
Assessment of perioperative cardiac risk using preoperative quantitative flow ratio in patients with coronary artery disease undergoing noncardiac surgery: a retrospective cohort study
Introduction
According to the World Health Organization (WHO), more than 300 million operations were conducted worldwide in 2012 alone. This number represents a 34% increase from 2004 and continues to rise. Notably, noncardiac surgeries (NCSs) constitute nearly 85% of all operations (1). Consequently, perioperative cardiovascular and cerebrovascular complications have emerged as a significant healthcare concern for patients undergoing surgery. In a national study conducted in the United States, it was found that 1 in 33 hospitalized patients undergoing NCS experience major adverse cardiovascular and cerebrovascular events (2). Several factors influence the risk of perioperative adverse cardiovascular events, including underlying medical conditions, preoperative clinical status, anesthesia, and the urgency, extent, type, and duration of surgery (3,4). Among these factors, coronary artery disease (CAD) stands out as a critical underlying condition that increases the risk of perioperative cardiovascular complications. For instance, in a Swiss cohort study involving 2,265 patients undergoing NCS, it was observed that 1 in 7 patients developed at least one adverse cardiovascular event within 30 days of the procedure. This risk was particularly pronounced among older patients and those with a history of heart disease, cardiovascular risk factors, or chronic kidney disease (5). As the global volume of operations continues to rise annually, NCS for patients with CAD has become increasingly common. Of all patients diagnosed with CAD, 18.2% underwent NCS during the 2012–2013 period (6). Several risk indices integrating high-risk factors associated with operations have been applied for the assessment of perioperative risk in NCS and have been validated over the past decade. These include the Revised Cardiac Risk Index (RCRI) (7,8), the American College of Surgery (ACS) National Surgical Quality Improvement Program (NSQIP) (9), The American University of Beirut AUB-HAS2 Cardiovascular Risk Index (AUB-HAS2) (10). However, most risk indices regard CAD as an independent risk factor due to the scope required and thus neglect the differences between patients with CAD, increasing the likelihood of false positives. Therefore, developing a means to completing the accurate preoperative evaluation of patients with CAD to mitigate the risk of postoperative adverse cardiovascular events after NCS is particularly urgent.
Invasive coronary angiography (ICA) is the primary method for diagnosing and determining the severity of CAD, but it is not recommended for patients undergoing NCS due to the potential for creating unnecessary and unpredictable delays in prescheduled surgical interventions (4,11,12). However, preoperative ICA is necessary for patients with CAD and can facilitate the development of follow-up treatment (13-15). However, the value of ICA in patients rescheduled to undergo NCS is incompletely understood. Related studies have identified myocardial ischemia as the major mechanism for adverse cardiovascular events after NCS. Patients with CAD are more vulnerable to myocardial ischemia due to the imbalance between oxygen supply and demand in the presence of coronary artery stenosis (16,17). Quantitative flow ratio (QFR) is a new angiography-based method for evaluating flow functionality in myocardial ischemia (18), which allows for the derivation of fractional flow reserve (FFR) without pressure wire or induced congestion and provides high diagnostic concordance with FFR in intermediate lesions (50–90% stenosis) (19). In assessment with FFR—which is considered the gold standard in the diagnosis of coronary hemodynamic disorders — vessels below the threshold of 0.75 are considered likely to induce myocardial ischemia, whereas values greater than 0.8 can exclude two-thirds of the adverse events caused by myocardial ischemia (20,21).
Recently, QFR has been applied in guiding the implantation of coronary stents and the diagnosis of myocardial infarction (22,23). However, a preoperative assessment for NCS in patients with CAD is still lacking. Our study thus aimed to validate the predictive value of QFR for major adverse cardiovascular events (MACEs) within 30 days after NCS and determine its utility when combined with pre-existing risk indices in patients with CAD. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-63/rc).
Methods
Study design and population
Through the admission system of The First Affiliated Hospital of Wenzhou Medical University, we obtained the information of patients who had undergone NCS within 1 year after receiving coronary angiography, consecutively enrolled these patients, and recorded their perioperative clinical endpoints. Patients in the study were followed up for endpoint events that occurred perioperatively (within 30 days after NCS), mainly through hospitalization records, outpatient clinic visits, and telephone interviews as conducted by specialists or nurses.
This single-center retrospective observational study was carried out in The First Affiliated Hospital of Wenzhou Medical University from January 2013 to December 2022. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Ethics Committee in Clinical Research (ECCR) of The First Affiliated Hospital of Wenzhou Medical University (No. KY-2022-006). The requirement for individual consent was waived due to the retrospective nature of the analysis.
Collection and definition of preoperative clinical data
Demographic, premedication, preoperative laboratory, preoperative coronary angiography, and NCS data were collected from the electronic medical record system. Surgical risk classification was based on the 2022 European Society of Cardiology (ESC) guidelines for the cardiovascular assessment and management of patients undergoing NCS (4), which classifies all types of surgery as low surgical risk, intermediate surgical risk, and high surgical risk. The RCRI is one of the currently recognized assessment models of clinical perioperative cardiac risk. The RCRI score is the number of the following high-risk factors that are present: perioperative high-risk surgery (defined as thoracic surgery, abdominal surgery, or large-vessel surgery above the groin), ischemic heart disease, pulmonary edema, cerebrovascular disease, history of insulin-dependent diabetes mellitus, and serum creatinine >2.0 mg/dL (8). The AUB-HAS2 used the number of the following six data elements that are present: age ≥75 years, history of cardiac disease, symptoms of angina pectoris or dyspnea, emergency surgery, vascular surgery, and hemoglobin <12 mg/dL (10).
Diagnosis of clinical diseases
In this study, CAD was defined stenosis greater than 50% in at least one vessel (and its major branches) and no vessel with stenosis of more than 90% on coronary angiography before cardiac surgery. Meanwhile, the exclusion criteria were as follows: major lesions less than 3 mm from the aorta, severely overlapping or tortuous vessels, myocardial bridge-induced stenosis, poor-quality angiographic images, and a narrow collateral downstream of the stenosis. Patients with CAD were enrolled in the study regardless of whether they had typical symptoms of chest tightness and chest pain as long as no myocardial infarction occurred 72 hours before NCS. NCS was defined as any surgical procedure that did not operate on the heart or its affiliated organs(such as the ascending aorta, aortic arch, and thoracic aorta) and could include the following operations: surgical specialties (vascular surgery, orthopedics, general surgery, gynecology, urology, neurosurgery, plastic surgery, ear, nose, and throat (ENT) surgery, thoracic surgery, ophthalmology) and endoscopic treatment (nasal endoscopy, laryngoscopy, digestive endoscopy, respiratory endoscopy, urethroscopies, colposcopy).
Assessment of QFR data based on coronary angiography
Two identical angiographic images before NCS with an angle difference ≥25° were transmitted to QFR analysis software (AngioPlus, Pulse Medical Imaging Technology, Shanghai, China) through the network, with the QFR being calculated offline based on Murray’s bifurcated fractal law. After the closest and most distal anatomical landmarks of the diseased vessel were identified as normal reference points, the vessel contours were automatically detected.; otherwise, manual correction of suboptimal images was required as indicated by the standard operating procedures (18). The procession of computation was performed and included the three-dimensional model reconstruction of the target vessel, reference vessel diameter confirmation, and acquisition of fixed QFR with fixed hyperemic inflow velocity. The measurements for all patients were acquired independently by a certified analyst who followed the standard procedures for maintaining the confidentiality of clinical data. If inaccurately measured, an analyst with 3 years of QFR measurement experience via training would review and correct images while also maintaining the confidentiality of clinical data. A diagram of QFR measurement is provided in Figure 1.

