white matter hyperintensities. Acta Neuropathol Commun 2023;11:2. [Crossref] [PubMed]
- Lampe L, Zhang R, Beyer F, Huhn S, Kharabian Masouleh S, Preusser S, Bazin PL, Schroeter ML, Villringer A, Witte AV. Visceral obesity relates to deep white matter hyperintensities via inflammation. Ann Neurol 2019;85:194-203. [Crossref] [PubMed]
- Hilal S, Ikram MA, Verbeek MM, Franco OH, Stoops E, Vanderstichele H, Niessen WJ, Vernooij MW. C-Reactive Protein, Plasma Amyloid-β Levels, and Their Interaction With Magnetic Resonance Imaging Markers. Stroke 2018;49:2692-8. [Crossref] [PubMed]
- Han YP, Tang X, Han M, Yang J, Cardoso MA, Zhou J, Simó R. Relationship between obesity and structural brain abnormality: Accumulated evidence from observational studies. Ageing Res Rev 2021;71:101445. [Crossref] [PubMed]
- Morys F, Dadar M, Dagher A. Association Between Midlife Obesity and Its Metabolic Consequences, Cerebrovascular Disease, and Cognitive Decline. J Clin Endocrinol Metab 2021;106:e4260-74. [Crossref] [PubMed]
- Wersching H, Duning T, Lohmann H, Mohammadi S, Stehling C, Fobker M, Conty M, Minnerup J, Ringelstein EB, Berger K, Deppe M, Knecht S. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology 2010;74:1022-9. [Crossref] [PubMed]
- Li Y, Li M, Zhang X, Shi Q, Yang S, Fan H, Qin W, Yang L, Yuan J, Jiang T, Hu W. Higher blood-brain barrier permeability is associated with higher white matter hyperintensities burden. J Neurol 2017;264:1474-81. [Crossref] [PubMed]
- Oosterman JM, Van Harten B, Weinstein HC, Scheltens P, Sergeant JA, Scherder EJ. White matter hyperintensities and working memory: an explorative study. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2008;15:384-99. [Crossref] [PubMed]
- Yamanaka T, Uchida Y, Sakurai K, Kato D, Mizuno M, Sato T, Madokoro Y, Kondo Y, Suzuki A, Ueki Y, Ishii F, Borlongan CV, Matsukawa N. Anatomical Links between White Matter Hyperintensity and Medial Temporal Atrophy Reveal Impairment of Executive Functions. Aging Dis 2019;10:711-8. [Crossref] [PubMed]
- Ciliz M, Sartor J, Lindig T, Pilotto A, Schäffer E, Weiss M, Scheltens P, Becker S, Hobert MA, Berg D, Liepelt-Scarfone I, Maetzler W. Brain-Area Specific White Matter Hyperintensities: Associations to Falls in Parkinson’s Disease. J Parkinsons Dis 2018;8:455-62. [Crossref] [PubMed]
- Mirza SS, Saeed U, Knight J, Ramirez J, Stuss DT, Keith J, Nestor SM, Yu D, Swardfager W, Rogaeva E, St George Hyslop P, Black SE, Masellis MAlzheimer's Disease Neuroimaging Initiative. APOE ε4, white matter hyperintensities, and cognition in Alzheimer and Lewy body dementia. Neurology 2019;93:e1807-19. [Crossref] [PubMed]
- Xue S, Shen T, Li M, Leng B, Yao R, Gao Y, Sun H, Li Z, Zhang J. Neuronal glutamate transporters are associated with cognitive impairment in obstructive sleep apnea patients without dementia. Neurosci Lett 2023;802:137168. [Crossref] [PubMed]
- Jokinen H, Laakso HM, Ahlström M, Arola A, Lempiäinen J, Pitkänen J, Paajanen T, Sikkes SAM, Koikkalainen J, Lötjönen J, Korvenoja A, Erkinjuntti T, Melkas S. Synergistic associations of cognitive and motor impairments with functional outcome in covert cerebral small vessel disease. Eur J Neurol 2022;29:158-67. [Crossref] [PubMed]
- Bolandzadeh N, Liu-Ambrose T, Aizenstein H, Harris T, Launer L, Yaffe K, Kritchevsky SB, Newman A, Rosano C. Pathways linking regional hyperintensities in the brain and slower gait. Neuroimage 2014;99:7-13. [Crossref] [PubMed]
- Zheng JJ, Delbaere K, Close JC, Sachdev PS, Wen W, Lord SR. White matter hyperintensities and impaired choice stepping reaction time in older people. Neurobiol Aging 2012;33:1177-85. [Crossref] [PubMed]
- Iandolo R, Avci E, Bommarito G, Sandvig I, Rohweder G, Sandvig A. Characterizing upper extremity fine motor function in the presence of white matter hyperintensities: A 7 T MRI cross-sectional study in older adults. Neuroimage Clin 2024;41:103569. [Crossref] [PubMed]
- VanGilder JL, Hooyman A, Peterson DS, Schaefer SY. Post-stroke cognitive impairments and responsiveness to motor rehabilitation: A review. Curr Phys Med Rehabil Rep 2020;8:461-8. [Crossref] [PubMed]


