
Discriminating bronchiolar adenoma from peripheral lung cancer by thin-section computed tomography (CT): a 2-center study
Introduction
Recently, bronchiolar adenoma (BA) has been recognized as a group of benign peripheral lung tumors in the 2021 World Health Organization (WHO) classification of lung tumors (1). Clinically, patients with BAs typically do not exhibit obvious symptoms, and the lesions are often incidentally detected during physical examinations or while diagnosing and treating other conditions (2). On computed tomography (CT) images, BA typically presents as a solitary, irregular, and small peripheral lung nodule, which may manifest as solid, part-solid, or ground-glass opacification (2-5). Due to its resemblance to peripheral lung cancer (PLC), particularly adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA), BA is prone to being misdiagnosed (3-6). Consequently, accurately diagnosing BAs poses a significant challenge.
Needle biopsy has traditionally been utilized to confirm the diagnosis of lung lesions prior to surgery (7-9). However, this procedure is invasive and carries certain risks (10,11). Furthermore, accurately diagnosing BAs through percutaneous or transbronchial biopsy is challenging due to their small size and peripheral distribution.
Making a correct diagnosis of BA via intraoperative frozen section is complex as some lesions lack typical pathological features and may resemble lung cancers (2,12-16). The preoperative or intraoperative misdiagnosis of BA may lead to unnecessary surgical resection or wide excision. Therefore, it is necessary to identify a non-traumatic method to effectively distinguish BA from lung cancer.
CT examination is an effective tool for detecting and diagnosing lung diseases. Currently, since it’s proposal in 2018, most of the studies on BA have focused on the pathological findings (2,7-9,12-16). Only a small number of articles in English have introduced the CT features of BAs and their differential diagnosis (3-5). Onishi et al. (4) firstly introduced the thin-section CT (TSCT) features of ciliated muconodular papillary tumors (a former name of BA), but only a few radiological indicators were studied. Cao et al. (3) distinguished BA from AIS and MIA by comparing their CT features, but only ground-glass nodules (GGNs) were included. Another study focused on CT texture analysis in distinguishing BA from AIS/MIA (5). The sample size of BAs in these studies was very small. Therefore, there is no comprehensive study in differential diagnosis of BA and PLC using TSCT based on a large sample, and the efficiency of CT in diagnosing BAs has not been verified.
In this study, the clinical and TSCT characteristics of BAs and PLCs from 2 hospitals were thoroughly evaluated with the aim of revealing their differences and identifying the key indicators of BAs. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-687/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Institutional Review Board of The First Affiliated Hospital of Chongqing Medical University (No. 2019-062) and The Second Affiliated Hospital of Army Medical University (No. 2020-research147-01). Additionally, due to the retrospective nature of this study, the requirement for informed consent was waived.
Patients
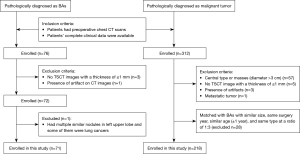
We retrospectively collected the data of patients in the Electronic Medical Record System who had pulmonary lesions that had been pathologically diagnosed as BA after surgical resection from March 2020 to May 2023. Meanwhile, patients with surgically resected and pathologically confirmed PLC were selected as the control group by individually matching them with the similar size, same surgery year, similar age (±1 year), and same type on CT images at a ratio of 3:1. Their chest CT data were collected and reviewed on the picture archiving and communication system (Vue PACS; Carestream, Rochester, NY, USA). In this study, the inclusion criteria of BAs and lung cancers were as follows: (I) patients had preoperative chest CT scans; (II) patients’ complete clinical data were available. The exclusion criteria were as follows: (I) the lung cancers were central type or masses (diameter >3 cm) on CT images because all of the BAs were nodules and located peripherally; (II) no TSCT images with a thickness of ≤1 mm; (III) presence of artifacts on CT images affecting evaluation; (IV) nodules were confirmed as metastatic tumors. After preliminarily searching, a total of 76 BAs were enrolled. Among the 76 BAs, 1 was excluded because this patient had multiple similar nodules in the left upper lobe and some of them were lung cancers, so it was difficult to confirm which was BA. Finally, a total of 75 BAs (36 from The First Affiliated Hospital of Chongqing Medical University and 39 from The Second Affiliated Hospital of Army Medical University) in 71 patients and 229 PLCs (109 from The First Affiliated Hospital of Chongqing Medical University, 120 from The Second Affiliated Hospital of Army Medical University) in 218 patients were included in this study (Figure 1).