To mitigate differences between vessels, we measured vessels with a reference diameter ≥2.5 mm based on visual observation and recorded the minimum QFR value obtained for each patient. According to the obtained QFR data, the patients were divided into a low-QFR group (QFR <0.75; n=122), a gray interval-QFR group (0.75≤ QFR ≤0.8; n=110), and a high-QFR group (QFR >0.8; n=697) according to the minimum QFR of the coronary artery stenosis.
Follow-up and clinical outcome definitions
The primary endpoint was MACEs, which were defined as a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, cardiopulmonary arrest, malignant ventricular arrhythmia (MVA), congestive heart failure, and revascularization. The secondary endpoints included cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, cardiopulmonary arrest, MVA, congestive heart failure and revascularization, bleeding, a perioperative major cardiac event (PMCE), and the primary outcome of AUB-HAS2. PMCE was defined as a composite of myocardial infarction, pulmonary edema, or cardiac death within 30 days after NCS. The primary outcome of AUB-HAS2 includes perioperative myocardial infarction, perioperative death, or perioperative stroke. Myocardial infarction was defined as the presence of one of the following factors: electrocardiography (ECG) indicating acute myocardial infarction (at least one of the following factors: ST elevation >1 mm in two or more contiguous leads, new left bundle branch, and new Q waves in two or more contiguous leads), or progressive elevation in troponin more than threefold the upper level of the reference range when accompanied by typical myocardial ischemia symptoms such as chest tightness and chest pain. Stroke was defined as the sudden onset of neurological deficits lasting more than 24 hours and confirmed by imaging.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation (SD) or as the median and interquartile range (25th–75th percentile), while categorical variables are presented as frequencies and percentages. Analysis of variance (ANOVA) or the Kruskal-Wallis test was used to compare the continuous variables between groups. Categorical variables were identified via the Pearson χ2 or Fisher exact test. Missing data were imputed via the replacement with the mean (or median) of similar items. The distribution of primary and secondary clinical outcomes in each group are described as number and percentages within the group. Patients lost to follow-up were excluded from the analysis. The log-rank test was used to analyze the prognostic differences and event-free survival rates of patients in the different QFR groups, and the temporal survival of MACEs distributions for the patients in different QFR groups was visualized by plotting the Kaplan-Meier curves. Univariate Cox regression was used to compare MACEs incidence and to obtain the relative hazard ratios (HRs) between the groups.
With MACEs as the endpoint event, univariate Cox regression analysis was used to obtain baseline clinical factors that significantly differed among the patients in various groups, including QFR ≤0.8, emergency surgery, history of chronic heart failure (CHF) history of diabetes, estimated glomerular filtration rate (eGFR), dialysis status, body mass index (BMI), hemoglobin, albumin, diuretics, angiotensin receptor-neurolysin inhibitor (ARNi), three-vessel disease, and number of vessels with QFR ≤0.8. Multivariate Cox regression analysis was used to obtain independent risk factors. HRs and 95% confidence intervals (CIs) were recorded. After the interaction between the factors was excluded via subgroup analysis, a final model (model 1) was constructed from the independent risk factors.
Model 1 was combined with the RCRI score and AUB-HAS2 score to evaluate the optimization in performance from the newly acquired independent risk factors for the existing risk indices. RCRI score has been extensively used to predict PMCEs, while the AUB-HAS2 score has been used to predict myocardial infarction, death, and stroke. We calculated the area under the curve (AUC), net reclassification improvement (NRI), and integrated discrimination improvement (IDI). In addition, to evaluate the reclassification of QFR in the original indices, ischemic heart disease was replaced by QFR as a parameter in RCRI while QFR was added to the AUB-HAS2 index. The receiver operating characteristic (ROC) curve was plotted, and the statistical differences in AUC were determined. Statistical significance was defined as a two-tailed P value <0.05. R v. 4.3.0 (The R Foundation for Statistical Computing, Vienna, Austria) and SPSS v. 25.0 (IBM Corp., Armonk, NY, USA) were used for all statistical analyses.
Results
Baseline characteristics
During the study period, 819 of 1,748 patients who underwent coronary angiography within 1 year before NCS were excluded (Figure 2), among whom 683 were excluded due to their degree of stenosis not being within the scope of QFR (preoperative coronary angiography indicating all stenoses <50% or at least one stenosis >90%) and 136 were excluded for other reasons such as autoimmune diseases, history of cardiac pacemaker implantation, history of heart bypass surgery, history of cardiopulmonary resuscitation, and tumor distant metastasis.
The median age of the remaining 929 patients was 68 years (IQR 62–74 years), and 72.0% were male. Notably, 565 (60.8%) of these patients had previously undergone percutaneous coronary intervention (PCI), 287 (30.9%) had a history of acute coronary syndrome (ACS), and 606 (65.2%) underwent surgery under general anesthesia at higher frequency than that observed in in the high-QFR group. Table 1 lists the demographic, premedication, preoperative laboratory, preoperative coronary angiography, and NCS data. It is worth noting that patients in the high-QFR group were younger and had significantly higher BMI values, but the prevalence of diabetes in this group was significantly lower than that of the other two groups. Compared with that of patients in the high-QFR group, the proportion of patients with three-vessel disease in the low-QFR group and gray interval-QFR group was significantly higher. Additionally, the following factors were significantly different between the three groups: a history of smoking; dialysis status; levels of hemoglobin, albumin, brain natriuretic peptide (BNP), high-sensitivity cardiac troponin T (hs-TNT), and D-dimer; eGFR; and the use of diuretics and β-blocker medication.
