
Role of clinical and multidetector computed tomography (MDCT) features in the prediction of patients with intestinal lipoma developing intussusception
Introduction
Lipomas are benign tumors that can occur in all organs. They often occur in subcutaneous tissue and less frequently in the gastrointestinal tract, accounting for only 5% of all gastrointestinal tract tumors (1-3). Gastrointestinal lipomas were first described by Bauer et al. in 1757 (3-6). They often appear as a single lesion, most commonly in the colon (65–75%), but also in the small intestine (20–25%), stomach, and esophagus (4,6-9).
Intestinal lipomas are slow-growing nonepithelial tumors that arise from adipocytes in the intestinal mucosa. Most intestinal lipomas are asymptomatic and discovered by chance during radiological or other examinations for symptoms arising from other causes (9). Lipomas without complications have been less studied due to their benign nature. In a small number of cases, intestinal lipomas present with massive bleeding, intestinal obstruction, intestinal perforation, and intussusception (3,5,10). Asymptomatic intestinal lipomas usually do not require removal (7), but surgical intervention is recommended for intestinal lipomas causing intussusception (5,9,11). Adult intussusception (AI) is uncommon, but lipoma is the most common benign cause (12,13). To date, studies on intussusception caused by intestinal lipoma have primarily been case reports (14-16), with few original articles available. There is a need to comprehensively analyze the clinical and multidetector computed tomography (MDCT) features of intestinal lipomas with and without intussusception and screen patients at high risk of intestinal lipoma-induced intussusception.
The purpose of this study is to share the clinical and MDCT features of intestinal lipomas in our institution to deepen the understanding of this entity. At the same time, the differences and similarities between intestinal lipomas with and without intussusception are compared to find risk factors for lipoma causing intussusception. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1530/rc).
Methods
Participants
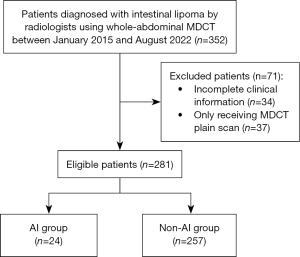
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by our institutional ethics committee (No. 2022[350]) and the requirement for written informed consent was waived for this retrospective study. A computerized search of our hospital’s imaging reporting system identified a total of 352 patients diagnosed with intestinal lipoma by whole-abdominal MDCT from the diaphragm to the symphysis pubis between January 2015 and August 2022. The MDCT diagnostic criteria for intestinal lipomas were low-density shadows, well-defined margins, no enhancement on enhanced scans, and computed tomography (CT) values similar to adipose tissue. Lipomas that had not been surgically confirmed should meet the criteria of having no thick septa, fat component greater than 75%, and no non-adipose mass-like areas (2,17-19). The exclusion criteria included patients with incomplete clinical information (n=34) and only receiving MDCT plain scan (n=37). Finally, 281 patients were included in the study (Figure 1). Reviewing the MDCT data of participants, patients with intestinal lipoma-induced intussusception were divided into the AI group and those without intussusception were divided into the non-AI group. Clinical and MDCT features were collected and compared between the 2 groups. For patients who underwent multiple whole-abdominal MDCT exams, images in which the lesion was first detected during treatment were selected.
MDCT examinations
All patients underwent plain scan and biphasic contrast-enhanced (hepatic arterial phase and portal venous phase) scans by a 64-row MDCT (LightSpeed; GE Healthcare, Milwaukee, WI, USA) or a 256-slice MDCT (Philips Healthcare, Cleveland, OH, USA) system. For enhanced scanning, a dual-head power injector was used to administer a flush of Iopromide (Ultravist; Bayer Schering Pharma, Berlin, Germany) at 370 mg iodine/mL followed by 30 mL of saline. The contrast agent and saline solution were injected at 3–4 mL/s through an 18-gauge plastic intravenous catheter placed in an antecubital vein. Contrast agent volumes were delivered at 2 mL/kg body weight, and the upper limit of dose was set to 120 mL for every patient. The arterial and portal phases were acquired at 20–25 seconds and 45–50 seconds after completion of the contrast agent administration, respectively. The scan parameters were as follows: 64×0.625 or 256×0.5 mm and table speed of 64 or 256 mm per rotation. The following were applied to all scans: pitch 0.984, matrix 512×512, field of view 180–240 mm, 3- or 5-mm-thick slice reconstructions, tube voltage 120 kV, and tube current 300 mA. Images were routinely reformatted with axial (5-mm-thick slice) and coronal (3-mm-thick slice) planes by using an off-line workstation (ADW4.3; General Electric Healthcare, USA).