CT examinations
All patients underwent chest CT examinations using one of the following CT scanners: SOMATOM Perspective (Siemens Healthineers, Erlangen, Germany), SOMATOM Definition Flash (Siemens Healthineers, Germany), SOMATOM Force (Siemens Healthineers, Germany), Discovery CT750 HD (GE Healthcare, Milwaukee, WI, USA), and Aquilion ONE pure ViSION (Canon Medical System, Tokyo, Japan). In order to minimize breathing artifacts, all CT scans were performed at the end of inspiration during a single breath-hold. The scan range was from the thoracic entrance to the costophrenic angle. The CT scan was acquired with the following settings: tube voltage, 110–130 kVp; tube current time, 50–140 mA (using automatic current modulation technology); scanning slice thickness, 5 mm; rotation time, 0.5 s; pitch, 1–1.1; collimation, 0.6 or 0.625 mm; reconstruction slice thickness and interval, 0.625 or 1 mm; matrix, 512×512. Plain CT scans were performed on all patients, and 76 (26.3%) of them (5 with BAs and 71 with PLCs) underwent contrast-enhanced CT scan with a total of 80–100 mL of nonionic iodinated contrast material (iopamidol, 320 mg/mL; Shanghai Bracco Sine Pharmaceutical Co., Ltd., Shanghai, China) at an injection rate of 3.0 mL/s, followed by 50 mL of saline solution via a power injector. Images with mediastinal (width, 350–400 HU; level, 20–40 HU) and lung (width, 1,200–1,600 HU; level, −500 to −700 HU) window settings were obtained.
Clinical data and image analysis
The patients’ clinical data were obtained by using the Electronic Medical Record System (Winning Health, Shanghai, China). Clinical data included patients’ age, gender, smoking history, clinical symptoms (cough, expectoration, hemoptysis, chest pain, back pain, and fever), and history of malignant tumor.
The patients’ chest TSCT data were analyzed on the PACS with lung window settings (width, 1,200–1,600 HU; level, −500 to −700 HU) and mediastinal window setting (width, 350–400 HU; level, 20–40 HU). All patients’ CT data were reviewed independently by 2 radiologists (Y.T. and T.W.X. with 6 and 10 years of experience in chest CT, respectively) who were blinded to clinical information and pathological diagnosis. Any interobserver discordance was resolved by reevaluating the images together or consulting with senior radiologists to reach a consensus.
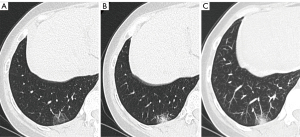
The following CT features of nodules were analyzed based on the non-contrast enhanced TSCT images: distribution in lobes and segments (upper, middle or lower lobe; apical, posterior, front, inner or outer, dorsal, basal or lingual segment), size (the mean of the longest diameter and the perpendicular diameter on axial CT images), CT pattern [pure GGNs (pGGNs), part-solid nodules (PSNs) or solid nodules (SNs)], mean CT value, density (homogeneous or heterogeneous), shape (regular or irregular), boundary (ill-defined or well-defined), margin (smooth or coarse), lobulation sign, spiculation sign, vacuole sign, air bronchogram sign, bronchial cut-off sign, central vessel sign and peripheral vessel sign and the type of vessels (pulmonary artery, pulmonary vein, or both), distance from lesion edge to pleura (D-ETP) (≤5 or >5 mm), distance from lesion center to pleura (D-CTP) (≤10 or >10 mm) (Figure 2), locally or totally attaching to pleura, pleural indentation sign, intrathoracic lymph node enlargement, and changes during follow-up. GGN was shown as a hazy opacity with the presence of the bronchial structures or underlying pulmonary vessels in high resolution CT (17). The difference between pGGN and PSN was the presence of solid components in it. The mean CT value was measured with a region of interest at the section with the most solid components (PSNs) or that with largest diameter of the lesion (pGGNs and SNs). The shape of oval or round was defined as regular; neither of them was defined as irregular. Well-defined boundary was defined as a clear tumor-lung interface, otherwise it was ill-defined. The margin was only evaluated for those lesions with well-defined boundary. Lobulation sign was defined as an abrupt bulging of the contour of the lesion (18). Spiculation sign was defined as linear strands that extended from the nodule surface into the lung parenchyma without reaching a pleural surface (19). Vacuole sign was defined as round or irregular air attenuation with a 1–2 mm diameter in a nodule (20). Air bronchogram sign was defined as a lucency along a regular bronchial wall within the lesion (21). In this study, central vessel sign was defined as vessels connecting with the middle part of a lesion margin that was opposite to the pleura; peripheral vessel sign was defined as vessels connecting with the other part of a lesion margin (Figure 3). Intrathoracic lymph node enlargement was defined as mediastinal or hilar lymph nodes with a diameter of at least 1 cm in short axes (22).

