
Ultrasound guided procedures in the musculoskeletal system: a narrative review with illustrative examples
Introduction
Ultrasound guidance is particularly useful for diagnostic and percutaneous interventional procedures across various anatomic locations of the human body. Its application in the musculoskeletal system has grown in recent years for several reasons. This is attributed to several factors: its established efficacy as a diagnostic tool for a range of musculoskeletal conditions (1,2), its cost-effectiveness and speed compared to magnetic resonance imaging (MRI) coupled with a high level of diagnostic agreement between the two techniques (especially for soft tissue pathology in the shoulder, foot, and ankle, as well as imaging of peripheral nerves, foreign bodies, or abnormalities near hardware) (3), and its broader adoption across both physician-led and non-physician specialties (4). In fact, for at least the past decade, most ultrasound-guided interventional procedures have been performed by specialists outside the field of radiology (5).
Ultrasound-guided procedures include, for instance, tumoral biopsies, diagnostic aspiration or therapeutic drainage of abscesses, hematomas, cysts, and collections, therapeutic injections of joints, nerves, and tendons, and barbotage of calcium deposits or extraction of foreign bodies. In most cases, these interventions are considered more accurate than palpation-guided interventions based solely on external anatomic landmarks. However, this superiority is not always backed by conclusive evidence (6,7).
In this article we review the established ultrasound-guided procedures in the musculoskeletal system and the most recent advances. We complemented the review findings with illustrative images based on our experience. We present this article in accordance with the Narrative Review reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-176/rc).
Methods
We performed a literature search in PubMed, Web of Science and EMBASE using MeSH and general terms related to interventional ultrasound and musculoskeletal system {e.g., (“Ultrasonography, Interventional”[Mesh]); “Musculoskeletal System”[Mesh]; “Biopsy [Mesh]”; “Drainage[Mesh]”; “Ultrasound-guided[All Fields]”}. The search was restricted to articles published in the last 10 years and written in English. Only reviews, systematic reviews, meta-analyses, and clinical trials in humans were included. General articles related to imaging techniques different from ultrasound, or spinal, thoracoabdominal and facial procedures were excluded (Table 1). After reading titles and abstracts, a total of 59 articles were selected for the review. The references of the selected studies were carefully examined to search for additional articles that could be of interest, and 61 more studies were added. Therefore, a total of 120 articles were included in the review.
Table 1
| Items | Specification |
|---|---|
| Date of search | 20 December 2023 |
| Databases and other sources searched | PubMed, Web of Science and EMBASE |
| Search terms used | “Ultrasonography, Interventional”[MeSH]; “Musculoskeletal System”[MeSH]; “Biopsy [MeSH]”; “Drainage[MeSH]”; “Ultrasound-guided[All Fields]” |
| Timeframe | 20 December 2013 to 20 December 2023 |
| Inclusion and exclusion criteria | Inclusion criteria: only reviews, systematic reviews, meta-analyses, and clinical trials in humans published in English |
| Exclusion criteria: general articles related to imaging techniques different from ultrasound, or spinal, thoracoabdominal and facial procedures | |
| Selection process | F.R.S., A.J.L.R.B. conducted the selection |
The included topics are described below under specific sections related to the main procedures and musculoskeletal conditions that can be approached through interventional techniques.
Musculoskeletal biopsies
The accuracy of ultrasound-guided biopsies is higher than that of palpation-guided biopsies, with values over 90% (8,9), and a significantly lower rate of complications compared to incisional biopsy (1% vs. 4%) (10). Biopsy tract contamination is much lower in ultrasound-guided biopsies than in incisional biopsies (1% vs. 30%) (11), shortening the time needed to begin definitive treatment (12). To achieve this, the use of automatic or semiautomatic coaxial biopsy systems is advocated, with core samples obtained through only one introductory needle (Figure 1). At least four core samples are recommended for soft tissue biopsies to increase the yield of the procedure (13). Additionally, the length of the specimen significantly impacts the yield, particularly for lesions smaller than 1 cm, where it tends to decrease (14,15).

Diagnostic aspiration or therapeutic drainage of abscesses or fluid collections
Aspiration or drainage of accessible collections can be facilitated by ultrasound guidance. The thickness of the needle or catheter depends on the thickness of the contents. For instance, a Baker’s cyst at the knee can typically be aspirated with a thin needle (>20 G). Morel-Lavallée lesions, post-traumatic degloving injuries caused by a shearing force, resulting in the separation of the hypodermis layer from the deeper fascia, are traditionally described at the thigh, although they may be seen at other sites, such as the knee, calf, and lumbar regions. While thin needles may suffice for draining a Morel-Lavallée lesion, the use of sclerosing agents like doxycycline, ethanol, or talc may be considered to reduce recurrence following drainage (Figure 2) (16).

Larger needles (<20 G) or even a catheter may be required for hematomas. Sometimes, hematomas are coagulated, and the introduction of fibrinolytics (e.g., urokinase) for different periods may be needed to fluidify the contents, facilitating aspiration (17).
Ganglion cysts, one of the most frequent benign soft tissue masses, usually originate from joints and tendon sheaths, although they may extend intratendinously, inside or around peripheral nerves, and intraosseously. Drainage of the cyst typically requires larger needles (14–19 G) due to the gelatinous texture of the content. To mitigate the risk of recurrence, performing multiple fenestrations on different sections of the cyst wall is advisable. The viscosity of the cyst’s contents can be decreased by injecting a small quantity of anesthetic into the cyst, thereby facilitating easier drainage (Figure 3) (18). Following drainage, the instillation of a steroid-anesthetic mixture is recommended to ensure the free movement of the mixture through the walls of the ganglion cyst, preventing refilling and the restoration of its pre-fenestration shape. Should the cyst refill, additional fenestrations may be necessary (18,19).

When an infection is suspected, one of the most common goals is to obtain a sample for microbiological analysis. This may be indicated in osteomyelitis with periosteal involvement (20), septic arthritis, or soft tissue abscess. In such cases, draining the contents and cleaning the residual cavity may require inserting a catheter for drainage until resolution. The catheter must be flushed at intervals with saline to prevent obstruction. This approach is curative in most scenarios (21). However, certain infections (e.g., tuberculosis) may involve an abscess cavity with a thickened wall that impedes the collapse of the cavity, necessitating surgical debridement as a definitive treatment (Figure 4).

Therapeutics injections
Ultrasound is very useful for guiding a wide array of procedures, including both diagnostic and therapeutic injections. These injections can be precisely administered into or around tendons, within joint spaces, or into the bursae.
Bursal injections
One of the most frequently injected sites is the subdeltoid bursa to treat external impingement syndrome. It appears to be more accurate than palpation-guided injections, although evidence about clinical superiority is conflicting. While some studies find no significant difference in efficacy between the two methods (22), others suggest that ultrasound-guided injections may lead to superior improvements (23-25). Moreover, it has been observed that the use of a high volume of ultrasound-guided local anesthetic and corticosteroids results in a quicker alleviation of pain compared to a lower volume (26). A loss of resistance during the injection serves as an indicator of correct needle placement. Ultrasound guidance allows for real-time confirmation of this through the observation of bursa distension and the fluid’s dispersal from the needle tip (27) (Figure 5).