Image analysis
MDCT images were reviewed retrospectively and independently by 2 radiologists with 6 and 10 years of experience, respectively, at the picture archiving and communication system (PACS) workstation. Continuous data were taken as the average of the measurements from 2 observers. When there was disagreement between the 2 observers on the categorical data, a consensus was reached under the guidance of another senior radiologist with 30 years of experience. MDCT features were analyzed considering the following terms: (I) number (single or multiple; when multiple lipomas were found in the AI group, the one that caused intussusception was included in the study; when there were multiple lipomas in the non-AI group, only the largest one was included in the study); (II) location; (III) size (maximum length and width of lipomas were measured on the axial images, maximum depth was measured on the coronal images, and volume was calculated using the following formula: 0.5233 × length × width × depth) (20); (IV) shape (based on the axial images, lipomas were divided into round/oval, drop-shaped, and irregular) (3); (V) density (homogeneous and heterogeneous); (VI) CT value (plain scan, hepatic arterial and portal venous phases); (VII) the presence or absence of lipoma-induced intussusception: if present, record its pattern (divided into target-sign, reniform-pattern and sausage-pattern according to the axial images) (13), type (enteric, ileocolic, colocolic) (21), and length (take the maximum length of the intussusception segment in the axial or coronal images, whichever longest) (22); (VIII) secondary changes included ascites and intestinal obstruction. Contrast enhancement is defined as the increase of CT value above 20 Hounsfield unit (HU). Intestinal obstruction is defined as intestinal distension [small intestine diameter greater than 2.5 cm (23) and large intestine diameter greater than 6 cm (24)].
Statistical analysis
The Shapiro-Wilk test was used to check the normality of continuous variables. Normal distributed data were expressed as means ± standard deviation (SD) and compared using 2 independent-samples t-test. Similarly, skewed distributed data were expressed as median and interquartile range (IQR) and analyzed with non-parametric test. Categorical variables were expressed as frequency and percentage, and Pearson’s chi-squared test or Fisher’s exact test was used for comparison. Owing to the small number of cases of lipoma-induced intussusception, univariate logistic regression was performed. For significant variables, receiver operating characteristic (ROC) curves were drawn and area under the ROC curves (AUC) were calculated. The optimal thresholds for continuous variables were determined by the highest Youden index and corresponding sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. Statistical analysis was performed by SPSS 25.0 statistical software package (IBM Corp., Armonk, NY, USA) and GraphPad prism 8 (GraphPad Software, San Diego, CA, USA). All tests were 2-sided, and a P value less than 0.05 was considered significant.
Results
Clinical characteristics
A total of 281 adult patients were included, with an average age of 68.0±11.3 years, including 166 females (59.1%) and 115 males (40.9%). The AI group included 11 females (45.8%) and 13 males (54.2%), with an average age of 66.9±11.0 years. The most common symptom was abdominal pain (17/24, 70.8%), followed by nausea/vomiting (9/24, 37.5%), and then hematochezia/melena (7/24, 29.2%). There were 155 females (60.3%) and 102 males (39.7%) in the non-AI group, with an average age of 68.1±11.3 years. The most common symptom was also abdominal pain (121/257, 47.1%), followed by abdominal distension (44/257, 17.1%), and then nausea/vomiting (38/257, 14.8%). There were significant differences in the incidence of abdominal pain (P=0.03), nausea/vomiting (P=0.009), hematochezia/melena (P=0.02), and abdominal tenderness (P<0.001) between the AI and non-AI groups, as detailed in Table 1.