Table 1
| Variables | QFR <0.75 (n=122) | 0.75≤ QFR ≤0.8 (n=110) | QFR >0.8 (n =697) | P value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, years | 70 (63, 75) | 69.5 (64, 76) | 68 (62, 74) | 0.045 |
| Male sex | 86 (70.5) | 86 (78.2) | 497 (71.3) | 0.303 |
| Body mass index, kg/m2 | 23.75±3.33 | 23.45±3.38 | 24.36±3.07 | 0.005 |
| Cancer | 37 (30.3) | 33 (30.0) | 190 (27.3) | 0.692 |
| Smoking history | 39 (32.0) | 56 (50.9) | 297 (42.6) | 0.013 |
| History of hypertension | 97 (79.5) | 89 (80.9) | 516 (74.0) | 0.164 |
| History of CHF | 17 (13.9) | 15 (13.6) | 66 (9.5) | 0.178 |
| History of atrial fibrillation | 7 (5.7) | 7 (6.4) | 48 (6.9) | 0.887 |
| History of stroke | 29 (23.8) | 22 (20.0) | 111 (15.9) | 0.082 |
| History of diabetes | 69 (56.6) | 58 (52.7) | 251 (36.0) | <0.001 |
| Oral hypoglycemic agent | 51 (41.8) | 38 (34.5) | 193 (27.7) | 0.004 |
| Insulin | 26 (21.3) | 25 (22.7) | 74 (10.6) | <0.001 |
| History of COPD | 12 (9.8) | 13 (11.8) | 73 (10.5) | 0.879 |
| Dialysis status | 4 (3.3) | 8 (7.3) | 17 (2.4) | 0.033 |
| Laboratory parameter | ||||
| Hemoglobin, g/L | 121 (110, 134.75) | 125.5 (114.25, 136.75) | 130 (117, 141) | < 0.001 |
| Albumin, g/L | 38.35 (35.02, 41.88) | 38.7 (35.7, 41.08) | 39.4 (36.6, 42.3) | 0.002 |
| D-dimer, mg/L | 0.65 (0.36, 1.09) | 0.56 (0.32, 1.16) | 0.48 (0.28, 0.88) | 0.004 |
| Myoglobin, g/L | 40.15 (31, 60) | 40.15 (32.55, 62.47) | 40.15 (29, 50.7) | 0.122 |
| hs-TNT, ng/L | 6.8 (0.02, 24.95) | 3.08 (0.01, 17.45) | 2.59 (0.01, 9.9) | < 0.001 |
| BNP, ng/L | 85.25 (51.83, 220.99) | 73.33 (45.44, 157.75) | 60 (38, 106.3) | < 0.001 |
| eGFR <60 mL/min/1.73 m2 | 80 (65.6) | 81 (73.6) | 558 (80.1) | 0.001 |
| CHD-related factors | ||||
| Three-vessel disease | 87 (71.3) | 58 (52.7) | 181 (26.0) | <0.001 |
| Minimum QFR before noncardiac surgery | 0.62 (0.52, 0.69) | 0.78 (0.76, 0.79) | 0.91 (0.87, 0.95) | < 0.001 |
| History of ACS before noncardiac surgery | ||||
| MI within 90 days | 19 (15.6) | 14 (12.7) | 52 (7.5) | 0.006 |
| OMI | 18 (14.8) | 14 (12.7) | 93 (13.3) | 0.889 |
| Unstable angina | 13 (10.7) | 13 (11.8) | 51 (7.3) | 0.168 |
| Previous PCI | ||||
| PCI beyond 3 months | 53 (43.4) | 41 (37.3) | 308 (44.2) | 0.396 |
| PTCA within 3 months | 11 (9.0) | 6 (5.5) | 29 (4.2) | 0.072 |
| Stenting within 3 months | 19 (15.6) | 18 (16.4) | 80 (11.5) | 0.203 |
| Medication | ||||
| Antiplatelets | 118 (96.7) | 106 (96.4) | 651 (93.4) | 0.205 |
| ACEI/ARBs | 67 (54.9) | 63 (57.3) | 351 (50.4) | 0.305 |
| ARNis | 8 (6.6) | 3 (2.7) | 22 (3.2) | 0.167 |
| Diuretics | 32 (26.3) | 17 (15.5) | 102 (14.6) | 0.006 |
| Beta blockers | 74 (60.7) | 48 (43.6) | 350 (50.2) | 0.029 |
| Statins | 120 (98.4) | 110 (100.0) | 689 (98.9) | 0.482 |
| Noncardiac surgery-related factors | ||||
| Emergency surgery | 5 (4.1) | 3 (2.7) | 14 (2.0) | 0.304 |
| Surgical risk | 0.646 | |||
| Low surgical risk | 58 (47.5) | 53 (48.2) | 358 (51.4) | |
| Intermediate surgical risk | 42 (34.4) | 35 (31.8) | 235 (33.7) | |
| High surgical risk | 22 (18.0) | 22 (20.0) | 104 (14.9) | |
| Anesthesia | 62 (50.8) | 71 (64.5) | 473 (67.9) | 0.001 |
| RCRI | 0.001 | |||
| 0–1 | 40 (32.8) | 40 (36.4) | 323 (46.3) | |
| 2 | 53 (43.4) | 47 (42.7) | 287 (41.2) | |
| ≥3 | 29 (23.8) | 23 (20.9) | 87 (21.5) | |
| AUB-HAS2 | 0.001 | |||
| 1 | 10 (8.2) | 10 (9.1) | 78 (11.2) | |
| 2 | 34 (28.0) | 44 (40.0) | 337 (48.3) | |
| 3 | 54 (44.4) | 35 (31.8) | 202 (29.0) | |
| >3 | 24 (19.7) | 21 (19.1) | 80 (11.5) |
Data are represented as median (interquartile range), mean ± standard deviation or number (%). QFR, quantitative flow ratio; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; hs-TNT, high-sensitivity cardiac troponin T; BNP, brain natriuretic peptide; eGFR, estimated glomerular filtration rate; CHD, coronary heart disease; ACS, acute coronary syndromes; MI, myocardial infarction; OMI, old myocardial infarction; PCI, percutaneous intervention; PTCA, percutaneous transluminal coronary angioplasty; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; ARNi, angiotensin receptor-neurolysin inhibitor; RCRI, Revised Cardiac Risk Index; AUB-HAS2, The American University of Beirut HAS2 Cardiovascular Risk Index.
Clinical outcomes in different groups stratified by QFR
The clinical outcomes for the three groups are shown in Table 2. During the 30-day follow-up following NCS, 67 (7.2%) of patients experienced MACEs. Kaplan-Meier survival curve tested by log-rank showed that there were significant differences in event incidence among the three groups. The incidence of QFR <0.8, 0.75≤ QFR ≤0.8 and QFR >0.8 were 32 (26.2%), 24 (21.8%) and 11 (1.6%), respectively (log-rank test P<0.001; Figure 3). Among the secondary outcomes, cardiovascular death, nonfatal myocardial infarction, congestive heart failure, nonfatal stroke, and PCI were significantly different, with the two groups with a lower QFR having higher incidences. Conversely, there was no significant difference in cardiopulmonary arrest, MVA, or bleeding between the three groups (Table 2).
Table 2
| Study outcome | QFR <0.75 (n=122) | 0.75≤ QFR ≤0.8 (n=110) | QFR >0.8 (n=697) | P value |
|---|---|---|---|---|
| Primary endpoint | ||||
| 30-day MACEsa | 32 (26.2) | 24 (21.8) | 11 (1.6) | <0.001 |
| Secondary endpoint (30 days) | ||||
| Cardiovascular death | 1 (0.8) | 5 (4.5) | 1 (0.1) | 0.004 |
| Cardiopulmonary arrest | 1 (0.8) | 2 (1.8) | 1 (0.1) | 0.116 |
| MVA | 1 (0.8) | 1 (0.9) | 1 (0.1) | 0.246 |
| Nonfatal myocardial infarction | 4 (3.3) | 3 (2.7) | 3 (0.4) | 0.014 |
| Congestive heart failure | 12 (9.8) | 9 (8.2) | 1 (0.1) | <0.001 |
| Nonfatal stroke | 10 (8.2) | 4 (3.6) | 5 (0.7) | <0.001 |
| Revascularization PCI | 6 (4.9) | 5 (4.5) | 0 (0.0) | – |
| Bleeding | 4 (3.3) | 5 (4.5) | 11 (1.6) | 0.109 |
| PMCEsb | 15 (12.3) | 14 (12.7) | 5 (0.7) | <0.001 |
| Death, myocardial infarction, or strokec | 14 (11.5) | 12 (10.9) | 9 (1.3) | <0.001 |
Data are presented as number (%). a, MACEs include cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, cardiopulmonary arrest, MVA, congestive heart failure, and revascularization; b, PMCEs include myocardial infarction, pulmonary edema, and cardiac death; c, the composite event of death, myocardial infarction, or stroke at 30 days after surgery was the original endpoint event of The American University of Beirut HAS2 Cardiovascular Risk Index. QFR, quantitative flow ratio; MACEs, major adverse cardiovascular events; MVA, malignant ventricular arrhythmia; PCI, percutaneous intervention; PMCE, perioperative major cardiac event.

To clarify differences in clinical outcomes among the different groups, we performed pairwise comparisons between them. Notably, there was no significant difference in the incidence of either the primary outcomes (log-rank P=0.325) or the secondary outcomes (Table 3) between the low-QFR group and the gray interval-QFR group. It was thus assumed that patients with QFR in the gray interval (0.75≤ QFR ≤0.8) had no difference in the risk of adverse cardiovascular events within 30 days after NCS as compared to those with QFR<0.75. However, when compared separately with the high-QFR group, the incidence was significantly higher in the group with a lower QFR value (QFR <0.75 vs. QFR >0.8: HR =20.70, P<0.001; QFR 0.75–0.8 vs. QFR >0.8: HR =15.99; P<0.001). The specific comparisons are shown in Table 3. The low-QFR group (QFR <0.75) and the gray interval-QFR group (0.75≤ QFR ≤0.8) were combined to form the QFR ≤0.8 group for the subsequent statistical analyses.
