
Anomalous origin of the right coronary artery from the proximal segment of the left circumflex artery combined with acute myocardial infarction: a rare type L II-P single coronary artery
Introduction
A single coronary artery (SCA) is an extremely rare anomaly that can occur in isolation or in the presence of other congenital malformations. Percutaneous coronary intervention (PCI) is used to clarify the true anatomic shape of the coronary artery. However, it can occasionally be difficult to determine the origin and distribution of coronary arteries in clinical practice. Congenital anatomic variation and chronic atherosclerosis-related occlusion hamper the ability to determine the origin and distribution of the coronary artery (1,2). PCI is not performed without the recognition of the true anatomic distribution of the coronary artery because possible extravascular operations may have disastrous consequences, including myocardial injury, coronary artery rupture, coronary artery hemorrhage, and pericardial tamponade (3). Here, we report a case in which the opening of the right coronary artery (RCA) originated from the proximal segment of the left circumflex (LCx) coronary artery. This isolated SCA was characterized as type L II-P according to the Lipton classification (4).
Case presentation
A 50-year-old male patient complained of general intermittent angina for 2 days. The condition of the patient worsened 7 hours before admission to the Beijing Friendship Hospital. He had a 6-year history of hypertension and a 10-year history of hyperlipidemia and had undergone lumbar plate implantation 5 years prior. He smoked approximately cigarettes 20 per day and consumed approximately 50–75 g of alcohol per day for 20 years. He had a family history of hypertension. His heart rate was 75 beats/minute, his blood pressure was 131/84 mmHg, and his body mass index was 22.15 kg/m2. There were no obvious positive signs for the heart or other systems. The electrocardiogram showed sinus rhythm with ST-segment depression in leads II, augmented vector foot, and V4–V6 with 0.1–0.15 mV (Figure 1). His peripheral myocardial injury markers were significantly elevated: his troponin I level was 2.709 ng/mL (reference 0–0.030 ng/mL), and his troponin T level was 0.259 ng/mL (reference 0–0.017 ng/mL). The final diagnosis was non-ST-segment elevated myocardial infarction (NSTEMI) and grade I Killip heart function. The left ventricular ejection fraction was 52%, as determined by the biplane Simpson method, with a decreased amplitude of left ventricular inferior and posterior wall motion. There were no abnormalities in any of the valves.

After discharge, antiplatelet agents, including aspirin (aspirin enteric-coated tablet, 100 mg per day), ticagrelor (ticagrelor tablet, 90 mg twice a day), low-molecular-weight heparin (LMWH; enoxaparin sodium, 6,000 IU, twice per day, for 3 days), statins (rosuvastatin calcium tablet, 20 mg once every night), beta-blockers (metoprolol tartrate tablet, 12.5 mg twice per day), ivabradine (ivabradine hydrochloride tablets, 5 mg twice per day), and angiotensin receptor blocker (ARBs; irbesartan tablet, 75 mg per day), were applied.
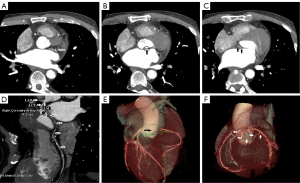
Selective coronary angiography (CAG) via distal radial access was immediately performed after admission to the hospital (Figure 2); however, selective cannulation of the RCA was impossible with a right Judkins catheter. The distal segment of the left anterior descending (LAD) branch was narrowed by 50–70%, the stenosis of the first diagonal and the second diagonal branch was 50–70%, that of the proximal segment of the LCx branch was 50–70%, and the distal LCx was completely occluded. Blood flow from the proximal LCx to the proximal RCA was observed. The middle RCA exhibited 70–90% stenosis, and the distal RCA exhibited 70–90% stenosis. A nonselective aortogram revealed the absence of the RCA in the right sinus of Valsalva, and it was found that the LCx supplied blood to the RCA through the traffic branch (Figure 2). After predilatation of the distal segment of the LCx, PCI was successfully performed without stent implantation. The patient’s chest pain was gradually relieved after PCI.

Subsequent coronary computed tomography angiography (CCTA) revealed that there was no origin of the RCA in the right sinus of Valsalva at 4 days after admission. The opening of the RCA originated from the proximal segment of the LCx and advanced posterior to the aortic root to reach the right atrioventricular groove, from which the RCA was normally distributed (Figure 3). The symptoms of chest pain disappeared after PCI via the LCx, the myocardial enzymes gradually decreased, and the electrocardiogram was stable. Although stenosis of the middle and distal RCA was significant, we did not perform PCI but rather implemented secondary prevention measures for coronary heart disease. The patient was discharged without complications on the seventh day after admission, and he has since experienced no recurrence of chest discomfort or exhibited any signs of myocardial ischemia. Regular outpatient visits were arranged. The left ventricular ejection fraction was 62.5% as determined by the biplane Simpson method, with no decrease in the amplitude of left ventricular wall motion. Cardiac magnetic resonance revealed late significant gadolinium enhancement in the apical segment of the inferior and lateral walls of the left ventricle at the follow-up 9 months after discharge.