Table 1
| Clinical characteristics | All (n=281) | AI group (n=24) | Non-AI group (n=257) | P value |
|---|---|---|---|---|
| Age (years) | 68.0±11.3 | 66.9±11.0 | 68.1±11.3 | 0.63 |
| Sex | 0.17 | |||
| Female | 166 (59.1) | 11 (45.8) | 155 (60.3) | |
| Male | 115 (40.9) | 13 (54.2) | 102 (39.7) | |
| BMI (kg/m2) | 22.6±3.7 | 21.3±3.7 | 22.8±3.7 | 0.07 |
| Previous abdominal surgery | 63 (22.4) | 5 (20.8) | 58 (22.6) | 0.85 |
| Digestive tract symptoms | 0.04 | |||
| No | 86 (30.6) | 3 (12.5) | 83 (32.3) | |
| Yes | 195 (69.4) | 21 (87.5) | 174 (67.7) | |
| Abdominal pain | 138 (49.1) | 17 (70.8) | 121 (47.1) | 0.03 |
| Abdominal distension | 47 (16.7) | 3 (12.5) | 44 (17.1) | 0.78 |
| Nausea/vomiting | 47 (16.7) | 9 (37.5) | 38 (14.8) | 0.009 |
| Exhaust and defecation stop | 5 (1.8) | 2 (8.3) | 3 (1.2) | 0.06 |
| Diarrhea | 18 (6.4) | 3 (12.5) | 15 (5.8) | 0.19 |
| Hematochezia/melena | 36 (12.8) | 7 (29.2) | 29 (11.3) | 0.02 |
| Changes in bowel habits | 25 (8.9) | 2 (8.3) | 23 (8.9) | >0.99 |
| Abdominal tenderness | 80 (28.5) | 16 (66.7) | 64 (24.9) | <0.001 |
| Comorbidities | ||||
| Hypertension | 67 (23.8) | 4 (16.7) | 63 (24.5) | 0.39 |
| Diabetes mellitus | 29 (10.3) | 0 | 29 (11.3) | 0.15 |
| Hyperlipidemia | 12 (4.3) | 2 (8.3) | 10 (3.9) | 0.27 |
| Digestive tract inflammation | 98 (34.9) | 7 (29.2) | 91 (35.4) | 0.54 |
| Gallbladder diseases | 41 (14.6) | 3 (12.5) | 38 (14.8) | >0.99 |
| Digestive tract tumors | 39 (13.9) | 1 (4.2) | 38 (14.8) | 0.22 |
| Others | 222 (79.0) | 20 (83.3) | 202 (78.6) | 0.59 |
| Treatment | <0.001 | |||
| Surgical treatment | 20 (7.1) | 8 (33.3) | 12 (4.7) | |
| Conservative treatment | 261 (92.9) | 16 (66.7) | 245 (95.3) |
Data are represented as mean ± SD or number (%). AI, adult intussusception; BMI, body mass index; SD, standard deviation.
MDCT features
In general, intestinal lipomas on MDCT appeared as single (231/281, 82.2%), round/oval (186/281, 66.2%), homogeneous hypodensity shadows (231/281, 82.2%), varying in size (from 0.3 to 9.2 cm), with the small intestine being the preferred site (224/281, 79.7%). The CT value of intestinal lipoma on plain scan ranged from −145.5 to −31.4 HU, and no enhancement was seen on the biphasic contrast-enhanced scans.