Other frequently injected bursae include the trochanteric and ischial bursae, and more rarely the iliopsoas, olecranon, and scapulothoracic bursae. Ultrasound-guided injections in the peritrochanteric area to treat trochanteric pain syndromes seem to be more effective compared to palpation-guided injections (28), although some authors consider palpation-guided injections the method of choice, reserving ultrasound for obese patients or after clinical injection failure (29). Local anesthetic and steroids should be placed between the gluteus minimus/medius tendons and the fascia lata. Opening of the space under the fascia lata is indicative of appropriate placement of the injectate.
Although frequently performed because of ischiogluteal bursitis or hamstring tendinosis, there is still limited evidence about the effectiveness of injections in the ischiogluteal bursa and/or peritendinous subgluteal area. In a previous case series, 9 out of 11 patients showed good results after injections (30).
Tendinous and peritendinous injections
Many tendons are common targets for ultrasound-guided procedures. Platelet-rich plasma (PRP) injections have been effective in alleviating pain and enhancing function in patients with partial rotator cuff tears (31) (Figure 6). Similarly, dextrose prolotherapy has shown utility in improving function in chronic rotator cuff lesions (32).

Ultrasound-guided injection of the long head of the biceps tendon is more accurate than palpation-guided injection (33). A recent study reported that dual injection of the long head of the biceps tendon and the subdeltoid bursa with local anesthetic and corticoids has extended duration of symptomatic relief than injection of the bursa alone (34).
At the elbow, lateral epicondylitis is the most usual place for injections, which can be peritendinous, intratendinous, or at the enthesis (tendon-bone interface) (35). In peritendinous injections, a minimum volume of 4 ml is recommended to effectively achieve hydro dissection of the tendon-fascia interface. Steroids or hyaluronic acid may be used for this purpose (36,37).
Intratendinous needling has also demonstrated high efficacy and superior results over corticosteroids in some reports (38), even with comparable therapeutic efficacy to open-release surgery (39). To avoid pain, we recommend injecting anesthetics proximal to the lateral epicondyle before performing the needling. Many products can be injected intratendinously, including local anesthetics (40), polidocanol (41), dextrose (42), or PRP (43). The latter has shown more beneficial effects in the long term than corticosteroids (43). It is recommended to use refrigerated centrifuges to maximize platelet concentration (44) and associate dry needling to promote angiogenesis. It is suggested to avoid local anesthetics at the site of PRP administration since they decrease platelet aggregation and release of growth factors and cytokines (45).
Finally, some authors have proposed scrapping or drilling the bone enthesis, mainly in chronic refractory tendinosis (46,47), although it is less frequently performed than the above-mentioned procedures (35).
At the wrist, de Quervain’s tenosynovitis is one of the most common conditions that can be treated by peritendinous injections. Longitudinal access with needling of the thickened tendon sheaths helps to alleviate symptoms, and corticosteroid injection alone has been associated with a high recurrence rate (48). In this regard, 15-day delayed 2 mL low molecular weight hyaluronic acid injection after the baseline corticosteroids injection reduced recurrence rate (49). A new technique, releasing acupotomy of the tendon sheath, is showing promising results. It is performed using an acupuncture needle with a flat-knife shaped tip (50) (Figure 6).
The same principle applies to trigger finger in the hand, carpal and cubital tunnels, and Dupuytren’s contracture. Tendon release by needling of the A1 pulley in trigger finger is superior to corticosteroid injections alone (51), with no differences between percutaneous release and open surgery (52). Needling the pulley with the lateral side of the needle bevel facilitates tearing the pulley for tendon release (53). Additionally, injecting corticosteroids into the tendon sheath combined with percutaneous needling surpasses the effectiveness of needling alone (54).
At the lower limb, the most frequently treated tendons are the patellar tendon (PT) at the knee, and the Achilles tendon at the ankle. Peritendinous corticosteroid injection for PT has been shown to be more effective than placebo (54). Most injections are performed at the proximal tendon/patellar insertion, and less frequently at the distal end/tibial insertion. To avoid the risk of skin atrophy, dilution of the corticosteroid with a mixture of local anesthetics and saline is recommended (55), especially if part of the injectate is placed at the superficial aspect of the tendon. Combination with dry needling may improve the efficiency of treatment with corticosteroids, PRP, or physical therapy (56,57). Other treatments used for patellar tendinopathy include prolotherapy, sclerosing injections with polidocanol, and hyaluronic acid, but no previous studies have compared their efficacy, and there is insufficient clinical evidence about the appropriateness of their use (58,59) (Figure 7).
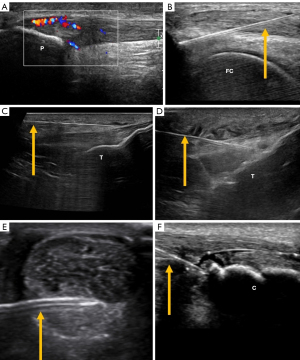
For the Achilles tendon, local anesthetics and corticosteroids are injected into the superficial and deep parts of the paratenon. In partial tears, PRP can be used. However, PRP injections and dry needling have shown similar short-term results at 3–6 months (60). Prolotherapy is another technique used to treat chronic tendinosis. This technique involves intratendinous injection of glucose to treat hypoechoic areas of tendinosis, achieving a reduction of pain in treated patients by more than 70% (61). In mid portion tendonitis, high volume corticosteroids injection and PRP in combination with eccentric exercises were found more effective in reducing pain and improving function than eccentric training alone (62) (Figure 7).
Intra-articular injections
Indications for intra-articular injections primarily include inflammatory arthritis and osteoarthritis. In the upper limb, the shoulder and wrist joints are most commonly targeted, while in the lower limb, the hip, knee, and ankle/foot joints receive the majority of injections.
Ultrasound-guided shoulder girdle injections provide more accurate needle placement control within the joint compared to landmark-guided injections (63), but, they have not demonstrated superiority in pain control and function (23). In cases of adhesive capsulitis, injections offer better short-term relief (2 weeks) compared to blind injections, although clinical differences vanish in longer follow-ups (64). Hydro dilatation, which involves the introduction of a high volume of fluid, has been successfully used to treat frozen shoulder (65). This method aims to rupture the joint capsule, thereby increasing joint capacity and mobility. However, some reports suggest that using lower volumes to achieve joint capsule distension without rupture yields better results (66). In this regard, a recent work showed that hydro dilatation with 20 ml of hyaluronic acid combined with physical therapy led to superior clinical benefit compared with physical therapy alone (67) (Figure 8).

Several joints in the wrist and hand, including the radiocarpal, distal radioulnar, metacarpophalangeal, interphalangeal, scapho-trapezium-trapezoidal, and first carpometacarpal joints, can be injected. Ultrasound guidance ensures higher accuracy in needle placement for wrist and hand joint injections compared to palpation guidance (Figure 8). For inflammatory arthritis, these injections are significantly less painful than palpation-guided methods and improve cost-effectiveness (68). Ultrasound-guided wrist injections provide superior improvement compared to palpation-guided injections (69). There are reports of more sustained improvement with hyaluronic acid (70) and PRP (71) compared to corticosteroids.