Table 3
| Study outcomes | QFR <0.75 vs. 0.75≤ QFR ≤0.8 | QFR <0.75 vs. QFR >0.8 | 0.75≤ QFR ≤0.8 vs. QFR >0.8 | |||||
|---|---|---|---|---|---|---|---|---|
| P value | HR | P value | HR | P value | HR | |||
| Primary endpoint | ||||||||
| 30-day MACEsa | 0.325 | – | <0.001 | 20.70 | <0.001 | 15.99 | ||
| Secondary endpoint (30 days) | ||||||||
| Cardiovascular death | 0.116 | – | 0.217 | 5.74 | 0.002 | 32.09 | ||
| Nonfatal myocardial infarction | 0.813 | – | 0.006 | 8.24 | 0.022 | 6.53 | ||
| Congestive heart failure | 0.650 | – | <0.001 | 71.25 | <0.001 | 59.12 | ||
| Nonfatal stroke | 0.166 | – | <0.001 | 11.71 | 0.015 | 5.14 | ||
| Revascularization PCI | 0.865 | – | – | – | – | – | ||
| PMCEsb | 0.956 | – | <0.001 | 18.24 | <0.001 | 18.86 | ||
| Death, myocardial infarction, or strokec | 0.918 | – | <0.001 | 9.23 | <0.001 | 8.83 | ||
a, MACEs include cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, cardiopulmonary arrest, MVA, congestive heart failure, and revascularization; b, PMCEs include myocardial infarction, pulmonary edema, and cardiac death; c, the composite event of death, myocardial infarction, and stroke at 30 days after surgery was the original endpoint event of The American University of Beirut HAS2 Cardiovascular Risk Index. HR, hazard ratio; QFR, quantitative flow ratio; MACEs, major adverse cardiovascular events; PCI, percutaneous intervention; PMCE, perioperative major cardiac event; MVA, malignant ventricular arrhythmia.
Subgroup analysis
Subgroup analyses were performed using Cox regression for the following variables: age, risk of surgery, a history of CHF, a history of diabetes, eGFR, the use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), diuretics, type of anesthesia, and whether patients had received stenting within the previous 3 months. The results of analyses revealed that no variable exhibited a significant interaction with QFR ≤0.8 (Figure 4); however, the subgroups showed significant differences in the baseline data for some factors.

Multivariate analysis of clinical outcomes of enrolled patients and construction of a new prediction model
Cox regression analysis was used to identify the independent predictors for MACEs 30 days after NCS. In the comparison of the risk between the low-QFR group and the gray interval-QFR group (as previously combined), no statistically significant difference was observed. The factors obtained from univariate Cox regression were included in the multivariate Cox regression (Table 4). Remarkably, QFR ≤0.8 emerged as an independent predictor significantly associated with the occurrence of MACEs (HR: 15.92, 95% CI: 5.96–42.51; P<0.001). Additionally, multivariate Cox regression analysis showed that a decrease in albumin (HR= 0.92, 95% CI: 0.87–0.98; P=0.008) and emergency surgery (HR= 4.12, 95% CI: 1.66–10.23; P=0.002) were independently associated with the risk of MACEs. A new prediction model (model 1) that included three independent risk factors (QFR ≤0.8, albumin, and emergency surgery) was constructed through multivariate Cox regression.
Table 4
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| QFR group | |||||
| >0.8 | Ref. | – | Ref. | – | |
| ≤0.8 | 18.50 (9.43–36.30) | <0.001 | 15.92 (5.96–42.51) | <0.001 | |
| Emergency surgery | 6.01 (2.74–13.16) | <0.001 | 4.12 (1.66–10.23) | 0.002 | |
| History of CHF | 2.95 (1.68–5.18) | <0.001 | 1.36 (0.68–2.68) | 0.383 | |
| History of diabetes | 2.10 (1.28–3.44) | 0.003 | 1.19 (0.70–2.03) | 0.529 | |
| eGFR, mL/min/1.73 m2 | 0.005 | ||||
| ≥60 | Ref. | – | Ref. | – | |
| <60 | 0.48 (0.29–0.80) | 0.005 | 1.05 (0.57–1.92) | 0.879 | |
| Dialysis status | 3.51 (1.52–8.14) | 0.003 | 1.65 (0.61–4.45) | 0.321 | |
| Body mass index, kg/m2 | 0.88 (0.82–0.96) | 0.002 | 0.98 (0.90–1.05) | 0.531 | |
| Hemoglobin, g/L | 0.98 (0.97–0.99) | <0.001 | 1.00 (0.99–1.02) | 0.765 | |
| Albumin, g/L | 0.88 (0.85–0.92) | <0.001 | 0.92 (0.87–0.98) | 0.008 | |
| Diuretics | 1.88 (1.08–3.27) | 0.025 | 1.36 (0.75–2.45) | 0.309 | |
| ARNi | 2.93 (1.26–6.78) | 0.012 | 1.96 (0.81–4.74) | 0.137 | |
| Three-vessel disease | 2.37 (1.45–3.86) | <0.001 | 0.83 (0.48–1.41) | 0.479 | |
| Number of vessels with QFR ≤0.8 | 3.09 (2.40–2.92) | <0.001 | 1.03 (0.56–1.88) | 0.932 | |
MACEs, major adverse cardiovascular events; HR, hazard ratio; CI, confidence interval; QFR, quantitative flow ratio; Ref., reference; CHF, chronic heart failure; eGFR, estimated glomerular filtration rate; ARNi, angiotensin receptor-neurolysin inhibitor.
Additive value of QFR and model 1 for the predictive value of RCRI and AUB-HAS2
The prognostic value of combining model 1 with RCRI score or AUB-HAS2 score to predict 30-day adverse cardiovascular events after NCS in patients with CAD is shown in Table 5. When MACEs were used as the end event, compared with the original RCRI scores, the addition of model 1 significantly improved reclassification in terms of NRI (0.201; 95% CI: 0.157–0.244; P<0.001), IDI (0.266; 95% CI: 0.083–0.450; P<0.001), and AUC (0.884; 95% CI: 0.848–0.920; P<0.001), with similar findings being observed for AUB-HAS2 scores (Table 5 for details). However, when the primary events were PMCEs, the addition of model 1 to the RCRI scores significantly improved NRI by 0.137 (P<0.001) and IDI by 30.3% (P<0.001). When the end events were the original endpoint event of AUB-HAS2, reclassification with the addition of model 1 to the AUB-HAS2 scores significantly improved the NRI to 0.098 (P<0.001) and the IDI by 25.9% (P<0.001).