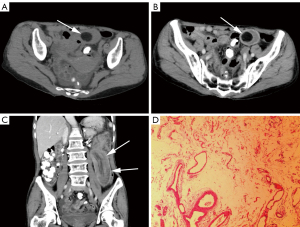
In the AI group, lipomas often presented single (20/24, 83.3%) (Figure 2), round/oval (19/24, 79.2%), and homogeneous hypodensity (14/24, 58.3%). Most of the lipomas that served as the lead point of intussusception were located at the apex of intussusceptum. Among 24 lipoma-induced intussusceptions, 17 intussusceptions (17/24, 70.8%) were enteric (Figure 2), 4 (4/24, 16.7%) were ileocolic, and 3 (3/24, 12.5%) were colocolic. A total of 18 (18/24, 75.0%) intussusception appearances were target-sign, and 6 (6/24, 25.0%) were sausage-pattern. The intussusception length in the AI group ranged from 2.2 to 39.5 cm, [median, 7.6 (4.8, 13.1) cm], and only 3 intussusceptions (3/24, 12.5%) were less than 3.5 cm (22) in length. The lipomas length and volume were 1.3 to 9.2 cm [median, 2.2 (1.7, 3.2) cm] and 0.7 to 144.1 cm³ [median, 4.1 (2.0, 11.1) cm3], respectively. Intestinal obstruction was only observed in 1 patient (1/24, 4.2%), and ascites was found in 8 patients (8/24, 33.3%).
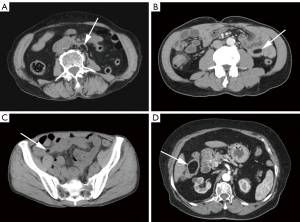
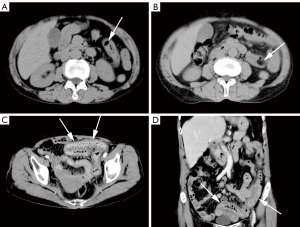
In the non-AI group, lipomas were mainly round/oval (167/257, 65.0%) in shape. In terms of density, 217 lipomas (217/257, 84.4%) were homogeneous hypodensity and 40 (40/257, 15.6%) were heterogeneous hypodensity (Figure 3). There were 57 single lipomas (Figure 4) in the duodenum, 90 in the jejunum, 22 in the ileum, 18 in the cecum, 11 in the ascending colon, 3 in the transverse colon, 5 in the descending colon, 4 in the sigmoid colon, and 1 in the rectum. Multiple lipomas (Figure 5) were present in 46 patients, with the largest occurring in the duodenum in 12 patients, jejunum in 24 patients, ileum in 2 patients, cecum in 3 patients, ascending colon in 4 patients, and transverse colon in 1 patient. There were 8 patients (8/257, 3.1%) with intestinal obstruction and 18 patients (18/257, 7.0%) with ascites, all caused by comorbidities other than lipoma.

There were statistically significant differences in the density (P=0.004), length (P<0.001), and volume (P<0.001) of lipomas between the 2 groups. The MDCT features were summarized in Table 2.
Table 2
| MDCT features | All (n=281) | AI group (n=24) | Non-AI group (n=257) | P value |
|---|---|---|---|---|
| Number | 0.99 | |||
| Single | 231 (82.2) | 20 (83.3) | 211 (82.1) | |
| Multiple | 50 (17.8) | 4 (16.7) | 46 (17.9) | |
| Location | 0.29* | |||
| Small bowel | 224 (79.7) | 17 (70.8) | 207 (80.5) | |
| Duodenum | 69 (24.6) | 0 | 69 (26.8) | |
| Jejunum | 127 (45.2) | 13 (54.2) | 114 (44.4) | |
| Ileum | 28 (10.0) | 4 (16.7) | 24 (9.3) | |
| Large bowel | 57 (20.3) | 7 (29.2) | 50 (19.5) | |
| Cecum | 21 (7.5) | 0 | 21 (8.2) | |
| Ascending colon | 17 (6.0) | 2 (8.3) | 15 (5.8) | |
| Transverse colon | 8 (2.8) | 4 (16.7) | 4 (1.6) | |
| Descending colon | 6 (2.1) | 1 (4.2) | 5 (1.9) | |
| Sigmoid | 4 (1.4) | 0 | 4 (1.6) | |
| Rectum | 1 (0.4) | 0 | 1 (0.4) | |
| Shape | 0.32 | |||