Hip injections serve as diagnostic tests or symptomatic treatments for various conditions, such as hip dysplasia, osteoarthritis, and inflammatory arthritis. Ultrasound-guided hip joint injections are more accurate than palpation-guided injections (72,73) (Figure 9). A positive response to intra-articular injection may help to predict a good outcome from hip arthroscopy for femoroacetabular impingement, although there is no universal agreement (74). It may also predict pain relief after hip surgery or replacement (75). Regarding injectates, corticosteroids are superior to hyaluronic acid in the short term, but there are no long-term differences. Similarly, no significant differences have been found between hyaluronic acid and PRP (76).

Intra-articular ultrasound-guided arthrocentesis or injection in the knee joint is more accurate than the palpation-guided technique, leading to improved fluid aspiration and therapeutic benefit (77) (Figure 9). Among the multiple approaches to the knee joint, the superomedial patellar seems to be the most accurate (78). Injections of corticosteroid and local anesthetics into the knee joint alleviate pain in the short and medium term and lead to functional improvement in inflammatory arthritis. While similar results have been observed in osteoarthritis, their effectiveness is more controversial (59).
Ultrasound-guided intra-articular injections of hyaluronic acid are safe and improve pain assessments and function in knee osteoarthritis (79), showing greater efficacy than corticosteroids in the long term (6 months) (80). It is common practice to combine both corticosteroids and hyaluronic acid in the same injection to enhance efficacy.
Regenerative drug injections (e.g., PRP) have shown clinical benefits in relieving pain and improving function in patients with knee osteoarthritis, although no clinical trials have confirmed this (59).
For ankle and foot injections, the usual targets include the tibiotalar, subtalar, midfoot, and forefoot joints. Ultrasound guidance is particularly useful for guiding injections in patients with large osteophytes and extremely narrowed joints, which may complicate palpation-guided or even fluoroscopy-guided injections (81) (Figure 9). Short-term benefits of corticosteroid therapeutic injections in the ankle and foot joints exceed 80% (82). Other injectates such as PRP and prolotherapy are useful, but evidence supporting their efficacy is limited (83). In fact, a recent work did not support the use of intraarticular PRP in patients with ankle osteoarthritis (84).
Nerve injections
Ultrasound-guided nerve blocks have recently gained popularity as an ancillary technique before interventional procedures, mostly performed by anesthesiologists. However, radiologists also apply these blocks before various imaging-guided interventional procedures, such as biopsies or thermal ablation. In the lower limb, the most commonly blocked nerves are the femoral, internal saphenous, sciatic, peroneal, and posterior tibial. In the upper limb, blocks can be performed on the brachial plexus, median, radial, or ulnar nerves (85) (Figure 10).
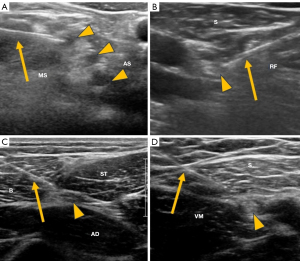
The main therapeutic targets of ultrasound-guided perineural injections in the upper limb include the suprascapular nerve for shoulder pain and the median nerve for carpal tunnel syndrome, showing superior benefits compared to palpation-guided injections (86). For chronic wrist pain, blocking the anterior and posterior interosseous nerves at the wrist can predict outcomes for surgical intervention (87) or ablative denervation of the wrist (88) (Figure 11).

For carpal tunnel syndrome, ultrasound-guided injections provide more favorable results for symptom severity and functional status in the short term compared to palpation-guided injections. However, there is no evidence regarding their effectiveness in the mid and long term (89). Hydro dissection with normal saline (90) or 5% dextrose (91) has proven to be as effective as injections with low or high doses of corticosteroids. Some studies found that carpal tunnel percutaneous release combined with corticosteroids injection achieves better outcomes than corticosteroids injection alone or carpal tunnel release alone (92-94).
Some of the main therapeutic targets of ultrasound-guided perineural injections in the lower limb include treating the lateral femoral cutaneous nerve in meralgia paresthetica and the Baxter nerve at the ankle in entrapment syndromes (95) (Figure 12). Corticosteroid injections around the lateral femoral cutaneous nerve provide relief in over 75% of cases (96).

For foot conditions, the posterior tibial nerve, and the plantar digital nerves are common targets in cases of tarsal tunnel syndrome, and Morton’s neuroma/bursitis (Figure 12). Ultrasound-guided injections for Morton’s neuroma showed higher success rates (69–89%) compared to injections based on anatomical landmarks (48–59%), in both short and long-term follow-ups (97,98).
Barbotage of calcific tendonitis
Hydroxyapatite deposits are most frequently treated percutaneously in the rotator cuff tendons, although they can also occur (and be treated) in the elbow and several other tendons (99). Typically, a thick needle (less than 20 G) is utilized to inject saline into non-fragmented calcifications with an intact peripheral rim. Upon relieving the pressure, the fluid refluxes into the syringe, clouded with calcium material (100). This procedure might require several syringes to completely wash out the calcification. In the resorptive phase, the calcium material may be soft enough to be better aspirated or washed through two needles communicating within the calcification (101), although some authors prefer the two needles technique for harder calcifications (102) (Figure 13). A systematic review showed that no existing evidence favours using a specific size or number of needles (103). About 90% of patients reported being symptom-free or experiencing significant improvement (104). In addition, corticosteroids injection in the bursa after barbotage improved pain in the 6 weeks following the procedure, and function in the 3 months after, but with no significant effect on calcification resorption (105). Barbotage also provides superior benefit than high-energy extracorporeal shock wave therapy in eliminating calcium deposits (106), greater reduction of pain (107), with less treatment sessions (106,107).

Foreign bodies withdrawal
Percutaneous ultrasound-guided foreign body removal is a minimally invasive, economical technique with a low risk of complications compared with surgical removal. Foreign bodies include splinters of wood, glass, plastic, or metal (108). Additionally, medical implants like contraceptive implants, Implanon, or Nexplanon rods may require ultrasound-guided removal in difficult cases, using a grasping micro forceps (109) (Figure 14).

Other indications of ultrasound-guided procedures in musculoskeletal conditions
Ultrasound offers the significant advantage of not using ionizing radiation for arthrography, unlike fluoroscopy (110). Most joints are easily accessible under ultrasound guidance, the most common being the shoulder, hip, knee, and wrist.
Radiofrequency ablation for neuropathic pain can also be performed safely under ultrasound guidance. This procedure is often applied in the upper limb, targeting the suprascapular nerve for shoulder pain (111). In the lower limb, it is used on the lateral femoral cutaneous nerve for meralgia paresthetica (112), the obturator nerve for hip pain (113), the geniculate nerves for knee pain (114), and for treating Morton’s neuroma (115). Nevertheless, its application to other nerves and pathologies is increasing. For instance, it is being increasingly used to treat muscle spasticity in both the upper and lower limbs (116) (Figure 15).

Pain relief lasts longer with continuous RF compared to pulsed RF, although the pain response generally diminishes around 6–9 months after the procedure. In addition, Pulsed RF is considered safer for treating mixed sensory and motor peripheral nerves (117).