All procedures in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Although there are some case reports of the RCA having an abnormal origin (5-7), in most cases, the RCA originates from the distal LCx, while cases in which the RCA originates from the proximal end of the LCx are rare. A variety of methods can be used to determine the true anatomic distribution of abnormal coronary arteries as long as patient safety is not compromised (8). In our case, in addition to the selected CAG, nonselective aortic root CAG and CCTA (9,10) were subsequently performed. Furthermore, it was essential to determine the anatomic structure of the coronary artery before PCI. If this case were determined to be occlusion of the RCA opening, the patient would likely have undergone PCI in an attempt to open the occluded RCA, which could have induced a catastrophic series of consequences.
Presence of SCA ostium, in which only one coronary artery originates from the aortic trunk and supplies the entire heart by a single coronary ostium, in the absence of other cardiac diseases is an extremely rare congenital disorder (11). The exact pathogenic mechanisms involved in the development of SCA syndrome are not known (12). One study reported that the SCA has an incidence of approximately 0.014–0.066% in the general population (13). A review examined 70,850 adult patients who had undergone diagnostic CAG and found an SCA incidence of 0.014% (14). This rare congenital anomaly as an isolated finding accounts for approximately 0.024% of the population (15). In one study analyzing 26,595 patients who underwent coronary arteriography, 56 had anomalies of the SCA (0.044% incidence) (16). Another study retrieved SCA data from 50,000 consecutive coronary angiographies and reported an incidence of 0.066% (11). In a series of studies, in approximately 0.23% of the patients with an SCA and no evidence of other congenital defects, 4382 consecutive coronary angiograms were found (15); subsequently, 4,250 coronary angiograms indicated no patients with an SCA (17). This difference, however, is probably due to a sample error caused by the rarity of the studied phenomenon (11).
One rare anatomical variant is the RCA originating directly from the distal segment of the LCx as a continuation of the LCx (18-20); another rare RCA anomaly is its arising from the mid-LAD segment distal to the first septal perforator and the first diagonal branch (21), with both constituting a single left coronary artery. Lipton et al. divided SCAs into three groups and nine patterns based on the course and distribution of the branches (15). In this case reported here, the SCA arose from the left sinus of Valsalva, and the RCA was delivered at the proximal segment of the LCx and passed posteriorly to the aorta. When the transverse trunk was located near the right sinus of Valsalva, it coursed in the atrioventricular sulcus in a manner similar to that of a normal RCA. This isolated SCA was characterized as type L II-P according to the Lipton classification (15). L II-P-type SCA is highly rare, with an estimated incidence of 0.002% (15). In most L II-type cases, the anomalous RCA originates from the left main coronary artery (LMCA) and passes between the aorta and pulmonary artery before reaching the right atrioventricular groove (16). To our knowledge, our case is the first reported variant of an anomalous origin of the RCA characterized as type L II-P with acute myocardial infarction (AMI).
Since most cases of congenital coronary artery anomalies are benign and clinically asymptomatic (22), these anomalies are discovered incidentally during CAG, enhanced CCTA, and cardiovascular magnetic resonance angiography. Isolated SCA is very rare and unlikely to cause serious clinical sequelae. However, SCA might contribute to the development of myocardial ischemia due to the relatively small proximal vessel, which may either become diseased or cause distal coronary arterial lesions to become more hemodynamically significant by reducing coronary blood flow, such as through resistance in series (17). Furthermore, SCA occlusion impairs the blood supply to almost the entire myocardium, possibly resulting in severe biventricular dysfunction (23). An anomalous coronary artery originating from the opposite coronary artery or coronary sinus of Valsalva between the aorta and the pulmonary artery is associated with increased risks of myocardial ischemia and sudden death in younger patients during exercise (16). Sudden death is strongly associated with anomaly of the LMCA, which arises from the right sinus of Valsalva and traverses two great arteries (Lipton R-IIB). High-risk anatomical characteristics include a slit-like ostium, acute take off angle, and an interarterial course (12).