| Round/oval | 186 (66.2) | 19 (79.2) | 167 (65.0) | |
| Drop-shaped | 33 (11.7) | 1 (4.2) | 32 (12.5) | |
| Irregular | 62 (22.1) | 4 (16.7) | 58 (22.6) | |
| Density | 0.004 | |||
| Homogeneous hypodensity | 231 (82.2) | 14 (58.3) | 217 (84.4) | |
| Heterogeneous hypodensity | 50 (17.8) | 10 (41.7) | 40 (15.6) | |
| Length (cm) | 1.3 (0.9, 1.8) | 2.2 (1.7, 3.2) | 1.2 (0.9, 1.7) | <0.001 |
| Volume (cm3) | 0.6 (0.3, 1.6) | 4.1 (2.0, 11.1) | 0.6 (0.2, 1.2) | <0.001 |
| CT value (HU) | ||||
| Plain scan | −89.8 (−100.8, −78.5) | −95.0 (−101.8, −84.5) | −89.5 (−100.5, −77.3) | 0.21 |
| Arterial phase | −88.5 (−98.0, −74.5) | −91.8 (−100.2, −83.3) | −88.4 (−98.0, −73.3) | 0.18 |
| Venous phase | −86.5 (−95.8, −74.0) | −89.7 (−99.9, −80.5) | −85.8 (−95.6, −72.6) | 0.11 |
Data are represented as mean (IQR) or number (%). *, there was no statistically significant difference in the lipoma location between the 2 groups (comparison between the small and large bowel). MDCT, multidetector computed tomography; AI, adult intussusception; CT, computed tomography; HU, Hounsfield unit; IQR, interquartile range.
Treatment and pathology
In the AI group, 8 patients (8/24, 33.3%) underwent surgical treatment. Small bowel segmental resection was performed after intraoperative intussusception reduction in 4 patients and right hemicolectomy without intussusception reduction was performed in the remaining 4 patients. There was no ischemic necrosis in the overlapped bowel. A total of 10 patients (10/24, 41.7%) received conservative treatment or other treatment for comorbidities and 6 (6/24, 25.0%) received outpatient treatment. In the non-AI group, 12 patients (12/257, 4.7%) received surgical treatment to obtain pathological evidence, including endoscopic submucosal dissection or endoscopic mucosal resection in 8 patients, laparoscopic tumor resection in 1 patient, and transabdominal tumor resection in 3 patients. In addition, 147 patients (147/257, 57.2%) were hospitalized due to other diseases and 98 (98/257, 38.1%) received ambulatory treatment. The difference in treatment between the 2 groups was statistically significant (P<0.001).
A total of 20 patients underwent surgical treatment and obtained pathological results. The gross specimens of 3 lipomas which were treated with nylon rope ligation were incomplete, whereas the remaining 17 lipomas obtained complete gross specimens. The length and volume obtained from gross specimens of these 17 lipomas were not statistically significant from those obtained from MDCT [length: 3.5 (2.4, 5.1) vs. 2.3 (2.0, 3.4) cm, P=0.08; volume: 6.3 (3.1, 23.4) vs. 5.0 (1.6, 11.2) cm3, P=0.55]. The gross specimens were grayish-yellow in 15 lipomas (15/17, 88.2%) and taupe in 2 lipomas (2/17, 11.8%). The location of 19 lipomas (19/20, 95.0%) diagnosed by surgery was consistent with those diagnosed by MDCT, and 1 lipoma (1/20, 5.0%) diagnosed by MDCT in the sigmoid colon was confirmed by colonoscopy as the descending colon.