Discussion
The use of ultrasound-guided interventional procedures has seen significant growth over the last decade, driven by advancements in the anatomical detailing capabilities of current ultrasound equipment and the real-time visibility of needles. These improvements facilitate precise injections or aspirations, ensuring that procedures target the correct anatomical sites. Consequently, ultrasound has become an indispensable tool across various medical specialties, as evidenced by numerous studies and reports (1-7,118).
Ultrasound-guided procedures outperform the landmark or palpation-guided procedures in most scenarios (22,23,25,33,62,71,77,97,98), despite some procedures are still accurately performed without ultrasound, being less expensive (22,29,119).
This narrative review encompasses 28 clinical trials and 9 meta-analyses, thereby reinforcing the reported data. However, data synthesis presents challenges due to the diversity of procedures, needle approaches, techniques, and dosages employed. Despite these limitations, this work provides a comprehensive overview of the vast potential of interventional musculoskeletal ultrasound (77,119,120).
Future research in interventional ultrasound should prioritize the standardization of techniques, procedures, drugs, and biological products to facilitate comparability of results.
Conclusions
Advances in ultrasound-guided procedures in the musculoskeletal system have enabled radiologists and other specialists to achieve high accuracy in diagnosing and treating various musculoskeletal conditions, including tumors, inflammatory and degenerative arthritis, tendinopathies, and bursal pathologies. This review addressed consolidated techniques and recent advances in interventional musculoskeletal ultrasound.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: With the arrangement by the Guest Editors and the editorial office, this article has been reviewed by external peers.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at: https://qims.amegroups.com/article/view/10.21037/qims-24-176/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-176/coif). The special issue “Advances in Diagnostic Musculoskeletal Imaging and Image-guided Therapy” was commissioned by the editorial office without any funding or sponsorship. F.R.S. served as the unpaid Guest Editor of the issue and serves as an unpaid editorial board member of Quantitative Imaging in Medicine and Surgery. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Finnoff JT. The Evolution of Diagnostic and Interventional Ultrasound in Sports Medicine. PM R 2016;8:S133-8. [Crossref] [PubMed]
- Finnoff JT, Hall MM, Adams E, Berkoff D, Concoff AL, Dexter W, Smith J. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. PM R 2015;7:151-68.e12. [Crossref] [PubMed]
- He L, Delzell P, Schils J. Comparison of MRI Findings After Musculoskeletal Ultrasound: An Opportunity to Reduce Redundant Imaging. J Am Coll Radiol 2018;15:1116-9. [Crossref] [PubMed]
- Kanesa-Thasan RM, Nazarian LN, Parker L, Rao VM, Levin DC. Comparative Trends in Utilization of MRI and Ultrasound to Evaluate Nonspine Joint Disease 2003 to 2015. J Am Coll Radiol 2018;15:402-7. [Crossref] [PubMed]
- Sharpe RE Jr, Nazarian LN, Levin DC, Parker L, Rao VM. The increasing role of nonradiologists in performing ultrasound-guided invasive procedures. J Am Coll Radiol 2013;10:859-63. [Crossref] [PubMed]
- Kane D, Koski J. Musculoskeletal interventional procedures: With or without imaging guidance? Best Pract Res Clin Rheumatol 2016;30:736-50. [Crossref] [PubMed]
- Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed 2011;39:121-31. [Crossref] [PubMed]
- Tan A, Rajakulasingam R, Saifuddin A. Diagnostic concordance between ultrasound-guided core needle biopsy and surgical resection specimens for histological grading of extremity and trunk soft tissue sarcoma. Skeletal Radiol 2021;50:43-50. [Crossref] [PubMed]
- Metz T, Heider A, Vellody R, Jarboe MD, Gemmete JJ, Grove JJ, Smith EA, Mody R, Newman EA, Dillman JR. Image-guided percutaneous core needle biopsy of soft-tissue masses in the pediatric population. Pediatr Radiol 2016;46:1173-8. [Crossref] [PubMed]
- Birgin E, Yang C, Hetjens S, Reissfelder C, Hohenberger P, Rahbari NN. Core needle biopsy versus incisional biopsy for differentiation of soft-tissue sarcomas: A systematic review and meta-analysis. Cancer 2020;126:1917-28. [Crossref] [PubMed]
- Barrientos-Ruiz I, Ortiz-Cruz EJ, Serrano-Montilla J, Bernabeu-Taboada D, Pozo-Kreilinger JJ. Are Biopsy Tracts a Concern for Seeding and Local Recurrence in Sarcomas? Clin Orthop Relat Res 2017;475:511-8. [Crossref] [PubMed]
- Kiefer J, Mutschler M, Kurz P, Stark GB, Bannasch H, Simunovic F. Accuracy of core needle biopsy for histologic diagnosis of soft tissue sarcoma. Sci Rep 2022;12:1886. [Crossref] [PubMed]
- Wu JS, Goldsmith JD, Horwich PJ, Shetty SK, Hochman MG. Bone and soft-tissue lesions: what factors affect diagnostic yield of image-guided core-needle biopsy? Radiology 2008;248:962-70. [Crossref] [PubMed]
- Kim SY, Chung HW. Small Musculoskeletal Soft-Tissue Lesions: US-guided Core Needle Biopsy--Comparative Study of Diagnostic Yields according to Lesion Size. Radiology 2016;278:156-63. [Crossref] [PubMed]
- Wong H, Tarr GP, Anand R, Atkinson N, Flint M, Clarke A, Symmans P, Doyle A. Diagnostic yield and concordance of image-guided biopsy in musculoskeletal lesions. Skeletal Radiol 2024;53:75-84. [Crossref] [PubMed]
- Singh R, Rymer B, Youssef B, Lim J. The Morel-Lavallée lesion and its management: A review of the literature. J Orthop 2018;15:917-21. [Crossref] [PubMed]
- Del Cura JL, Zabala R, Corta I. Ultrasound-guided interventional procedures in the musculoskeletal system. Radiologia 2010;52:525-33. [Crossref] [PubMed]
- Schallert EK, Cano MC, Ditzler MG, Jadhav SP, Jose J, Kan JH. Percutaneous ultrasound-guided ganglion fenestration in children: initial results. Skeletal Radiol 2021;50:1169-75. [Crossref] [PubMed]
- Zeidenberg J, Aronowitz JG, Landy DC, Owens PW, Jose J. Ultrasound-guided aspiration of wrist ganglions: a follow-up survey of patient satisfaction and outcomes. Acta Radiol 2016;57:481-6. [Crossref] [PubMed]