Statistical analysis
The patients’ clinical data and CT features of nodules were analyzed using the statistical software SPSS 21.0 (IBM Corp., Armonk, NY, USA) and MedCalc (MedCalc Software, Ostend, Belgium). Continuous variables were expressed as mean ± standard deviation (SD); categorical variables were expressed as number and percentage. The intraclass correlation coefficient (ICC) was used to assess the interobserver agreement of continuous variables, and Cohen’s kappa or Fleiss’ kappa coefficient was used to assess the interobserver agreement of categorical variables. Interobserver agreement based on ICC was classified as poor (<0.50), moderate (0.50−0.74), good (0.75−0.89), or excellent (≥0.90). Interobserver agreement based on kappa coefficients was categorized as slight (≤0.20), fair (0.21−0.40), moderate (0.41−0.60), substantial (0.61−0.80), or almost perfect (≥0.81). The Kolmogorov-Smirnov test was used to assess the normal distribution of the continuous variables. In order to compare differences in variables between BAs and PLCs, Student’s t-test was used for normally distributed data (patient age), Mann-Whitney U-test was used for non-normally distributed data (nodule size and mean CT value), and Pearson’s Chi-squared test was used for sex, clinical symptoms, history of malignant tumor, smoking history, lesion location, CT pattern, uniformity of density, shape, boundary, margin, lobulation sign, spiculation sign, vacuole sign, air bronchogram sign, bronchial cut-off sign, central vessel sign and peripheral vessel sign and the corresponding type of vessels, D-ETP (≤5 or >5 mm), D-CTP (≤10 or >10 mm), local or total attachment to pleura, pleural indentation sign, intrathoracic lymph node enlargement, and changes during follow-up. Variables with statistical differences were further included in logistic regression analysis to determine independent factors for predicting BAs. A 2-sided P value of <0.05 was considered indicative of a statistically significant difference.
Results
Patients’ clinical characteristics
Among the 71 patients with 75 BAs, 1 had 3 lesions and 2 had 2 lesions. Meanwhile, a total of 29 concomitant nodules in 24 patients (1 had 3 lesions and 3 had 2 lesions) were confirmed as PLCs. Among the 218 patients with 229 PLCs, 7 had 2 lesions and 2 had 3 lesions. The 229 PLCs included 76 (33.2%) invasive adenocarcinomas, 87 (38.0%) MIAs, 58 (25.3%) AISs, 5 (2.2%) invasive mucinous adenocarcinoma, 1 (0.4%) squamous cell carcinomas, 1 (0.4%) atypical carcinoid, and 1 (0.4%) acinar adenocarcinoma. Table 1 summarizes the patients’ clinical characteristics. The BAs and PLCs were all more common in women (60.6% and 67.9%, respectively, P=0.257). Similar to patients with PLCs, more individuals had no clinical symptoms in those with BAs (84.9% and 91.5%, respectively, P=0.152).
Table 1
| Characteristics | Patients with BAs (n=71) |
Patients with PLCs (n=218) |
P value |
|---|---|---|---|
| Number of lesions | 75 | 229 | – |
| Age (years) | 55.38±13.56 | 53.67±11.64 | 0.302 |
| Sex | 0.257 | ||
| Female | 43 (60.6) | 148 (67.9) | |
| Male | 28 (39.4) | 70 (32.1) | |
| Clinical symptoms | 0.152 | ||
| Yes | 6 (8.5) | 33 (15.1) | |
| No | 65 (91.5) | 185 (84.9) | |
| History of malignant tumor | 0.508 | ||
| Yes | 5 (7.0) | 21 (9.6) | |
| No | 66 (93.0) | 197 (90.4) | |
| Smoking history | 0.867 | ||
| Yes | 14 (19.7) | 45 (20.6) | |
| No | 57 (80.3) | 173 (79.4) | |
Data are expressed as number (percentage) or mean ± standard deviation. BAs, bronchiolar adenomas; PLCs, peripheral lung cancers.
The pathological characteristics of BAs
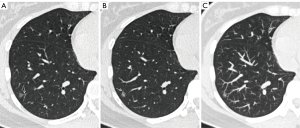
In the BAs, the characteristic histological features included a bilayered bronchiolar-type epithelium with a continuous basal cell layer. The luminal cell layer typically exhibited abundant mucinous and ciliated cells. Immunohistochemical (IHC) staining commonly showed positivity for markers such as p63, p40, and CK5/6 in the basal cell layer (Figure 4). Among the 75 BAs in the study, 53 underwent IHC staining, revealing positive rates of 93.9% (31/33) for p40, 93.9% (46/49) for p63, 89.4% (42/47) for CK5/6, 100% (49/49) for Ki-67, 89.6% (43/48) for TTF-1, and 66.7% (16/24) for Napsin-A.

Interobserver agreement
Table 2 summarizes the interobserver agreement for the CT features. For the continuous variables, agreements were all good (ICC: 0.75–0.89). For the categorical indicators, agreement for uniformity of density was substantial (Kappa coefficient: 0.61–0.80), and other agreements were almost perfect (Kappa coefficient ≥0.81).