Table 5
| Event and model | AUC | IDI | NRI | |||||
|---|---|---|---|---|---|---|---|---|
| Index (95% CI) | P value | Index (95% CI) | P value | Index (95% CI) | P value | |||
| 30-day MACEs | ||||||||
| RCRI | 0.574 (0.504–0.643) | Ref. | – | Ref. | – | Ref. | ||
| RCRI + model 1a | 0.884 (0.848–0.920) | <0.001 | 0.266 (0.083–0.450) | <0.001 | 0.201 (0.157–0.244) | <0.001 | ||
| AUB-HAS2 | 0.612 (0.548–0.677) | Ref. | Ref. | Ref. | ||||
| AUB-HAS2 + model 1a | 0.886 (0.852–0.921) | <0.001 | 0.300 (0.123–0.476) | <0.001 | 0.199 (0.154–0.244) | <0.001 | ||
| APMCEsb | ||||||||
| RCRI | 0.560 (0.467–0.652) | Ref. | Ref. | Ref. | ||||
| RCRI + model 1a | 0.837 (0.777–0.897) | <0.001 | 0.303 (0.120–0.487) | <0.001 | 0.137 (0.091–0.182) | <0.001 | ||
| Death, myocardial infarction, or stroke at 30 days after surgeryc | ||||||||
| AUB-HAS2 | 0.605 (0.519–0.691) | Ref. | Ref. | Ref. | ||||
| AUB-HAS2 + model 1a | 0.840 (0.782–0.897) | <0.001 | 0.259 (0.076–0.443) | <0.001 | 0.098 (0.071–0.125) | <0.001 | ||
a, model 1 includes QFR, albumin, and emergency surgery; b, PMCEs include myocardial infarction, pulmonary edema, and cardiac death; c, the composite event of death, myocardial infarction, and stroke at 30 days after surgery was the original endpoint event of the AUB-HAS2. QFR, quantitative flow ratio; RCRI, Revised Cardiac Risk Index; AUB-HAS2, The American University of Beirut HAS2 Cardiovascular Risk Index; AUC, area under curve; CI, confidence interval; IDI, integrated discrimination improvement; NRI, net reclassification improvement; MACEs, major adverse cardiovascular events; Ref., reference; PMCE, perioperative major cardiac event.
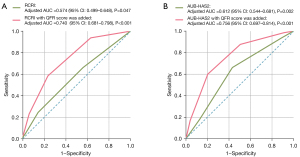
To optimize the prediction efficacy based on the simplified RCRI score and AUB-HAS2 score, the risk factor of ischemic heart disease in the RCRI score was replaced by QFR ≤0.8 to form a new score. The analysis showed that the AUC of the new score was 0.166 higher than the original one (adjusted AUC =0.740; 95% CI: 0.681–0.798; P<0.001; Figure 5A). In addition, QFR ≤0.8 was added to the original AUB-HAS2 score as a parameter, yielding an AUC 0.144 higher than that of the original score (adjusted AUC =0.756; 95% CI: 0.697–0.814; P<0.001; Figure 5B).
Discussion
This study demonstrated that in patients with CAD, the presence of diseased coronary arteries with QFR <0.8 was independently associated with MACEs in the perioperative period (within 30 days after NCS). Interestingly, the risk of MACEs in the perioperative period after NCS showed no significant difference between patients with a minimum QFR in all diseased vessels (QFRmin) in the “gray zone” (0.75≤ QFRmin ≤0.8) and patients with QFRmin <0.75. In addition, we found that incorporating independent risk factors including QFR into established post-NCS cardiovascular risk prediction models (i.e., RCRI and AUB-HAS2 score) significantly improved the predictive accuracy of these scores for adverse cardiovascular events. Replacing ischemic heart disease with QFR <0.8 as an independent risk factor in the RCRI score led to a reduction in false positives and a further optimization of risk stratification within the score. Thus, QFR can be used as an accurate and efficient indicator to better identify high-risk patients in the population of patients with CAD requiring NCS.
The main cause of perioperative cardiovascular or cerebrovascular events is myocardial ischemia, which has a multifactorial etiology and pathogenesis. For patients undergoing NCS, intraoperative sympathetic activation and increased fluid transfer can occur due to surgical trauma and proinflammatory and hypercoagulable states (24). In addition, perioperative anesthesia and analgesia may lead to hemodynamic perturbations (25). These are important triggers of perioperative myocardial ischemia in the operation process. CAD increases the risk of perioperative myocardial ischemia via two primary mechanisms: The limited and obstructed flow caused by stenosis and the withdrawal of anti-ischemic cardiovascular drugs (such as β-blockers) imposes an oxygen supply-demand imbalance on the myocardium (26-28). Second, susceptible atherosclerotic plaques more readily undergo acute thrombosis (16,29). Both Helwani et al. and Sheth et al. found that most cardiovascular-related adverse events are triggered by demand ischemia, with only a small proportion being due to an acute thrombotic event (30,31). There is reason to assume that the oxygen supply-demand mismatch caused by coronary stenosis in perioperative myocardial ischemia plays a more important role than does thrombosis.
As the core of cardiovascular and cerebrovascular events occurring after NCS, myocardial ischemia is a complex pathological mechanism, and coronary stenosis is the immediate cause of myocardial ischemia for patients with CAD, whose diagnosis mainly depends on radiological imaging. Noninvasive radiography has been studied extensively due to its simplicity, but some related controversies persist. Brown et al. showed that the occurrence of cardiac events after NCS is best predicted by the risk extent of the myocardium as reflected by myocardial perfusion imaging (MPI) (32). However, a study on 629 individuals showed that MPI had weak predictive ability and failed to improve upon traditional predictors in the classification of cardiac complication risk (33). Coronary computed tomographic angiography (CCTA), as a noninvasive method, is recommended as an initial evaluation for stable patients with low clinical likelihood or no previous diagnosis for CAD. Studies by Sheth et al. and Walpot et al., have demonstrated the feasibility of image analysis based on CCTA for predicting cardiovascular events after NCS while improving the risk stratification of RCRI score (34,35). However, CCTA exhibits significantly reduced diagnostic accuracy in patients with severe calcification or prior stent implantation (36). Additionally, atrial fibrillation or other causes of tachycardia require higher radiation doses to achieve optimal image clarity with CCTA, thereby increasing procedural risks for diagnosed patients (37). For these patients, the ESC guidelines recommend ICA for a more precise diagnosis (38).
ICA has rarely been reported in predicting adverse events after NCS due to cumbersome operations and trauma. However, as the gold standard for diagnosis and treatment, there is no substitute for ICA in patients with CAD (14,15). The 2022 ESC guidelines for NCS outline the indications for coronary angiography before NCS, which are similar to those of nonsurgical vascular imaging scenarios (39). The ECS guidelines are as follows: (I) for patients with chronic coronary syndrome (CCS) who exhibit typical angina refractory to medical therapy or with low exercise tolerance and whose initial clinical assessment indicates a high risk of adverse events, ICA is preferred over CCTA for diagnosis. (II) In patients with ACS, prompt ICA is recommended, especially for those with ST-segment elevation myocardial infarction (STEMI), extreme high-risk factors (hemodynamic instability or cardiogenic shock, recurrent or refractory angina posttreatment, life-threatening arrhythmias, mechanical complications of myocardial infarction, heart failure significantly associated with ACS, or periodic dynamic ST-segment or T-wave changes, especially intermittent ST-segment elevation), or those with high-risk factors [non-STEMI, Global Registry of Acute Coronary Events (GRACE) score >140, dynamic ST-segment or T-wave changes, or transient ST-segment elevation] (15,38,40,41).
Rough anatomical assessments from CCTA (coronary stenosis exceeding 70%) unduly overestimate the risk of cardiovascular events by more than fivefold, leading patients to undergo inappropriate coronary revascularization and the opportunity for the optimal NCS (42). For patients with CCTA indicating 50–90% coronary artery stenosis or multivessel disease, further assessment with intravascular physiology during ICA is required to assess the matching of severity of stenosis with hemodynamic significance (38). QFR, as a kind of FFR based on computerized three-dimensional reconstruction, can effectively reflect the hemotologic function in coronary artery lesions of moderate severity (50–90%) and impression of myocardial perfusion without drug-induced congestion or guidewire (18,43-45). van Diemen et al. found that QFR had a higher diagnostic performance than did MPI in vessel-specific significant CAD (46). In previous studies, QFR has been used to evaluate the functional relevance of coronary lesions in patients with severe aortic valve stenosis (SAS) before transcatheter aortic valve implantation (TAVI) (47). Li et al. reported a case of myocardial infarction with nonobstructive coronary arteries (MINOCAs) assessed by QFR after bronchoscopy (48). However, no previous studies have used coronary flow function indicators to predict the risk of NCS. Remarkably, our study is the first to use QFR to predict the risk of perioperative adverse cardiovascular events for NCS in patients with CAD and thus holds certain clinical significance.