- Paliwal AK, Sahdev R, Deshwal A, Ram B. Role of ultrasound in the diagnosis of paediatric acute osteomyelitis. J Ultrason 2021;21:34-40. [Crossref] [PubMed]
- Yacoub WN, Sohn HJ, Chan S, Petrosyan M, Vermaire HM, Kelso RL, Towfigh S, Mason RJ. Psoas abscess rarely requires surgical intervention. Am J Surg 2008;196:223-7. [Crossref] [PubMed]
- Fan D, Liu X, Ma J, Zhang S, Sun J, Li Y, Jiang B, Zhang L. Ultrasound Guidance Is Not Superior in Subacromial Bursa and Intraarticular Injections but Superior in Bicipital Groove: A Meta-analysis of Randomized Controlled Trials. Arthroscopy 2022;38:1642-57. [Crossref] [PubMed]
- Aly AR, Rajasekaran S, Ashworth N. Ultrasound-guided shoulder girdle injections are more accurate and more effective than landmark-guided injections: a systematic review and meta-analysis. Br J Sports Med 2015;49:1042-9. [Crossref] [PubMed]
- Wu T, Song HX, Dong Y, Li JH. Ultrasound-guided versus blind subacromial-subdeltoid bursa injection in adults with shoulder pain: A systematic review and meta-analysis. Semin Arthritis Rheum 2015;45:374-8. [Crossref] [PubMed]
- Azadvari M, Emami-Razavi SZ, Torfi F, Nazar NSB, Malekirad AA. Ultrasound-guided versus blind subacromial bursa corticosteroid injection for paraplegic spinal cord injury patients with rotator cuff tendinopathy: a randomized, single-blind clinical trial. Int J Neurosci 2021;131:445-52. [Crossref] [PubMed]
- Klontzas ME, Vassalou EE, Zibis AH, Karantanas AH. The effect of injection volume on long-term outcomes of US-guided subacromial bursa injections. Eur J Radiol 2020;129:109113. [Crossref] [PubMed]
- McGill KC, Patel R, Chen D, Okwelogu N. Ultrasound-guided bursal injections. Skeletal Radiol 2023;52:967-78. [Crossref] [PubMed]
- Sconfienza LM, Adriaensen M, Alcala-Galiano A, Allen G, Aparisi Gómez MP, Aringhieri G, et al. Clinical indications for image-guided interventional procedures in the musculoskeletal system: a Delphi-based consensus paper from the European Society of Musculoskeletal Radiology (ESSR)-part IV, hip. Eur Radiol 2022;32:551-60. [Crossref] [PubMed]
- Mitchell WG, Kettwich SC, Sibbitt WL Jr, Sibbitt RR, Muruganandam M, Rolle NA, Hayward WA, Fields RA, Roldan LP, Emil NS, Fangtham M, Bankhurst AD. Outcomes and cost-effectiveness of ultrasound-guided injection of the trochanteric bursa. Rheumatol Int 2018;38:393-401. [Crossref] [PubMed]
- Roh YH, Yoo SJ, Choi YH, Yang HC, Nam KW. Effects of Inflammatory Disease on Clinical Progression and Treatment of Ischiogluteal Bursitis: A Retrospective Observational Study. Malays Orthop J 2020;14:32-41. [Crossref] [PubMed]
- Kim SJ, Kim EK, Kim SJ, Song DH. Effects of bone marrow aspirate concentrate and platelet-rich plasma on patients with partial tear of the rotator cuff tendon. J Orthop Surg Res 2018;13:1. [Crossref] [PubMed]
- Seven MM, Ersen O, Akpancar S, Ozkan H, Turkkan S, Yıldız Y, Koca K. Effectiveness of prolotherapy in the treatment of chronic rotator cuff lesions. Orthop Traumatol Surg Res 2017;103:427-33. [Crossref] [PubMed]
- Hashiuchi T, Sakurai G, Morimoto M, Komei T, Takakura Y, Tanaka Y. Accuracy of the biceps tendon sheath injection: ultrasound-guided or unguided injection? A randomized controlled trial. J Shoulder Elbow Surg 2011;20:1069-73. [Crossref] [PubMed]
- Wang JC, Chang KV, Wu WT, Han DS, Özçakar L. Ultrasound-Guided Standard vs Dual-Target Subacromial Corticosteroid Injections for Shoulder Impingement Syndrome: A Randomized Controlled Trial. Arch Phys Med Rehabil 2019;100:2119-28. [Crossref] [PubMed]
- Ricci V, Mezian K, Cocco G, Tamborrini G, Fari G, Zunica F, Chang KV, Kara M, Özçakar L. Ultrasonography for Injecting (Around) the Lateral Epicondyle: EURO-MUSCULUS/USPRM Perspective. Diagnostics (Basel) 2023.
- Pellegrino R, Paolucci T, Brindisino F, Mondardini P, Di Iorio A, Moretti A, Iolascon G. Effectiveness of High-Intensity Laser Therapy Plus Ultrasound-Guided Peritendinous Hyaluronic Acid Compared to Therapeutic Exercise for Patients with Lateral Elbow Tendinopathy. J Clin Med 2022;11:5492. [Crossref] [PubMed]
- Coombes BK, Bisset L, Brooks P, Khan A, Vicenzino B. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia: a randomized controlled trial. JAMA 2013;309:461-9. [Crossref] [PubMed]
- Uygur E, Aktaş B, Yilmazoglu EG. The use of dry needling vs. corticosteroid injection to treat lateral epicondylitis: a prospective, randomized, controlled study. J Shoulder Elbow Surg 2021;30:134-9. [Crossref] [PubMed]
- Bureau NJ, Tétreault P, Grondin P, Freire V, Desmeules F, Cloutier G, Julien AS, Choinière M. Treatment of chronic lateral epicondylosis: a randomized trial comparing the efficacy of ultrasound-guided tendon dry needling and open-release surgery. Eur Radiol 2022;32:7612-22. [Crossref] [PubMed]
- Zeisig E, Ohberg L, Alfredson H. Extensor origin vascularity related to pain in patients with Tennis elbow. Knee Surg Sports Traumatol Arthrosc 2006;14:659-63. [Crossref] [PubMed]
- Zeisig E, Ohberg L, Alfredson H. Sclerosing polidocanol injections in chronic painful tennis elbow-promising results in a pilot study. Knee Surg Sports Traumatol Arthrosc 2006;14:1218-24. [Crossref] [PubMed]
- Sconfienza LM, Adriaensen M, Albano D, Aparisi Gómez MP, Bazzocchi A, Beggs I, et al. Clinical indications for image-guided interventional procedures in the musculoskeletal system: a Delphi-based consensus paper from the European Society of Musculoskeletal Radiology (ESSR)-Part II, elbow and wrist. Eur Radiol 2020;30:2220-30. [Crossref] [PubMed]
- Huang K, Giddins G, Wu LD. Platelet-Rich Plasma Versus Corticosteroid Injections in the Management of Elbow Epicondylitis and Plantar Fasciitis: An Updated Systematic Review and Meta-analysis. Am J Sports Med 2020;48:2572-85. [Crossref] [PubMed]
- Amable PR, Carias RB, Teixeira MV, da Cruz Pacheco I, Corrêa do Amaral RJ, Granjeiro JM, Borojevic R. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther 2013;4:67. [Crossref] [PubMed]