Table 2
| CT features | Metric | 95% CI | P value |
|---|---|---|---|
| Diameter | 0.880 | 0.847–0.906 | <0.001 |
| Types on CT images | 0.816 | 0.751–0.881 | <0.001 |
| Mean CT value | 0.883 | 0.847–0.911 | <0.001 |
| Uniformity of density | 0.707 | 0.615–0.799 | <0.001 |
| Shape | 0.813 | 0.748–0.878 | <0.001 |
| Boundary | 0.838 | 0.728–0.948 | <0.001 |
| Margin | 0.874 | 0.776–0.972 | <0.001 |
| Lobulation sign | 0.863 | 0.775–0.951 | <0.001 |
| Spiculation sign | 0.892 | 0.823–0.961 | <0.001 |
| Vacuole sign | 0.855 | 0.741–0.969 | <0.001 |
| Air bronchogram | 0.846 | 0.715–0.977 | <0.001 |
| Bronchial cut-off sign | 0.853 | 0.651–1.055 | <0.001 |
| Central vessel sign | 0.850 | 0.797–0.957 | <0.001 |
| Type of central vessels | 0.877 | 0.797–0.957 | <0.001 |
| Peripheral vessel sign | 0.845 | 0.772–0.918 | <0.001 |
| Type of peripheral vessels | 0.851 | 0.794–0.908 | <0.001 |
| D-ETP | 0.882 | 0.843–0.911 | <0.001 |
| D-CTP | 0.892 | 0.856–0.918 | <0.001 |
| Attaching to pleura | 0.829 | 0.745–0.913 | <0.001 |
| Pleural indentation sign | 0.850 | 0.754–0.946 | <0.001 |
| Intrathoracic lymph node enlargement | 0.853 | 0.704–1.002 | <0.001 |
Metric represents intraclass correlation coefficient for continuous variables and kappa coefficient for categorical variables. CT, computed tomography; CI, confidence interval; D-ETP, distance from lesion edge to pleura; D-CTP, distance from lesion center to pleura.
Comparison of CT features of BAs and PLCs
The CT features of BAs and PLCs are summarized in Table 3. Compared with PLCs, more BAs located in the basal segments of the lower lobes and had irregular shape (each P<0.001). Attaching to pleura and vacuole sign were more common in BAs than in PLCs (each P<0.05). Lobulation and spiculation sign were all less common in BAs than in PLCs (each P<0.05). Central vessel sign and peripheral vessel sign were both more common in BAs than in PLCs (P<0.05). For the BAs and PLCs with central vessel sign and peripheral vessel sign, both the pulmonary artery and pulmonary vein were more common in the former (each P<0.001). The proportions of lesions with D-ETP ≤5 mm and D-CTP ≤10 mm in BAs were significantly higher than those in PLCs (each P<0.001) (Figure 4).
Table 3
| Features | BAs (n=75) | PLCs (n=229) | P value |
|---|---|---|---|
| Distribution in lobe | |||
| Upper lobe | 11 (14.7) | 133 (58.1) | <0.001 |
| Middle lobe | 3 (4.0) | 21 (9.2) | 0.180 |
| Lower lobe | 61 (81.3) | 75 (32.8) | <0.001 |
| Distribution in segment | |||
| Apical/posterior segment | 8 (10.7) | 86 (37.6) | <0.001 |
| Front segment | 3 (4.0) | 33 (14.4) | 0.015 |
| Inner and outer segment | 3 (4.0) | 21 (9.2) | 0.150 |
| Dorsal segment | 11 (14.7) | 33 (14.4) | 0.956 |
| Lingual segment | 0 (0) | 14 (6.1) | 0.061 |
| Basal segment | 50 (66.7) | 42 (18.3) | <0.001 |
| Diameter (mm) | 6.86±3.86 | 6.86±3.13 | 0.301 |
| Types on CT images | 0.906 | ||
| SN | 36 (48.0) | 104 (45.4) | |
| PSN | 26 (34.7) | 81 (35.4) | |
| pGGN | 13 (17.3) | 44 (19.2) | |
| Mean CT value (HU) | −322.81±190.71 | −287.08±236.11 | 0.282 |
| Uniformity of density | 0.533 | ||
| Homogeneous | 30 (40.0) | 101 (44.1) | |
| Heterogeneous | 45 (60.0) | 128 (55.9) | |
| Shape | <0.001 | ||
| Irregular | 32 (42.7) | 39 (17.0) | |
| Regular | 43 (57.3) | 190 (83.0) | |
| Boundary | 0.827 | ||
| Well-defined | 64 (85.3) | 193 (84.3) | |
| Ill-defined | 11 (14.7) | 36 (15.7) | |
| Margin* | 0.177 | ||
| Smooth | 47 (73.4) | 124 (64.2) | |
| Coarse | 17 (26.6) | 69 (35.8) | |
| Lobulation sign | 4 (5.3) | 35 (15.3) | 0.025 |
| Spiculation sign | 5 (6.7) | 52 (22.7) | 0.002 |
| Vacuole sign | 16 (21.3) | 20 (8.7) | 0.003 |
| Air bronchogram | 7 (9.3) | 17 (7.4) | 0.595 |
| Bronchial cut-off sign | 0 (0) | 7 (3.1) | 0.276 |