Univariate logistic regression and ROC curves
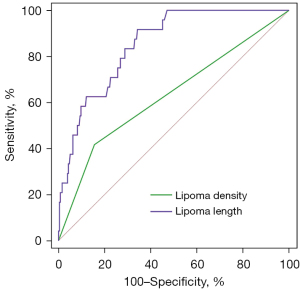
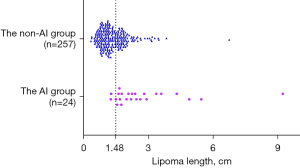
In the univariate logistic regression, lipoma density and length were identified as possible risk factors for lipoma-induced intussusception (Table 3). Of all lipomas with heterogeneous hypodensity, only 20.0% (10/50, PPV) belonged to the AI group. Of those with homogeneous hypodensity, 93.9% (217/231, NPV) belonged to the non-AI group. ROC curves showed that the AUC of lipoma density and length were 0.631 [95% confidence interval (CI): 0.503–0.758] and 0.854 (95% CI: 0.790–0.917) (Table 4, Figure 6). According to the highest Youden index, the optimal threshold value for lipoma length was 1.5 cm, with a sensitivity of 91.7% and a specificity of 65.8% (Figure 7).
Table 3
| Variables | B | SE | Wald | P value | Odds ratio (95% CI) |
|---|---|---|---|---|---|
| Sexa | 0.586 | 0.429 | 1.862 | 0.17 | 1.796 (0.775–4.164) |
| BMI | −0.130 | 0.068 | 3.631 | 0.06 | 0.878 (0.768–1.004) |
| Digestive tract symptomsb | 1.206 | 0.631 | 3.646 | 0.06 | 3.339 (0.969–11.511) |
| Lipoma densityc | 1.355 | 0.448 | 9.127 | 0.003 | 3.875 (1.609–9.331) |
| Lipoma length | 1.168 | 0.248 | 22.155 | <0.001 | 3.216 (1.977–5.231) |
a, the reference category was female; b, the reference was no digestive tract symptoms; c, the reference category was homogeneous hypodensity. B, regression coefficient; SE, standard error; CI, confidence interval; BMI, body mass index.
Table 4
| Variables | AUC (95% CI) | SE | P value |
|---|---|---|---|
| Lipoma density | 0.631 (0.503–0.758) | 0.065 | 0.03 |
| Lipoma length | 0.854 (0.790–0.917) | 0.032 | <0.001 |
AUC, area under the curve; CI, confidence interval; SE, standard error.
Discussion
The present study highlights several differences between intestinal lipomas with and without intussusception: clinical symptoms, signs, lipoma density and length. Moreover, we note that some features of intestinal lipomas may have changed in the era of widespread use of MDCT.
Intestinal lipomas are benign tumors of mesenchymal origin and account for 10% of gastrointestinal benign tumors (2). The peak age for intestinal lipoma is 50–70 years, and it is more common in women than men (6,7,25-27). Overall, this observation is consistent with our findings. Most intestinal lipomas are asymptomatic and long standing, with occasional clinical manifestations being occult and changeable (26). The most common symptom reported in patients with intestinal lipomas previously was abdominal pain, and other symptoms such as constipation, changes in bowel habits, and hemorrhage had also been reported (3), which was basically consistent with our patients’ symptoms. The clinical manifestations for patients with lipoma-induced intussusception are also quite vague and nonspecific (9). Our study showed that that more patients in the AI group had digestive tract symptoms than those in the non-AI group, mainly manifested in abdominal pain, nausea/vomiting, hematochezia/melena, and abdominal tenderness.
Although intestinal lipomas lack specific clinical manifestations, they can be diagnosed easily by MDCT due to their fairly typical adipose tissue attenuation (6,13). When lipoma is the lead point of intussusception, radiologists can make the right diagnosis based on the typical bowel-within-bowel appearance and adipose tissue attenuation (12,28). Lipomas consist of mature fat cells arranged in lobules separated by thin fibrous septa, with branched fibrous tissue and capillaries between the lobules. Lipomas in the AI-group showed a higher proportion of heterogeneous hypodensity, which might be connected to the larger size and more mixed internal components. Meanwhile, the range of CT values in plain scan for all intestinal lipomas in our study was −145.5 to −31.4 HU, which is approximately consistent with previous reports (3,29).