Due to the considerable difference between anatomical obstruction and physiological obstruction (49), QFR, which can identify the stenosis caused by myocardial ischemia, can substantially improve the diagnostic performance of coronary angiography, especially in borderline lesions and asymptomatic lesions (19). According to a meta-analysis, the FFR-assisted strategy used in patients with stable CAD with intermediate stenosis can reduce revascularization by one-half, with fewer adverse events (50). In our study, we demonstrated that QFR can restratify the perioperative cardiovascular risk of NCS in patients with CAD with borderline disease. Interestingly, we also found that patients with lesions in the gray zone were exposed to the same perioperative risk as were patients with QFR <0.75. Previous studies have shown that for patients with CAD with borderline lesions, a QFR <0.75 consistently indicates inducible ischemia and warrants aggressive PCI (51,52). However, for patients with stenosis in the gray zone, the influence of coronary ischemia on prognosis, similarly to the treatment plan, has yet to be determined. Udelsman et al. and Halter et al. confirmed that the hypothalamic-pituitary-adrenal and renin-angiotensin axes activated during NCS-induced transient coronary thrombosis or spasm (53,54). We hypothesized that this process leads to further ischemia of the myocardium perfused by the lesions in the gray zone; however, further research is needed to confirm this. In addition, Ellis et al. found that distal inadequate collateralization is also a cause of cardiovascular events after NCS in patients with CAD (24). QFR, which has been approved for the diagnose of microcirculation dysfunction, is expected to be used for the early diagnosis of distal coronary perfusion disorders (55).
In recent years, computed tomography-derived fractional flow reserve (FFRCT) has emerged as a noninvasive index of coronary artery flow function (56). Krievins et al. used FFRCT to evaluate and inform preoperative intervention in in patients undergoing lower extremity revascularization surgery, which reduced the incidence of cardiovascular events 1 year after surgery (57). However, similar to that of conventional CCTA, the diagnostic accuracy of FFRCT decrease in cases of tachycardia and severe calcification. In a study by Tanigaki et al., QFR demonstrated higher diagnostic accuracy than did FFRCT when FFR was used as the reference standard (58). Nonetheless, this does not negate the potential of using FFRCT for preoperative assessment in NCS. With the advancements in CT technology, larger-scale clinical studies are needed to further validate the application of FFRCT.
Risk scores such as RCRI and AUB-HAS2 have played a key role in predicting perioperative cardiovascular risk in patients undergoing NCS (7,10). However, using the RCRI to predict perioperative risk has certain limitations. A recent study showed that 35% of patients with an RCRI score of 0 experienced PMCEs (59). For the original RCRI score, ischemic heart disease is defined as a history of a positive exercise test, history of myocardial infarction, chest pain secondary to myocardial ischemia, and ECG with pathological Q waves or use of nitrate therapy; however, under these criteria, asymptomatic patients with CAD and pathogenic myocardial ischemia can be easily overlooks (7). In our study, QFR was used to replace the CAD-related indicators in the original score, which optimized the risk stratification and avoided surgery in those with occult myocardial ischemia. Furthermore, Rapp-Kesek et al. found that decreased albumin was associated with an increased risk for infection after surgery (60). This finding, when considered in conjunction with our results, suggests that combining QFR with other traditional risk measures such as albumin, emergency surgery and RCRI, may be useful for predicting cardiovascular events before major NCS.
In this study, for patients with QFR ≤0.8, intervention therapy before surgical procedures was deemed necessary. Coronary artery bypass grafting, drug-eluting balloon angioplasty, and stent implantation could be opted for, with the latter being the primary recommended approach in guidelines and requiring regular postoperative dual-antiplatelet therapy. To balance perioperative bleeding and thrombotic risk to patients not at high risk of in-stent thrombosis, a delay in NCS until 1 month after stenting (3 months for patients with ACS) and a maintenance dose of aspirin (75 mg) is suggested. For other patients, a multidisciplinary decision involving cardiology, anesthesia, and surgical teams is necessary to formulate a treatment plan (39). Moreover, detailed prospective cohort studies are needed to clarify treatment strategies and outcomes for patients with QFR ≤0.8.
Limitation
Our study involved several limitations which should be addressed. First, due to the retrospective nature of the study, inconsistent time intervals between coronary angiography and NCS for each patient caused by the progression of CAD might have affected the results. Second, due to the single-center, observational study design, QFR could not be measured for all participants, and there may be some selection bias and influence of confounders. Third, the trial did not examine the effect of unstable plaque rupture and thromboembolism on the incidence of postoperative adverse events. These factors should be considered because they can also explain the occurrence of adverse events from pathophysiological mechanisms, and preoperative intravenous ultrasound examination of patients may be a productive research direction. Fourth, many patients had multivessel disease, and we did not investigate the impact of nonculprit artery disease on adverse events after NCS. We aim to further investigate this area in future research to better understand its implications. Finally, this study included patients during the coronavirus disease 2019 (COVID-19) pandemic, and thus we excluded many patients who died from viral infection, which resulted in a small sample size. Despite these limitations, this preliminary study proved the clinical utility of QFR in evaluating the preoperative risk for cardiovascular events after NCS in patients with CAD. Additional studies on long-term patient outcomes and the design of prospective randomized controlled studies to demonstrate the clinical efficacy of pre-NCS QFR-directed interventional therapy will further expand the value of QFR.
Conclusions
Our study provides compelling evidence that QFR ≤0.8 can serve as an independent predictor of perioperative (within 30 days) adverse cardiovascular outcomes in patients with CAD undergoing NCS. Furthermore, gray-zone lesions (0.75≤ QFR ≤0.8) were not statistically different from lesions with QFR <0.75 in terms of risk. The addition of QFR improved the predictive value of the RCRI score and AUB-HAS2 scores. Given the high predictive performance of QFR, it is worth validating its clinical benefit in large prospective clinical trials.
Acknowledgments
We are grateful for the assistance of the investigators of The First Affiliated Hospital of Wenzhou Medical University and for the support of participants.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-63/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-63/coif). H.Z. received grants from the National Natural Science Foundation of China (grant No. 82271620 and 80222146) and the Zhejiang Provincial Natural Science Foundation of China (grant No. LY22H0200). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee in Clinical Research of The First Affiliated Hospital of Wenzhou Medical University (No. KY-2022-006). The requirement for individual consent was waived due to the retrospective nature of the analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weiser TG, Haynes AB, Molina G, Lipsitz SR, Esquivel MM, Uribe-Leitz T, Fu R, Azad T, Chao TE, Berry WR, Gawande AA. Size and distribution of the global volume of surgery in 2012. Bull World Health Organ 2016;94:201-209F. [Crossref] [PubMed]
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative Major Adverse Cardiovascular and Cerebrovascular Events Associated With Noncardiac Surgery. JAMA Cardiol 2017;2:181-7. [Crossref] [PubMed]
- Lurati Buse GA, Schumacher P, Seeberger E, Studer W, Schuman RM, Fassl J, Kasper J, Filipovic M, Bolliger D, Seeberger MD. Randomized comparison of sevoflurane versus propofol to reduce perioperative myocardial ischemia in patients undergoing noncardiac surgery. Circulation 2012;126:2696-704. [Crossref] [PubMed]
- Gencer B, Gale CP, Aktaa S, Halvorsen S, Beska B, Abdelhamid M, Mueller C, Tutarel O, McGreavy P, Schirmer H, Geissler T, Sillesen H, Niessner A, Zacharowski K, Mehilli J, Potpara T. European Society of Cardiology quality indicators for the cardiovascular pre-operative assessment and management of patients considered for non-cardiac surgery. Developed in collaboration with the European Society of Anaesthesiology and Intensive Care. Eur Heart J Qual Care Clin Outcomes 2023;9:331-41. [PubMed]
- Sazgary L, Puelacher C, Lurati Buse G, Glarner N, Lampart A, Bolliger D, et al. Incidence of major adverse cardiac events following non-cardiac surgery. Eur Heart J Acute Cardiovasc Care 2020; Epub ahead of print. [Crossref] [PubMed]
- Smilowitz NR, Gupta N, Guo Y, Beckman JA, Bangalore S, Berger JS. Trends in cardiovascular risk factor and disease prevalence in patients undergoing non-cardiac surgery. Heart 2018;104:1180-6. [Crossref] [PubMed]
- Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KK, Ludwig LE, Pedan A, Goldman L. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100:1043-9. [Crossref] [PubMed]
- Davis C, Tait G, Carroll J, Wijeysundera DN, Beattie WS. The Revised Cardiac Risk Index in the new millennium: a single-centre prospective cohort re-evaluation of the original variables in 9,519 consecutive elective surgical patients. Can J Anaesth 2013;60:855-63. [Crossref] [PubMed]
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013;217:833-42.e1-3.