| Central vessel sign | 0.003 | ||
| Yes | 61 (81.3) | 144 (62.9) | |
| Type of vessels | |||
| Pulmonary artery | 53 (86.9) | 58 (40.3) | <0.001 |
| Pulmonary vein | 6 (9.8) | 63 (43.8) | <0.001 |
| Both | 2 (3.3) | 23 (16.0) | 0.011 |
| No | 14 (18.7) | 85 (37.1) | |
| Peripheral vessel sign | <0.001 | ||
| Yes | 69 (92.0) | 152 (66.4) | |
| Type of vessels | |||
| Pulmonary artery | 7 (10.1) | 86 (56.6) | <0.001 |
| Pulmonary vein | 60 (87.0) | 58 (38.2) | <0.001 |
| Both | 2 (2.9) | 8 (5.3) | 0.664 |
| No | 6 (8.0) | 77 (33.6) | |
| D-ETP | <0.001 | ||
| ≤5 mm | 57 (76.0) | 94 (41.0) | |
| >5 mm | 18 (24.0) | 135 (59.0) | |
| D-CTP | <0.001 | ||
| ≤10 mm | 58 (77.3) | 108 (47.2) | |
| >10 mm | 17 (22.7) | 121 (52.8) | |
| Attaching to pleura* | 25 (33.3) | 42 (18.3) | 0.007 |
| Locally | 18 (72.0) | 30 (71.4) | 0.96 |
| Totally | 7 (28.0) | 12 (28.6) | |
| Pleural indentation sign | 8 (10.7) | 32 (14.0) | 0.462 |
| Intrathoracic lymph node enlargement | 2 (2.7) | 10 (4.4) | 0.753 |
| Follow-up | 24 (32) | 57 (24.9) | |
| Increase in size | 3 (12.5) | 13 (22.8) | 0.448 |
Data are expressed as number (percentage) or mean ± standard deviation. *, this indicator is only evaluated in some of the patients. CT, computed tomography; BAs, bronchiolar adenomas; PLCs, peripheral lung cancers; SN, solid nodule; PSN, part-solid nodule; pGGN, pure ground glass nodule; HU, Hounsfield unit; D-ETP, distance from lesion edge to pleura; D-CTP, distance from lesion center to pleura.
Logistic regression analysis for BAs and PLCs
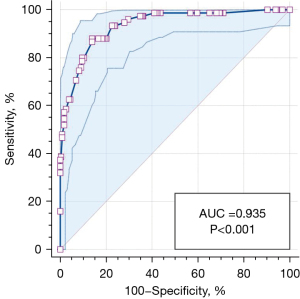
Table 4 shows the clinical and CT characteristics that were shown to independently discriminate BAs from PLCs via binary logistic regression analysis. Compared with PLCs, distributing in the basal segment of lower lobe, irregular shape, central vessel sign with pulmonary artery, peripheral vessel sign with pulmonary vein, and D-ETP ≤5 mm were found to be significantly independent indicators of BAs (Figure 5). The sensitivity, specificity, and the area under the curve (AUC) of this model in diagnosing BAs were 88.0%, 86.03%, and 0.935 [95% confidence interval (CI): 0.901–0.960] (P<0.001), respectively (Figure 6).
Table 4
| Variables | Odds ratio (95% CI) | P value |
|---|---|---|
| Distribution in basal segment | <0.001 | |
| No | 1 | |
| Yes | 17.835 (6.977–45.588) | |
| Shape | 0.001 | |
| Regular | 1 | |
| Irregular | 4.765 (1.877–12.099) | |
| Negative of spiculation sign | 0.002 | |
| No | 1 | |
| Yes | 7.436 (2.063–26.809) | |
| Central vessel sign (pulmonary artery) | 0.003 | |
| No | 1 | |
| Yes | 3.576 (1.557–8.211) | |
| Peripheral vessel sign (pulmonary vein) | <0.001 | |
| No | 1 | |
| Yes | 12.444 (4.934–31.383) | |
| D-ETP | <0.001 | |
| >5 mm | 1 | |
| ≤5 mm | 5.535 (2.346–13.057) | |
BAs, bronchiolar adenomas; CI, confidence interval; D-ETP, distance from lesion edge to pleura.
Discussion
Following their proposal, BAs have gradually received attention in recent years, with more research on the pathology but relatively few radiological comparative studies on large samples. In view of the similarity of BAs and PLCs on CT images, further study regarding their differential diagnosis is needed. This study collected a relatively large number of BAs and compared them with common PLCs. It was found that the BAs and PLCs had many significant differences in TSCT features. Compared with PLCs, BAs usually presented some unique characteristics. They were frequently located in the basal segments of lower lobes, and usually had an irregular shape, central vessel sign with pulmonary artery, peripheral vessel sign with pulmonary vein, and a short D-ETP (≤5 mm). These distinct features are helpful for describing the characteristics of BAs and differentiating them from PLCs.