- Bausset O, Magalon J, Giraudo L, Louis ML, Serratrice N, Frere C, Magalon G, Dignat-George F, Sabatier F. Impact of local anaesthetics and needle calibres used for painless PRP injections on platelet functionality. Muscles Ligaments Tendons J 2014;4:18-23. [Crossref] [PubMed]
- McShane JM, Nazarian LN, Harwood MI. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J Ultrasound Med 2006;25:1281-9. [Crossref] [PubMed]
- Yoo SH, Cha JG, Lee BR. Ultrasound-guided percutaneous bone drilling for the treatment of lateral epicondylitis. Eur Radiol 2018;28:390-7. [Crossref] [PubMed]
- Saaiq M. Management Outcome of de Quervain's Disease with Corticosteroid Injection Versus Surgical Decompression. Arch Bone Jt Surg 2021;9:167-73. [PubMed]
- Orlandi D, Corazza A, Fabbro E, Ferrero G, Sabino G, Serafini G, Silvestri E, Sconfienza LM. Ultrasound-guided percutaneous injection to treat de Quervain's disease using three different techniques: a randomized controlled trial. Eur Radiol 2015;25:1512-9. [Crossref] [PubMed]
- Shen Y, Zhou Q, Sun X, Qiu Z, Jia Y, Li S, Zhang W. The ultrasound-guided percutaneous release technique for De Quervain's disease using an acupotomy. Front Surg 2023;9:1034716. [Crossref] [PubMed]
- Abdoli A, Hashemizadeh Aghda SM, Jalil Abrisham SM. Comparing the Corticosteroid Injection and A1 Pulley Percutaneous Release in Treatment of Trigger Finger: A Clinical Trial. J Hand Surg Asian Pac Vol 2021;26:207-13. [Crossref] [PubMed]
- Wang J, Zhao JG, Liang CC. Percutaneous release, open surgery, or corticosteroid injection, which is the best treatment method for trigger digits? Clin Orthop Relat Res 2013;471:1879-86. [Crossref] [PubMed]
- Lapègue F, André A, Meyrignac O, Pasquier-Bernachot E, Dupré P, Brun C, Bakouche S, Chiavassa-Gandois H, Sans N, Faruch M. US-guided Percutaneous Release of the Trigger Finger by Using a 21-gauge Needle: A Prospective Study of 60 Cases. Radiology 2016;280:493-9. [Crossref] [PubMed]
- Zan X, Zhou WP, Wang Y, Xu M, Zhou FS, Fang XM. Combination of ultrasound-guided percutaneous A1 pulley release and intra-tendon sheath injection improves the therapeutic outcomes in adult trigger finger patients. Med Ultrason 2023;25:153-60. [Crossref] [PubMed]
- Fredberg U, Bolvig L, Pfeiffer-Jensen M, Clemmensen D, Jakobsen BW, Stengaard-Pedersen K. Ultrasonography as a tool for diagnosis, guidance of local steroid injection and, together with pressure algometry, monitoring of the treatment of athletes with chronic jumper's knee and Achilles tendinitis: a randomized, double-blind, placebo-controlled study. Scand J Rheumatol 2004;33:94-101. [Crossref] [PubMed]
- Morton S, Chan O, King J, Perry D, Crisp T, Maffulli N, Morrissey D. High volume image-guided Injections for patellar tendinopathy: a combined retrospective and prospective case series. Muscles Ligaments Tendons J 2014;4:214-9. [Crossref] [PubMed]
- Dragoo JL, Wasterlain AS, Braun HJ, Nead KT. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med 2014;42:610-8. [Crossref] [PubMed]
- Sharif F, Ahmad A, Gilani SA. Effectiveness of ultrasound guided dry needling in management of jumper's knee: a randomized controlled trial. Sci Rep 2023;13:4736. [Crossref] [PubMed]
- Sconfienza LM, Adriaensen M, Albano D, Alcala-Galiano A, Allen G, Aparisi Gómez MP, et al. Clinical indications for image-guided interventional procedures in the musculoskeletal system: a Delphi-based consensus paper from the European Society of Musculoskeletal Radiology (ESSR)-part V, knee. Eur Radiol 2022;32:1438-47. [Crossref] [PubMed]
- Martínez-Martínez A, Ruiz-Santiago F, García-Espinosa J. Platelet-rich plasma: myth or reality? Radiologia (Engl Ed) 2018;60:465-75. [Crossref] [PubMed]
- Bello Baez A, Nieto Morales ML, Mora Guanche P, Cavada Laza A, Pérez Méndez LI. Can Achilles tendinosis be treated effectively with lidocaine and glucose infiltrations, and if so, is the effect lasting? A longitudinal, observational on 27 consecutive patients. Radiologia (Engl Ed) 2023;65:S41-9. [Crossref] [PubMed]
- Boesen AP, Hansen R, Boesen MI, Malliaras P, Langberg H. Effect of High-Volume Injection, Platelet-Rich Plasma, and Sham Treatment in Chronic Midportion Achilles Tendinopathy: A Randomized Double-Blinded Prospective Study. Am J Sports Med 2017;45:2034-43. [Crossref] [PubMed]
- Raeissadat SA, Rayegani SM, Langroudi TF, Khoiniha M. Comparing the accuracy and efficacy of ultrasound-guided versus blind injections of steroid in the glenohumeral joint in patients with shoulder adhesive capsulitis. Clin Rheumatol 2017;36:933-40. [Crossref] [PubMed]
- Lee HJ, Lim KB, Kim DY, Lee KT. Randomized controlled trial for efficacy of intra-articular injection for adhesive capsulitis: ultrasonography-guided versus blind technique. Arch Phys Med Rehabil 2009;90:1997-2002. [Crossref] [PubMed]
- Gavant ML, Rizk TE, Gold RE, Flick PA. Distention arthrography in the treatment of adhesive capsulitis of the shoulder. J Vasc Interv Radiol 1994;5:305-8. [Crossref] [PubMed]
- Kim K, Lee KJ, Kim HC, Lee KJ, Kim DK, Chung SG. Capsule preservation improves short-term outcome of hydraulic distension in painful stiff shoulder. J Orthop Res 2011;29:1688-94. [Crossref] [PubMed]
- Wu SY, Hsu PC, Tsai YY, Huang JR, Wang KA, Wang JC. Efficacy of combined ultrasound-guided hydrodilatation with hyaluronic acid and physical therapy in patients with adhesive capsulitis: A randomised controlled trial. Clin Rehabil 2024;38:202-15. [Crossref] [PubMed]
- Sibbitt WL Jr, Band PA, Chavez-Chiang NR, Delea SL, Norton HE, Bankhurst AD. A randomized controlled trial of the cost-effectiveness of ultrasound-guided intraarticular injection of inflammatory arthritis. J Rheumatol 2011;38:252-63. [Crossref] [PubMed]
- Dubreuil M, Greger S, LaValley M, Cunnington J, Sibbitt WL Jr, Kissin EY. Improvement in wrist pain with ultrasound-guided glucocorticoid injections: a meta-analysis of individual patient data. Semin Arthritis Rheum 2013;42:492-7. [Crossref] [PubMed]
- Monfort J, Rotés-Sala D, Segalés N, Montañes FJ, Orellana C, Llorente-Onaindia J, Mojal S, Padró I, Benito P. Comparative efficacy of intra-articular hyaluronic acid and corticoid injections in osteoarthritis of the first carpometacarpal joint: results of a 6-month single-masked randomized study. Joint Bone Spine 2015;82:116-21. [Crossref] [PubMed]