- Dakik HA, Sbaity E, Msheik A, Kaspar C, Eldirani M, Chehab O, Abou Hassan O, Mailhac A, Makki M, Tamim H. AUB-HAS2 Cardiovascular Risk Index: Performance in Surgical Subpopulations and Comparison to the Revised Cardiac Risk Index. J Am Heart Assoc 2020;9:e016228. [Crossref] [PubMed]
- Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila-Roman VG, Gerhard-Herman MD, Holly TA, Kane GC, Marine JE, Nelson MT, Spencer CC, Thompson A, Ting HH, Uretsky BF, Wijeysundera DN. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2215-45. [Crossref] [PubMed]
- Duceppe E, Parlow J, MacDonald P, Lyons K, McMullen M, Srinathan S, Graham M, Tandon V, Styles K, Bessissow A, Sessler DI, Bryson G, Devereaux PJ. Canadian Cardiovascular Society Guidelines on Perioperative Cardiac Risk Assessment and Management for Patients Who Undergo Noncardiac Surgery. Can J Cardiol 2017;33:17-32. [Crossref] [PubMed]
- Multimodality Writing Group for Stable Ischemic Heart Disease. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Card Fail 2014;20:65-90. [Crossref] [PubMed]
- Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, Maisch B, Mayosi B, Pavie A, Ristic AD, Sabaté Tenas M, Seferovic P, Swedberg K, Tomkowski WESC Scientific Document Group. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921-64. [Crossref] [PubMed]
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský PESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. [Crossref] [PubMed]
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999;8:133-9. [Crossref] [PubMed]
- Biccard BM, Rodseth RN. The pathophysiology of peri-operative myocardial infarction. Anaesthesia 2010;65:733-41. [Crossref] [PubMed]
- Tu S, Westra J, Yang J, von Birgelen C, Ferrara A, Pellicano M, Nef H, Tebaldi M, Murasato Y, Lansky A, Barbato E, van der Heijden LC, Reiber JHC, Holm NR, Wijns WFAVOR Pilot Trial Study Group. Diagnostic Accuracy of Fast Computational Approaches to Derive Fractional Flow Reserve From Diagnostic Coronary Angiography: The International Multicenter FAVOR Pilot Study. JACC Cardiovasc Interv 2016;9:2024-35. [Crossref] [PubMed]
- Xu B, Tu S, Qiao S, Qu X, Chen Y, Yang J, Guo L, Sun Z, Li Z, Tian F, Fang W, Chen J, Li W, Guan C, Holm NR, Wijns W, Hu S. Diagnostic Accuracy of Angiography-Based Quantitative Flow Ratio Measurements for Online Assessment of Coronary Stenosis. J Am Coll Cardiol 2017;70:3077-87. [Crossref] [PubMed]
- Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J, Koolen JJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996;334:1703-8. [Crossref] [PubMed]
- Legalery P, Schiele F, Seronde MF, Meneveau N, Wei H, Didier K, Blonde MC, Caulfield F, Bassand JP. One-year outcome of patients submitted to routine fractional flow reserve assessment to determine the need for angioplasty. Eur Heart J 2005;26:2623-9. [Crossref] [PubMed]
- Xu B, Tu S, Song L, Jin Z, Yu B, Fu G, et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): a multicentre, randomised, sham-controlled trial. Lancet 2021;398:2149-59. [Crossref] [PubMed]
- Guan C, Johnson NP, Zhang R, Xie L, Chu M, Zhao Y, Qiao Z, Yuan S, Sun Z, Dou K, Tu S, Song L, Qiao S, Xu B. Quantitative flow ratio as a continuous predictor of myocardial infarction. EuroIntervention 2023;19:e374-82. [Crossref] [PubMed]
- Ellis SG, Hertzer NR, Young JR, Brener S. Angiographic correlates of cardiac death and myocardial infarction complicating major nonthoracic vascular surgery. Am J Cardiol 1996;77:1126-8. [Crossref] [PubMed]
- Dawood MM, Gutpa DK, Southern J, Walia A, Atkinson JB, Eagle KA. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol 1996;57:37-44. [Crossref] [PubMed]
- Botto F, Alonso-Coello P, Chan MT, Villar JC, Xavier D, Srinathan S, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014;120:564-78. [Crossref] [PubMed]
- Devereaux PJ, Szczeklik W. Myocardial injury after non-cardiac surgery: diagnosis and management. Eur Heart J 2020;41:3083-91. [Crossref] [PubMed]
- Cao D, Chandiramani R, Capodanno D, Berger JS, Levin MA, Hawn MT, Angiolillo DJ, Mehran R. Non-cardiac surgery in patients with coronary artery disease: risk evaluation and periprocedural management. Nat Rev Cardiol 2021;18:37-57. [Crossref] [PubMed]
- Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation 2009;119:2936-44. [Crossref] [PubMed]
- Helwani MA, Amin A, Lavigne P, Rao S, Oesterreich S, Samaha E, Brown JC, Nagele P. Etiology of Acute Coronary Syndrome after Noncardiac Surgery. Anesthesiology 2018;128:1084-91. [Crossref] [PubMed]
- Sheth T, Natarajan MK, Hsieh V, Valettas N, Rokoss M, Mehta S, Jolly S, Tandon V, Bezerra H, Devereaux PJ. Incidence of thrombosis in perioperative and non-operative myocardial infarction. Br J Anaesth 2018;120:725-33. [Crossref] [PubMed]
- Brown KA, Rowen M. Extent of jeopardized viable myocardium determined by myocardial perfusion imaging best predicts perioperative cardiac events in patients undergoing noncardiac surgery. J Am Coll Cardiol 1993;21:325-30. [Crossref] [PubMed]
- Yao Y, Quirk T, French M, Dharmalingam A, Collins N. Myocardial perfusion imaging failed to improve patient risk classification compared with the revised cardiac risk index for early cardiac complications after major non-cardiac surgery. Intern Med J 2022;52:1203-14. [Crossref] [PubMed]
- Sheth T, Chan M, Butler C, Chow B, Tandon V, Nagele P, Mitha A, Mrkobrada M, Szczeklik W, Faridah Y, Biccard B, Stewart LK, Heels-Ansdell D, Devereaux PJCoronary Computed Tomographic Angiography and Vascular Events in Noncardiac Surgery Patients Cohort Evaluation Study Investigators. Prognostic capabilities of coronary computed tomographic angiography before non-cardiac surgery: prospective cohort study. BMJ 2015;350:h1907. [Crossref] [PubMed]
- Walpot J, Massalha S, Jayasinghe P, Sadaf M, Clarkin O, Godkin L, et al. Normalized Subendocardial Myocardial Attenuation on Coronary Computed Tomography Angiography Predicts Postoperative Adverse Cardiovascular Events: Coronary CTA VISION Substudy. Circ Cardiovasc Imaging 2022;15:e012654. [Crossref] [PubMed]