The BAs were relatively more often found in female patients, there could be more than 1/multiple in one individual, and they showed occasional coexistence with lung cancers, as reported in previous studies (2,15). The present findings are consistent with those of previous reports. These characteristics of BAs are similar to those of PLCs, especially the neoplastic GGNs (23,24). In this study, clinical symptoms were all less common in patients with BAs and PLCs, which was inconsistent with previous findings (2,3,5), which may be related to the small diameter of the lesions. Besides the clinical symptoms, patients with BAs and those with PLCs displayed a high degree of consistency in clinical characteristics. Therefore, we concluded that the clinical indicators have limited significance in telling them apart.
On CT images, both BAs and PLCs can manifest as pGGNs, PSNs, or SNs, which contributes to the challenge of distinguishing between them. In this study, BAs were more frequently observed in the lower lobes, particularly in the basal segments, whereas PLCs were more commonly located in the upper lobes. This distribution pattern aligns with findings from previous studies (2-5,25,26). Furthermore, BAs were more likely to present with irregular shapes, whereas typical signs of lung cancer such as lobulation and spiculation were rarely observed in them. These distinctions imply that smaller irregular nodules lacking the typical features of PLCs and located in the basal segments are more likely to be BAs. However, the vacuolar sign, as a common sign of lung cancers, was more common in BAs in this study, which is consistent with previous research (3). Actually, the vacuole sign represents spared parenchyma, normal or ecstatic bronchi, or focal emphysema; its occurrence in BAs may be related to the structural abnormalities caused by slow growth in benign lesions.
In addition to the traditional morphological features on CT images, this study also identified new indicators that were effective for differentiating BAs and PLCs. It was revealed that BAs tended to have shorter D-ETP and D-CTP compared to PLCs. This indicated that BAs are typically located closer to pleura, which may be relevant to their origin. Pathologically, BAs originate from the epithelium of the bronchioles, which are densely distributed in the sub-pleural zone. Furthermore, the presence of central vessel sign was more commonly observed in BAs than in PLCs, and pulmonary arteries were more prevalent in BAs. Similarly, peripheral vessel sign was more frequently detected in BAs than in PLCs, with pulmonary veins being the primary type of vessels in BAs. This observation could be attributed to the fact that bronchioles often accompany pulmonary arteries, whereas pulmonary veins typically run within interlobular septa. Therefore, the location and type of connected vessels can serve as important clues for distinguishing between BAs and PLCs.
Intrapulmonary lymph nodes (IPLNs) are benign lesions that are frequently detected as incidental findings on high-resolution chest CT scans. Typical CT characteristics of IPLNs include a noncalcified solitary nodule with well-defined margins, a round, oval, or polygonal shape, located within 15 mm of the pleura, and often positioned below the carina level (27), resembling some features seen in BAs. When distinguishing between BAs and IPLNs becomes challenging, an irregular shape and the presence of central or peripheral vessel signs can be crucial in making a differential diagnosis.
Despite an increasing number of reported cases of malignant transformation of BAs in recent years (2,8,28-33), there is still no consensus on their benign and malignant characteristics. However, to date, there have been no reports of recurrence or metastasis following treatment, regardless of the surgical method employed. Lymph node enlargement is typically attributed to the invasion or spread of either inflammatory cells or tumor cells (34). In this study, only 2 BA patients exhibited intrathoracic lymph node enlargement. Its incidence rate was similar to that in PLCs, which may be related to the fact that a majority of the enrolled PLCs were MIA (38.0%) and AIS (25.3%). Nevertheless, when a peripheral pulmonary nodule presents with intrathoracic lymph node enlargement, the possibility of lung cancer should be considered as a primary concern because it more commonly presents with lymph node metastasis.
This study had 3 limitations. Firstly, although our sample size of BAs from 2 centers was the largest among relevant studies, it was still relatively small considering the diversity of BAs. The differences between BAs and PLCs revealed in this study should be verified in clinical practice. Secondly, the comparison in this study was limited to BAs and PLCs, and potential differences between BAs and other benign nodules remain unknown. Lastly, certain indicators on CT images, such as central vessel sign with pulmonary artery and peripheral vessel sign with pulmonary vein, were newly defined in this study. Therefore, their efficacy in differential diagnosis requires further validation in clinical practice.