- Malahias MA, Roumeliotis L, Nikolaou VS, Chronopoulos E, Sourlas I, Babis GC. Platelet-Rich Plasma versus Corticosteroid Intra-Articular Injections for the Treatment of Trapeziometacarpal Arthritis: A Prospective Randomized Controlled Clinical Trial. Cartilage 2021;12:51-61. [Crossref] [PubMed]
- Hoeber S, Aly AR, Ashworth N, Rajasekaran S. Ultrasound-guided hip joint injections are more accurate than landmark-guided injections: a systematic review and meta-analysis. Br J Sports Med 2016;50:392-6. [Crossref] [PubMed]
- Balog TP, Rhodehouse BB, Turner EK, Slevin JM, Bush LA, Grassbaugh JA, Marchant BG. Accuracy of Ultrasound-Guided Intra-articular Hip Injections Performed in the Orthopedic Clinic. Orthopedics 2017;40:96-100. [Crossref] [PubMed]
- Takla A, Gunatilake K, Ma N, Moaveni A. Can intra-articular hip injections predict arthroscopy outcomes for femoroacetabular impingement syndrome? A systematic review. J Orthop 2024;50:122-9. [Crossref] [PubMed]
- Jentzsch T, Meyer YK, Unterfrauner I, Rosskopf AB, Pfirrmann CW, Zingg PO. Can pre-operative intraarticular injection predict pain relief after total hip arthroplasty? BMC Musculoskelet Disord 2023;24:19. [Crossref] [PubMed]
- Berney M, McCarroll P, Glynn L, Lenehan B. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci 2021;190:1021-5. [Crossref] [PubMed]
- Wu T, Dong Y. Song Hx, Fu Y, Li JH. Ultrasound-guided versus landmark in knee arthrocentesis: A systematic review. Semin Arthritis Rheum 2016;45:627-32. [Crossref] [PubMed]
- Peng PW, Shankar H. Ultrasound-guided interventional procedures in pain medicine: a review of anatomy, sonoanatomy, and procedures. Part V: knee joint. Reg Anesth Pain Med 2014;39:368-80. [Crossref] [PubMed]
- Kianmehr N, Hasanzadeh A, Naderi F, Khajoei S, Haghighi A. A randomized blinded comparative study of clinical response to surface anatomy guided injection versus sonography guided injection of hyaloronic acid in patients with primary knee osteoarthritis. Int J Rheum Dis 2018;21:134-9. [Crossref] [PubMed]
- He WW, Kuang MJ, Zhao J, Sun L, Lu B, Wang Y, Ma JX, Ma XL. Efficacy and safety of intraarticular hyaluronic acid and corticosteroid for knee osteoarthritis: A meta-analysis. Int J Surg 2017;39:95-103. [Crossref] [PubMed]
- Ruiz Santiago F, Moraleda Cabrera B, Láinez Ramos-Bossini AJ. Ultrasound guided injections in ankle and foot. J Ultrasound 2024;27:153-9. [Crossref] [PubMed]
- Grice J, Marsland D, Smith G, Calder J. Efficacy of Foot and Ankle Corticosteroid Injections. Foot Ankle Int 2017;38:8-13. [Crossref] [PubMed]
- Sconfienza LM, Adriaensen M, Albano D, Alcala-Galiano A, Allen G, Aparisi Gómez MP, et al. Clinical indications for image-guided interventional procedures in the musculoskeletal system: a Delphi-based consensus paper from the European Society of Musculoskeletal Radiology (ESSR)-part VI, foot and ankle. Eur Radiol 2022;32:1384-94. [Crossref] [PubMed]
- Paget LDA, Reurink G, de Vos RJ, Weir A, Moen MH, Bierma-Zeinstra SMA, Stufkens SAS, Kerkhoffs GMMJ, Tol JL. Effect of Platelet-Rich Plasma Injections vs Placebo on Ankle Symptoms and Function in Patients With Ankle Osteoarthritis: A Randomized Clinical Trial. JAMA 2021;326:1595-605. [Crossref] [PubMed]
- Ashken T, Bowness J, Macfarlane AJR, Turbitt L, Bellew B, Bedforth N, et al. Recommendations for anatomical structures to identify on ultrasound for the performance of intermediate and advanced blocks in ultrasound-guided regional anesthesia. Reg Anesth Pain Med 2022;47:762-72. [Crossref] [PubMed]
- Sconfienza LM, Adriaensen M, Albano D, Allen G, Aparisi Gómez MP, Bazzocchi A, et al. Clinical indications for image guided interventional procedures in the musculoskeletal system: a Delphi-based consensus paper from the European Society of Musculoskeletal Radiology (ESSR)-part III, nerves of the upper limb. Eur Radiol 2020;30:1498-506. [Crossref] [PubMed]
- Hofmeister EP, Moran SL, Shin AY. Anterior and posterior interosseous neurectomy for the treatment of chronic dynamic instability of the wrist. Hand (N Y) 2006;1:63-70. [Crossref] [PubMed]
- Smeraglia F, Berritto D, Basso MA, Mosillo G, Grassi R, Mariconda M. Percutaneous Radiofrequency Ablation of the Posterior and Anterior Interosseous Nerves for Chronic Wrist Pain: A Novel Technique. Tech Hand Up Extrem Surg 2020;25:89-93. [Crossref] [PubMed]
- Yang FA, Shih YC, Hong JP, Wu CW, Liao CD, Chen HC. Ultrasound-guided corticosteroid injection for patients with carpal tunnel syndrome: a systematic review and meta-analysis of randomized controlled trials. Sci Rep 2021;11:10417. [Crossref] [PubMed]
- Salman Roghani R, Holisaz MT, Tarkashvand M, Delbari A, Gohari F, Boon AJ, Lokk J. Different doses of steroid injection in elderly patients with carpal tunnel syndrome: a triple-blind, randomized, controlled trial. Clin Interv Aging 2018;13:117-24. [Crossref] [PubMed]
- Wu YT, Ke MJ, Ho TY, Li TY, Shen YP, Chen LC. Randomized double-blinded clinical trial of 5% dextrose versus triamcinolone injection for carpal tunnel syndrome patients. Ann Neurol 2018;84:601-10. [Crossref] [PubMed]
- Zhang S, Wang F, Ke S, Lin C, Liu C, Xin W, Wu S, Ma C. The Effectiveness of Ultrasound-Guided Steroid Injection Combined with Miniscalpel-Needle Release in the Treatment of Carpal Tunnel Syndrome vs. Steroid Injection Alone: A Randomized Controlled Study. Biomed Res Int 2019;2019:9498656. [Crossref] [PubMed]
- Guo XY, Xiong MX, Lu M, Cheng XQ, Wu YY, Chen SY, Chen K, Zhou QD, Wang L, Tan L, Quan JR, He FD, Chen Q. Ultrasound-guided needle release of the transverse carpal ligament with and without corticosteroid injection for the treatment of carpal tunnel syndrome. J Orthop Surg Res 2018;13:69. [Crossref] [PubMed]
- Guo XY, Xiong MX, Zhao Y, He FD, Cheng XQ, Wu YY, Chen K, Lu M. Comparison of the Clinical Effectiveness of Ultrasound-Guided Corticosteroid Injection with and without Needle Release of the Transverse Carpal Ligament in Carpal Tunnel Syndrome. Eur Neurol 2017;78:33-40. [Crossref] [PubMed]