Coronary artery anomalies are rarely diagnosed throughout life and are most common during autopsy, mainly because they are difficult to identify by routine examination or clinical testing. Selective CAG may be inadequate for identifying coronary anomalies in detail and for characterizing the surrounding structures. CCTA is considered a powerful tool and the gold standard for assessing the relevant spatial information between a coronary artery variant and its surrounding structures, providing detailed structural information preoperatively as guidance for corrective PCI or surgical procedures and shortening the time of image acquisition (24-27). Notably, the process of delivering the RCA at the proximal segment of the LCx has been confused with that of delivering the lateral branch from the LCx to the RCA with occlusion of the RCA orifice. Therefore, multidetector computed tomography (MDCT) may be a good, sensitive diagnostic technique; in our case study, the origins and course of the RCA were well demonstrated via MDCT as was the relationship between the aorta and pulmonary artery.
Identifying anomalies is of paramount importance when intervention is necessary, including interventional or surgical therapy (7,24). The strategy for PCI should be carefully planned to avoid complications when there is an anatomical variation in the coronary artery. PCI for SCA is technically challenging and carries a high risk. In this circumstance, the proximal RCA is severely tortuous, and it is difficult to pass the balloon and stent. Therefore, it is technically challenging to treat similar lesions, and the operational experience under these conditions needs to be further summarized. Invasive angiography (such as intravascular ultrasound and optical coherence examination) could also be used to determine whether there is an occluded site within the coronary artery (1) while evaluating the severity of coronary artery disease and formulating PCI strategies. Since this patient in this case study experienced no recurrence of chest discomfort, we did not perform PCI of the middle or distal RCA but rather implemented secondary prevention measures for coronary heart disease.
Conclusions
To our knowledge, this is the first report of a rare variation of the RCA originating from the proximal LCx with the AMI. It is hoped this can serve to help cardiovascular interventionists and cardiac surgeons identify this congenital variation and be fully aware of its anatomical types. During coronary angioplasty or cardiac surgery, lack of familiarity with this variant may lead to unexpected complications.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-86/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Adam EL, Generoso G, Bittencourt MS. Anomalous Coronary Arteries: When to Follow-up, Risk Stratify, and Plan Intervention. Curr Cardiol Rep 2021;23:102. [Crossref] [PubMed]
- Eid AH, Itani Z, Al-Tannir M, Sayegh S, Samaha A. Primary congenital anomalies of the coronary arteries and relation to atherosclerosis: an angiographic study in Lebanon. J Cardiothorac Surg 2009;4:58. [Crossref] [PubMed]
- Karacsonyi J, Vemmou E, Nikolakopoulos I, Ungi I, Abi Rafeh N, ElGuindy A, Azzalini L, Burke MN, Brilakis ES. Current challenges and prevention strategies for chronic total occlusion (CTO) complications. Expert Rev Cardiovasc Ther 2021;19:337-47. [Crossref] [PubMed]
- Bhattarai V, Mahat S, Sitaula A, Neupane NP, Rajlawot K, Jha SK, Chettry S. A rare case of isolated single coronary artery, Lipton's type LIIB diagnosed by computed tomography coronary angiography. Radiol Case Rep 2022;17:4704-9. [Crossref] [PubMed]
- Tyczyński P, Kukuła K, Pietrasik A, Bochenek T, Dębski A, Oleksiak A, Marona M, Lelek M, Stępińska J, Witkowski A. Anomalous origin of culprit coronary arteries in acute coronary syndromes. Cardiol J 2018;25:683-90. [PubMed]
- Phan NT, Nguyen HT, Nguyen TT, Ly ND, Le NT. St Elevation Myocardial Infarction in a Patient with an Anomalous Right Coronary Artery Originating from the Distal Left Circumflex. Int Med Case Rep J 2019;12:379-82. [Crossref] [PubMed]
- D'Abramo M, Saltarocchi S, Saade W, Chourda E, De Orchi P, Miraldi F. Setting things "right": right internal mammary artery on anomalous right coronary artery - a case report. J Int Med Res 2021;49:3000605211054438. [Crossref] [PubMed]