Conclusions
BA is a type of pulmonary nodules that is gradually being recognized. As a special kind of peripheral pulmonary nodule, it frequently needs to be differentiated from PLCs. Compared with PLCs, BAs usually present some unique characteristics. Any type of peripheral pulmonary nodules located in basal segments of lower lobes with irregular shape, central vessel sign with pulmonary artery, peripheral vessel sign with pulmonary vein, and D-ETP ≤5 mm, but lacking spiculation sign, should be highly suspected of BAs. Follow-up may be the preferred approach for further management.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-687/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-687/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of The First Affiliated Hospital of Chongqing Medical University (No. 2019-062) and The Second Affiliated Hospital of Army Medical University (No. 2020-research147-01). Due to the retrospective nature of this study, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, Dacic S, Jain D, Kerr KM, Lantuejoul S, Noguchi M, Papotti M, Rekhtman N, Scagliotti G, van Schil P, Sholl L, Yatabe Y, Yoshida A, Travis WD. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J Thorac Oncol 2022;17:362-87. [Crossref] [PubMed]
- Sun J, Zhao W, Zhang C, Zheng E, Jiang X, Wang L, Hayashi T, Sasaki E, Tachibana M, Zhao G. Multiple bronchiolar adenomas/ciliated muconodular papillary tumors of the bilateral lung with tumor budding and potential malignant transformation into squamous cell carcinoma: a case report and literature review. Transl Lung Cancer Res 2023;12:1611-24. [Crossref] [PubMed]
- Cao L, Wang Z, Gong T, Wang J, Liu J, Jin L, Yuan Q. Discriminating between bronchiolar adenoma, adenocarcinoma in situ and minimally invasive adenocarcinoma of the lung with CT. Diagn Interv Imaging 2020;101:831-7. [Crossref] [PubMed]
- Onishi Y, Kusumoto M, Motoi N, Watanabe H, Watanabe SI. Ciliated Muconodular Papillary Tumor of the Lung: Thin-Section CT Findings of 16 Cases. AJR Am J Roentgenol 2020;214:761-5. [Crossref] [PubMed]
- Sun J, Liu K, Tong H, Liu H, Li X, Luo Y, Li Y, Yao Y, Jin R, Fang J, Chen X. CT Texture Analysis for Differentiating Bronchiolar Adenoma, Adenocarcinoma In Situ, and Minimally Invasive Adenocarcinoma of the Lung. Front Oncol 2021;11:634564. [Crossref] [PubMed]
- Si MJ, Tao XF, Du GY, Cai LL, Han HX, Liang XZ, Zhao JM. Thin-section computed tomography-histopathologic comparisons of pulmonary focal interstitial fibrosis, atypical adenomatous hyperplasia, adenocarcinoma in situ, and minimally invasive adenocarcinoma with pure ground-glass opacity. Eur J Radiol 2016;85:1708-15. [Crossref] [PubMed]
- Sun Y, Liu M, Jiang Z, Li B. Bronchiolar adenoma with diffuse pulmonary nodules: a extremely rare case report and review of literature. BMC Pulm Med 2020;20:192. [Crossref] [PubMed]
- Zhao L, Willson CM, Givens NT, Zhu Z, Wakefield MR, Wang Y, Yang W, Fang Y. A rare case of ciliated muconodular papillary tumor accompanied with adenocarcinoma in situ. BMC Pulm Med 2021;21:223. [Crossref] [PubMed]
- Maroongroge S, Weissferdt A, Buszek S, Rice DC, Smith BD, Gandhi SJ. Management of Bronchial Adenoma/Ciliated Muconodular Papillary Tumor with Definitive Stereotactic Body Radiation Therapy (SBRT): A Case Report. Clin Lung Cancer 2022;23:e335-8. [Crossref] [PubMed]
- Merritt RE, Shrager JB. Indications for surgery in patients with localized pulmonary infection. Thorac Surg Clin 2012;22:325-32. [Crossref] [PubMed]
- Ozeki N, Iwano S, Taniguchi T, Kawaguchi K, Fukui T, Ishiguro F, Fukumoto K, Nakamura S, Hirakawa A, Yokoi K. Therapeutic surgery without a definitive diagnosis can be an option in selected patients with suspected lung cancer. Interact Cardiovasc Thorac Surg 2014;19:830-7. [Crossref] [PubMed]
- Chu HH, Park SY, Cha EJ. Ciliated muconodular papillary tumor of the lung: The risk of false-positive diagnosis in frozen section. Human Pathology: Case Reports 2017;7:8-10.