- Vavere AL, Arbab-Zadeh A, Rochitte CE, Dewey M, Niinuma H, Gottlieb I, Clouse ME, Bush DE, Hoe JW, de Roos A, Cox C, Lima JA, Miller JM. Coronary artery stenoses: accuracy of 64-detector row CT angiography in segments with mild, moderate, or severe calcification--a subanalysis of the CORE-64 trial. Radiology 2011;261:100-8. [Crossref] [PubMed]
- Vorre MM, Abdulla J. Diagnostic accuracy and radiation dose of CT coronary angiography in atrial fibrillation: systematic review and meta-analysis. Radiology 2013;267:376-86. [Crossref] [PubMed]
- Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407-77. [Crossref] [PubMed]
- Halvorsen S, Mehilli J, Cassese S, Hall TS, Abdelhamid M, Barbato E, et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J 2022;43:3826-924. [Crossref] [PubMed]
- Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J 2023;44:3720-826. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. [Crossref] [PubMed]
- Dowsley TF, Sheth T, Chow BJW. Complementary pre-operative risk assessment using coronary computed tomography angiography and nuclear myocardial perfusion imaging in non-cardiac surgery: A VISION-CTA sub-study. J Nucl Cardiol 2020;27:1331-7. [Crossref] [PubMed]
- De Bruyne B, Pijls NH, Paulus WJ, Vantrimpont PJ, Sys SU, Heyndrickx GR. Transstenotic coronary pressure gradient measurement in humans: in vitro and in vivo evaluation of a new pressure monitoring angioplasty guide wire. J Am Coll Cardiol 1993;22:119-26. [Crossref] [PubMed]
- Lopez-Palop R, Pinar E, Lozano I, Saura D, Picó F, Valdés M. Utility of the fractional flow reserve in the evaluation of angiographically moderate in-stent restenosis. Eur Heart J 2004;25:2040-7. [Crossref] [PubMed]
- Silber S, Albertsson P, Avilés FF, Camici PG, Colombo A, Hamm C, Jørgensen E, Marco J, Nordrehaug JE, Ruzyllo W, Urban P, Stone GW, Wijns WTask Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur Heart J 2005;26:804-47. [Crossref] [PubMed]
- van Diemen PA, de Winter RW, Schumacher SP, Everaars H, Bom MJ, Jukema RA, et al. The diagnostic performance of quantitative flow ratio and perfusion imaging in patients with prior coronary artery disease. Eur Heart J Cardiovasc Imaging 2023;25:116-26. [Crossref] [PubMed]
- Mejía-Rentería H, Nombela-Franco L, Paradis JM, Lunardi M, Lee JM, Amat-Santos IJ, Veiga Fernandez G, Kalra A, Bansal EJ, de la Torre Hernandez JM, Rodés-Cabau J, Ribichini FL, Escaned J. Collaborators. Angiography-based quantitative flow ratio versus fractional flow reserve in patients with coronary artery disease and severe aortic stenosis. EuroIntervention 2020;16:e285-92. [Crossref] [PubMed]
- Li M, Liu Y, Wang H. Diagnosis and prognosis of myocardial infarction in a patient without obstructive coronary artery disease during bronchoscopy: a case study and literature review. BMC Cardiovasc Disord 2020;20:185. [Crossref] [PubMed]
- Toth G, Hamilos M, Pyxaras S, Mangiacapra F, Nelis O, De Vroey F, Di Serafino L, Muller O, Van Mieghem C, Wyffels E, Heyndrickx GR, Bartunek J, Vanderheyden M, Barbato E, Wijns W, De Bruyne B. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J 2014;35:2831-8. [Crossref] [PubMed]
- Johnson NP, Tóth GG, Lai D, Zhu H, Açar G, Agostoni P, et al. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol 2014;64:1641-54. [Crossref] [PubMed]
- Bech GJ, De Bruyne B, Bonnier HJ, Bartunek J, Wijns W, Peels K, Heyndrickx GR, Koolen JJ, Pijls NH. Long-term follow-up after deferral of percutaneous transluminal coronary angioplasty of intermediate stenosis on the basis of coronary pressure measurement. J Am Coll Cardiol 1998;31:841-7. [Crossref] [PubMed]
- Pijls NH. Is it time to measure fractional flow reserve in all patients? J Am Coll Cardiol 2003;41:1122-4. [Crossref] [PubMed]
- Halter JB, Pflug AE, Porte D Jr. Mechanism of plasma catecholamine increases during surgical stress in man. J Clin Endocrinol Metab 1977;45:936-44. [Crossref] [PubMed]
- Udelsman R, Norton JA, Jelenich SE, Goldstein DS, Linehan WM, Loriaux DL, Chrousos GP. Responses of the hypothalamic-pituitary-adrenal and renin-angiotensin axes and the sympathetic system during controlled surgical and anesthetic stress. J Clin Endocrinol Metab 1987;64:986-94. [Crossref] [PubMed]
- Sheng X, Qiao Z, Ge H, Sun J, He J, Li Z, Ding S, Pu J. Novel application of quantitative flow ratio for predicting microvascular dysfunction after ST-segment-elevation myocardial infarction. Catheter Cardiovasc Interv 2020;95:624-32. [Crossref] [PubMed]
- Dai X, Hou Y, Tang C, Lu Z, Shen C, Zhang L, Zhang J. Long-term prognostic value of the serial changes of CT-derived fractional flow reserve and perivascular fat attenuation index. Quant Imaging Med Surg 2022;12:752-65. [Crossref] [PubMed]
- Krievins D, Zellans E, Latkovskis G, Erglis A, Zvaigzne L, Kumsars I, Rumba R, Stradins P, Jegere S, Zarins CK. Pre-operative Diagnosis of Silent Coronary Ischaemia May Reduce Post-operative Death and Myocardial Infarction and Improve Survival of Patients Undergoing Lower Extremity Surgical Revascularisation. Eur J Vasc Endovasc Surg 2020;60:411-20. [Crossref] [PubMed]
- Tanigaki T, Emori H, Kawase Y, Kubo T, Omori H, Shiono Y, Sobue Y, Shimamura K, Hirata T, Matsuo Y, Ota H, Kitabata H, Okubo M, Ino Y, Matsuo H, Akasaka T. QFR Versus FFR Derived From Computed Tomography for Functional Assessment of Coronary Artery Stenosis. JACC Cardiovasc Interv 2019;12:2050-9. [Crossref] [PubMed]
- Roshanov PS, Sessler DI, Chow CK, Garg AX, Walsh MW, Lam NN, Hildebrand AM, Biccard BM, Acedillo RR, MacNeil SD, Lee VW, Szczeklik W, Mrkobrada M, Thabane L, Devereaux PJ. Predicting Myocardial Injury and Other Cardiac Complications After Elective Noncardiac Surgery with the Revised Cardiac Risk Index: The VISION Study. Can J Cardiol 2021;37:1215-24. [Crossref] [PubMed]
- Rapp-Kesek D, Ståhle E, Karlsson TT. Body mass index and albumin in the preoperative evaluation of cardiac surgery patients. Clin Nutr 2004;23:1398-404. [Crossref] [PubMed]