There were significant differences in the lipoma length and volume between the 2 groups. In addition, the length and volume obtained from complete gross specimens in 17 lipomas were not statistically significant compared with those measured by MDCT. However, lipoma volume was rarely calculated in routine imaging work, so it was not included in the univariate logistic regression analysis in this study. Our data suggested that lipoma length was a risk factor for intussusception caused by intestinal lipoma, and the longer the length, the higher the risk of causing intussusception. The threshold value for lipoma length was 1.5 cm, and the sensitivity and specificity for determining whether an intestinal lipoma could cause an intussusception were 91.7% and 65.8%, respectively. Previous reports (4,5,7,30,31) have asserted that intestinal lipomas longer than 2.0 cm could cause clinical symptoms. Our threshold value was 1.5 cm, slightly shorter than the previously reported 2.0 cm, suggesting that more attention should be paid to this less appreciated benign lesion. Some authors (4,32) have reported that endoscopy is a reliable treatment method for intestinal lipoma, even for those longer than 2.0 cm in length, providing clinicians with more treatment options.
The conventional view holds that colon lipomas are more common than small intestine lipomas (2,7,8,25). The most common sites of colonic lipomas are the ascending colon and cecum, followed by the transverse colon, descending colon, sigmoid colon, and rectum (6). The distal ileum is the most common location for small intestine lipomas (2,3,25,26,33). In contrast to these studies, there were many more lipomas in the small intestine than in the colon in our series, with the jejunum being the most common site in both the AI and non-AI groups. Besides, multiple lipomas were mostly detected in the small intestine in our study, which was inconsistent with the reports of previous studies that multiple lipomas mainly occurred in the cecum (3,8). Limited sample size or regional differences may be responsible for these discrepancies. However, we cannot rule out the possibility that more and more lipomas in the small intestine are being discovered with the advancement and extensive utilization of imaging technology. Even though the jejunum and ileum cannot be completely and accurately distinguished based on MDCT images, the small and large bowel can be accurately identified.
Lipomas are soft in texture, and their shape can change in response to intestinal movement and compression (1,3). Therefore, it was not surprising that our study found no significant difference in lipoma shape between the 2 groups.
There are several limitations to this study. First, the study was retrospective, which might have resulted in incomplete information for some patients and inevitably intrinsic selection bias. Second, the majority of lipomas in the non-AI group did not undergo surgery to confirm radiological diagnosis, but this was difficult to avoid in studies that include asymptomatic lipomas as study participants. Third, it only included a small number of patients in a single institution. Fourth, patients had some concomitant diseases, and the clinical symptoms and signs they exhibited might not necessarily have resulted from intestinal lipomas with and without intussusception.
Conclusions
Lipomas, as benign tumors, are rarely taken seriously by patients and doctors until they cause serious symptoms. This is the first study to systematically analyze risk factors for causing intussusception in patients with intestinal lipomas. Our study identifies lipoma density and length as risk factors for lipoma-induced intussusception and 1.5 cm as the threshold value for lipoma length. In addition, in the MDCT era, the predilection site of intestinal lipomas may have changed from the colon to the small intestine.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1530/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1530/coif). The authors declare that the study was supported by the grant from the Clinical Medical Research Project of Army Medical University (No. 2022XLC08). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by our institutional ethics committee [No. 2022(350)] and the requirement for written informed consent was waived for this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hu Q, Wu J, Sun Y. Intussusception Related to Small Intestinal Lipomas: A Case Report and Review of the Literature. Front Surg 2022;9:915114. [Crossref] [PubMed]