- Sconfienza LM, Adriaensen M, Albano D, Alcala-Galiano A, Allen G, Aparisi Gómez MP, et al. Clinical indications for image-guided interventional procedures in the musculoskeletal system: a Delphi-based consensus paper from the European Society of Musculoskeletal Radiology (ESSR)-part VII, nerves of the lower limb. Eur Radiol 2022;32:1456-64. [Crossref] [PubMed]
- Klauser AS, Abd Ellah MM, Halpern EJ, Sporer I, Martinoli C, Tagliafico A, Sojer M, Taljanovic MS, Jaschke WR. Meralgia paraesthetica: Ultrasound-guided injection at multiple levels with 12-month follow-up. Eur Radiol 2016;26:764-70. [Crossref] [PubMed]
- Ruiz Santiago F, Prados Olleta N, Tomás Muñoz P, Guzmán Álvarez L, Martínez Martínez A. Short term comparison between blind and ultrasound guided injection in morton neuroma. Eur Radiol 2019;29:620-7. [Crossref] [PubMed]
- Santiago FR, Muñoz PT, Ramos-Bossini AJL, Martínez AM, Olleta NP. Long-term comparison between blind and ultrasound-guided corticoid injections in Morton neuroma. Eur Radiol 2022;32:8414-22. [Crossref] [PubMed]
- Albano D, Viglino U, Messina C, Fusco S, Gitto S, Lacelli F, Sconfienza LM. US-guided percutaneous irrigation of extra-shoulder calcific tendinitis. Br J Radiol 2024;97:267-73. [Crossref] [PubMed]
- Messina C, Sconfienza LM. Ultrasound-Guided Percutaneous Irrigation of Calcific Tendinopathy. Semin Musculoskelet Radiol 2016;20:409-13. [Crossref] [PubMed]
- Serafini G, Sconfienza LM, Lacelli F, Silvestri E, Aliprandi A, Sardanelli F. Rotator cuff calcific tendonitis: short-term and 10-year outcomes after two-needle us-guided percutaneous treatment--nonrandomized controlled trial. Radiology 2009;252:157-64. [Crossref] [PubMed]
- Farin PU, Jaroma H, Soimakallio S. Rotator cuff calcifications: treatment with US-guided technique. Radiology 1995;195:841-3. [Crossref] [PubMed]
- Lanza E, Banfi G, Serafini G, Lacelli F, Orlandi D, Bandirali M, Sardanelli F, Sconfienza LM. Ultrasound-guided percutaneous irrigation in rotator cuff calcific tendinopathy: what is the evidence? A systematic review with proposals for future reporting. Eur Radiol 2015;25:2176-83. [Crossref] [PubMed]
- del Cura JL, Torre I, Zabala R, Legórburu A. Sonographically guided percutaneous needle lavage in calcific tendinitis of the shoulder: short- and long-term results. AJR Am J Roentgenol 2007;189:W128-34. [Crossref] [PubMed]
- Darrieutort-Laffite C, Varin S, Coiffier G, Albert JD, Planche L, Maugars Y, Cormier G, Le Goff B. Are corticosteroid injections needed after needling and lavage of calcific tendinitis? Randomised, double-blind, non-inferiority trial. Ann Rheum Dis 2019;78:837-43. [Crossref] [PubMed]
- Louwerens JKG, Sierevelt IN, Kramer ET, Boonstra R, van den Bekerom MPJ, van Royen BJ, Eygendaal D, van Noort A. Comparing Ultrasound-Guided Needling Combined With a Subacromial Corticosteroid Injection Versus High-Energy Extracorporeal Shockwave Therapy for Calcific Tendinitis of the Rotator Cuff: A Randomized Controlled Trial. Arthroscopy 2020;36:1823-1833.e1. [Crossref] [PubMed]
- Del Castillo-González F, Ramos-Alvarez JJ, Rodríguez-Fabián G, González-Pérez J, Jiménez-Herranz E, Varela E. Extracorporeal shockwaves versus ultrasound-guided percutaneous lavage for the treatment of rotator cuff calcific tendinopathy: a randomized controlled trial. Eur J Phys Rehabil Med 2016;52:145-51. [PubMed]
- Pan P. Ultrasound-Guided Percutaneous Removal of Soft-Tissue Foreign Bodies in Children. J Indian Assoc Pediatr Surg 2021;26:436-8. [Crossref] [PubMed]
- Jacques T, Brienne C, Henry S, Baffet H, Giraudet G, Demondion X, Cotten A. Minimally invasive removal of deep contraceptive implants under continuous ultrasound guidance is effective, quick, and safe. Eur Radiol 2022;32:1718-25. [Crossref] [PubMed]
- Messina C, Banfi G, Aliprandi A, Mauri G, Secchi F, Sardanelli F, Sconfienza LM. Ultrasound guidance to perform intra-articular injection of gadolinium-based contrast material for magnetic resonance arthrography as an alternative to fluoroscopy: the time is now. Eur Radiol 2016;26:1221-5. [Crossref] [PubMed]
- Mermekli A, Reddy P, McKean D, Abdelsalam H, Teh J, Mansour R. Ultrasound-guided continuous radiofrequency ablation of the suprascapular nerve for chronic shoulder pain secondary to osteoarthritis: a retrospective cohort study. Eur Radiol 2022;32:6230-7. [Crossref] [PubMed]
- Fowler IM, Tucker AA, Mendez RJ. Treatment of meralgia paresthetica with ultrasound-guided pulsed radiofrequency ablation of the lateral femoral cutaneous nerve. Pain Pract 2012;12:394-8. [Crossref] [PubMed]
- Cheney CW, Ahmadian A, Brennick C, Zheng P, Mattie R, McCormick ZL, Nagpal A. Radiofrequency Ablation for Chronic Hip Pain: A Comprehensive, Narrative Review. Pain Med 2021;22:S14-9. [Crossref] [PubMed]
- Huang Y, Deng Q, Yang L, Ma J, Wang Z, Huang D, Luo L, Zhou H. Efficacy and Safety of Ultrasound-Guided Radiofrequency Treatment for Chronic Pain in Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Pain Res Manag 2020;2020:2537075. [Crossref] [PubMed]
- Chuter GS, Chua YP, Connell DA, Blackney MC. Ultrasound-guided radiofrequency ablation in the management of interdigital (Morton's) neuroma. Skeletal Radiol 2013;42:107-11. [Crossref] [PubMed]
- Pascoal A, Lourenço C, Ermida FN, Costa A, Carvalho JL. Ultrasound-Guided Percutaneous Radiofrequency Thermal Neuroablation for the Treatment of Adductor and Rectus Femoris Spasticity. Cureus 2023;15:e33422. [Crossref] [PubMed]
- Rohof OJ. Radiofrequency treatment of peripheral nerves. Pain Pract 2002;2:257-60. [Crossref] [PubMed]
- Adler RS. What is the place of ultrasound in MSK imaging? Skeletal Radiol 2024; Epub ahead of print. [Crossref] [PubMed]
- Simoni P, Grumolato M, Malaise O, Preziosi M, Pasleau F, de Lemos Esteves F. Are blind injections of gleno-humeral joint (GHJ) really less accurate imaging-guided injections? A narrative systematic review considering multiple anatomical approaches. Radiol Med 2017;122:656-75. [Crossref] [PubMed]
- Lee JY, Park Y, Park KD, Lee JK, Lim OK. Effectiveness of ultrasound-guided carpal tunnel injection using in-plane ulnar approach: a prospective, randomized, single-blinded study. Medicine (Baltimore) 2014;93:e350. [Crossref] [PubMed]