- Willner J, Moreira AL. Squamous overgrowth and metaplasia: an expanded spectrum of bronchiolar adenomas. Histopathology 2023;83:166-7. [Crossref] [PubMed]
- Shirsat H, Zhou F, Chang JC, Rekhtman N, Saqi A, Argyropoulos K, Azour L, Simms A, Melamed J, Hung YP, Roden AC, Mino-Kenudson M, Moreira AL, Narula N. Bronchiolar Adenoma/Pulmonary Ciliated Muconodular Papillary Tumor. Am J Clin Pathol 2021;155:832-44. [Crossref] [PubMed]
- Ding B, Shang Z, Xiang Z, Han Y. Clinicopathologic Features and Frozen Diagnostic Pitfalls of Bronchiolar Adenoma/Ciliated Muconodular Papillary Tumors (BA/CMPTs). Am J Surg Pathol 2023;47:431-9. [Crossref] [PubMed]
- Guo Y, Shi Y, Tong J. Bronchiolar adenoma: A challenging diagnosis based on frozen sections. Pathol Int 2020;70:186-8. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Xu DM, van Klaveren RJ, de Bock GH, Leusveld A, Zhao Y, Wang Y, Vliegenthart R, de Koning HJ, Scholten ET, Verschakelen J, Prokop M, Oudkerk M. Limited value of shape, margin and CT density in the discrimination between benign and malignant screen detected solid pulmonary nodules of the NELSON trial. Eur J Radiol 2008;68:347-52. [Crossref] [PubMed]
- Li WJ, Lv FJ, Tan YW, Fu BJ, Chu ZG. Benign and malignant pulmonary part-solid nodules: differentiation via thin-section computed tomography. Quant Imaging Med Surg 2022;12:699-710. [Crossref] [PubMed]
- Fan L, Liu SY, Li QC, Yu H, Xiao XS. Pulmonary malignant focal ground-glass opacity nodules and solid nodules of 3cm or less: comparison of multi-detector CT features. J Med Imaging Radiat Oncol 2011;55:279-85. [Crossref] [PubMed]
- Xiang W, Xing Y, Jiang S, Chen G, Mao H, Labh K, Jia X, Sun X. Morphological factors differentiating between early lung adenocarcinomas appearing as pure ground-glass nodules measuring ≤10 mm on thin-section computed tomography. Cancer Imaging 2014;14:33. [Crossref] [PubMed]
- Shi W, Zhou L, Peng X, Ren H, Wang Q, Shan F, Zhang Z, Liu L, Shi Y. HIV-infected patients with opportunistic pulmonary infections misdiagnosed as lung cancers: the clinicoradiologic features and initial application of CT radiomics. J Thorac Dis 2019;11:2274-86. [Crossref] [PubMed]
- Seow WJ, Matsuo K, Hsiung CA, Shiraishi K, Song M, Kim HN, et al. Association between GWAS-identified lung adenocarcinoma susceptibility loci and EGFR mutations in never-smoking Asian women, and comparison with findings from Western populations. Hum Mol Genet 2017;26:454-65. [PubMed]
- Sato Y, Fujimoto D, Morimoto T, Uehara K, Nagata K, Sakanoue I, Hamakawa H, Takahashi Y, Imai Y, Tomii K. Natural history and clinical characteristics of multiple pulmonary nodules with ground glass opacity. Respirology 2017;22:1615-21. [Crossref] [PubMed]
- Lin RY, Lv FJ, Fu BJ, Li WJ, Liang ZR, Chu ZG. Features for Predicting Absorbable Pulmonary Solid Nodules as Depicted on Thin-Section Computed Tomography. J Inflamm Res 2021;14:2933-9. [Crossref] [PubMed]
- Ren Z, Ding H, Cai Z, Mu Y, Wang L, Pan S. Development and validation of a prediction model for malignant pulmonary nodules: A cohort study. Medicine (Baltimore) 2021;100:e28110. [Crossref] [PubMed]
- Schreuder A, Jacobs C, Scholten ET, van Ginneken B, Schaefer-Prokop CM, Prokop M, Typical CT. Features of Intrapulmonary Lymph Nodes: A Review. Radiol Cardiothorac Imaging 2020;2:e190159. [Crossref] [PubMed]
- Lau KW, Aubry MC, Tan GS, Lim CH, Takano AM. Ciliated muconodular papillary tumor: a solitary peripheral lung nodule in a teenage girl. Hum Pathol 2016;49:22-6. [Crossref] [PubMed]
- Udo E, Furusato B, Sakai K, Prentice LM, Tanaka T, Kitamura Y, Tsuchiya T, Yamasaki N, Nagayasu T, Nishio K, Fukuoka J. Ciliated muconodular papillary tumors of the lung with KRAS/BRAF/AKT1 mutation. Diagn Pathol 2017;12:62. [Crossref] [PubMed]
- Miyai K, Takeo H, Nakayama T, Obara K, Aida S, Sato K, Matsukuma S. Invasive form of ciliated muconodular papillary tumor of the lung: A case report and review of the literature. Pathol Int 2018;68:530-5. [Crossref] [PubMed]
- Han X, Hao J, Ding S, Wang EH, Wang L. Bronchiolar Adenoma Transforming to Invasive Mucinous Adenocarcinoma: A Case Report. Onco Targets Ther 2021;14:2241-6. [Crossref] [PubMed]
- Chen F, Ren F, Zhao H, Xu X, Chen J. Mucinous adenocarcinoma caused by cancerization from a ciliated multinodular papilloma tumor: A case report. Thorac Cancer 2021;12:1629-33. [Crossref] [PubMed]
- Li X, Wu Y, Hui D, Luo X, Wu W, Zhang J, Chen H. Multiple bronchiolar adenomas with malignant transformation and CCNE1 mutation: a case report and literature review. J Cardiothorac Surg 2021;16:307. [Crossref] [PubMed]
- Bazemore AW, Smucker DR. Lymphadenopathy and malignancy. Am Fam Physician 2002;66:2103-10. [PubMed]