- Nagashima K, Hiro T, Fukamachi D, Okumura Y, Watanabe I, Hirayama A, et al. Anomalous origin of the coronary artery coursing between the great vessels presenting with a cardiovascular event (J-CONOMALY Registry). Eur Heart J Cardiovasc Imaging 2020;21:222-30. [PubMed]
- Erol C, Seker M. Coronary artery anomalies: the prevalence of origination, course, and termination anomalies of coronary arteries detected by 64-detector computed tomography coronary angiography. J Comput Assist Tomogr 2011;35:618-24. [Crossref] [PubMed]
- Gao X, Wang W, Zhang X, Zhao H, Liu R. A rare V-shaped course variant of the posterior right diagonal artery: a case description and literature analysis. Quant Imaging Med Surg 2023;13:2001-7. [Crossref] [PubMed]
- Desmet W, Vanhaecke J, Vrolix M, Van de Werf F, Piessens J, Willems J, de Geest H. Isolated single coronary artery: a review of 50,000 consecutive coronary angiographies. Eur Heart J 1992;13:1637-40. [Crossref] [PubMed]
- Mahapatro AK, Patro AS, Sujatha V, Sinha SC. Isolated single coronary artery presenting as acute coronary syndrome: case report and review. Int J Angiol 2014;23:143-6. [Crossref] [PubMed]
- Turkmen S, Yolcu M, Sertcelik A, Ipek E, Dokumaci B, Batyraliev T. Single coronary artery incidence in 215,140 patients undergoing coronary angiography. Folia Morphol (Warsz) 2014;73:469-74. [Crossref] [PubMed]
- Tuncer C, Batyraliev T, Yilmaz R, Gokce M, Eryonucu B, Koroglu S. Origin and distribution anomalies of the left anterior descending artery in 70,850 adult patients: multicenter data collection. Catheter Cardiovasc Interv 2006;68:574-85. [Crossref] [PubMed]
- Lipton MJ, Barry WH, Obrez I, Silverman JF, Wexler L. Isolated single coronary artery: diagnosis, angiographic classification, and clinical significance. Radiology 1979;130:39-47. [Crossref] [PubMed]
- Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn 1990;21:28-40. [Crossref] [PubMed]
- Engel HJ, Torres C, Page HL Jr. Major variations in anatomical origin of the coronary arteries: angiographic observations in 4,250 patients without associated congenital heart disease. Cathet Cardiovasc Diagn 1975;1:157-69. [Crossref] [PubMed]
- Chung SK, Lee SJ, Park SH, Lee SW, Shin WY, Jin DK. An extremely rare variety of anomalous coronary artery: right coronary artery originating from the distal left circumflex artery. Korean Circ J 2010;40:465-7. [Crossref] [PubMed]
- Pourafkari L, Taban M, Ghaffari S. Anomalous origin of right coronary artery from distal left circumflex artery: a case study and a review of its clinical significance. J Cardiovasc Thorac Res 2014;6:127-30. [PubMed]
- Bagheri B, Piran R, Nabati M, Jalalian R, Dabirian M. Anomalous origin of the right coronary artery from the left circumflex coronary artery: an extremely rare coronary anomaly. Scott Med J 2015;60:e14-6. [Crossref] [PubMed]
- Dahdouh Z, Roule V, Fadel BM, Grollier G. Anomalous right coronary artery originating from the mid left anterior descending artery. Indian Heart J 2015;67:604-6. [Crossref] [PubMed]
- Spindola-Franco H, Grose R, Solomon N. Dual left anterior descending coronary artery: angiographic description of important variants and surgical implications. Am Heart J 1983;105:445-55. [Crossref] [PubMed]
- Patil S, Rachaiah JM, Ramalingam R, Manjunath CN, Subramanyam K. Percutaneous Coronary Intervention of Hidden Coronary Artery-Unusual Type of Isolated Single Coronary Artery. J Clin Diagn Res 2016;10:OD03-4. [Crossref] [PubMed]
- Kunimoto S, Sato Y, Kunimasa T, Kasamaki Y, Takayama T, Matsumoto N, Kasama S, Yoda S, Saito S, Hirayama A. Double left anterior descending artery arising from the left and right coronary arteries: depiction at multidetector-row computed tomography. Int J Cardiol 2009;132:e54-6. [Crossref] [PubMed]
- Gao X, Li H, Chen H. Myocardial ischemia due to a type IV dual LAD with the long LAD arising from the right sinus of valsalva: a case report and literature review. Intern Med 2015;54:2619-23. [Crossref] [PubMed]
- Albuquerque F, de Araújo Gonçalves P, Marques H, Ferreira A, Freitas P, Lopes P, Gonçalves M, Dores H, Cardim N. Anomalous origin of the right coronary artery with interarterial course: a mid-term follow-up of 28 cases. Sci Rep 2021;11:18666. [Crossref] [PubMed]
- Bollempalli H, Divakaran VG, Kontak AC, Lee PC. Anomalous Origin of All Coronary Arteries from Right Sinus of Valsalva. Tex Heart Inst J 2020;47:170-2. [Crossref] [PubMed]