- Mouaqit O, Hasnai H, Chbani L, Benjelloun B, El Bouhaddouti H, Ibn El Majdoub K, Toughrai I, Laalim SA, Oussaden A, Maazaz K, Amarti A, Taleb KA. Adult intussusceptions caused by a lipoma in the jejunum: report of a case and review of the literature. World J Emerg Surg 2012;7:28. [Crossref] [PubMed]
- Kouritas VK, Baloyiannis I, Koukoulis G, Mamaloudis I, Zacharoulis D, Efthimiou M. Spontaneous expulsion from rectum: a rare presentation of intestinal lipomas. World J Emerg Surg 2011;6:19. [Crossref] [PubMed]
- Lee KJ, Kim GH, Park DY, Shin NR, Lee BE, Ryu DY, Kim DU, Song GA. Endoscopic resection of gastrointestinal lipomas: a single-center experience. Surg Endosc 2014;28:185-92. [Crossref] [PubMed]
- Tascilar O, Cakmak GK, Gün BD, Uçan BH, Balbaloglu H, Cesur A, Emre AU, Comert M, Erdem LO, Aydemir S. Clinical evaluation of submucosal colonic lipomas: decision making. World J Gastroenterol 2006;12:5075-7. [Crossref] [PubMed]
- Zhou XC, Hu KQ, Jiang Y. A 4-cm lipoma of the transverse colon causing colonic intussusception: A case report and literature review. Oncol Lett 2014;8:1090-2. [Crossref] [PubMed]
- Mantzoros I, Raptis D, Pramateftakis MG, Kanellos D, Psomas S, Makrantonakis A, Tsachalis T, Angelopoulos S. Colonic lipomas: our experience in diagnosis and treatment. Tech Coloproctol 2011;15:S71-3. [Crossref] [PubMed]
- Jiang RD, Zhi XT, Zhang B, Chen ZQ, Li T. Submucosal Lipoma: a Rare Cause of Recurrent Intestinal Obstruction and Intestinal Intussusception. J Gastrointest Surg 2015;19:1733-5. [Crossref] [PubMed]
- Menegon Tasselli F, Urraro F, Sciaudone G, Bagaglini G, Pagliuca F, Reginelli A, Ferraraccio F, Cappabianca S, Selvaggi F, Pellino G. Colonic Lipoma Causing Bowel Intussusception: An Up-to-Date Systematic Review. J Clin Med 2021;10:5149. [Crossref] [PubMed]
- Ferrara F, Duburque C, Quinchon JF, Gaudissart Q. Laparoscopic resection of small bowel lipoma causing obscure gastrointestinal bleeding. Updates Surg 2012;64:153-6. [Crossref] [PubMed]
- Genchellac H, Demir MK, Ozdemir H, Unlu E, Temizoz O. Computed tomographic and magnetic resonance imaging findings of asymptomatic intra-abdominal gastrointestinal system lipomas. J Comput Assist Tomogr 2008;32:841-7. [Crossref] [PubMed]
- Al-Radaideh AM, Omari HZ, Bani-Hani KE. Adult intussusception : A 14-year retrospective study of clinical assessment and computed tomography diagnosis. Acta Gastroenterol Belg 2018;81:367-72. [PubMed]
- Kim YH, Blake MA, Harisinghani MG, Archer-Arroyo K, Hahn PF, Pitman MB, Mueller PR. Adult intestinal intussusception: CT appearances and identification of a causative lead point. Radiographics 2006;26:733-44. [Crossref] [PubMed]
- Vagholkar K, Chavan R, Mahadik A, Maurya I. Lipoma of the Small Intestine: A Cause for Intussusception in Adults. Case Rep Surg 2015;2015:856030. [Crossref] [PubMed]
- Boyack I, Vu D, Patel P, Opsha O. Colocolic intussusception secondary to submucosal lipoma. Am J Emerg Med 2020;38:1697.e1-3. [Crossref] [PubMed]
- Shimazaki J, Nakachi T, Tabuchi T, Suzuki S, Ubukata H, Tabuchi T. Laparoscopic management of an octogenarian adult intussusception caused by an ileal lipoma suspected preoperatively: a case report. World J Surg Oncol 2015;13:75. [Crossref] [PubMed]
- Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology 2002;224:99-104. [Crossref] [PubMed]
- Cheng Y, Ko AT, Huang JH, Lee BC, Yang RS, Liang CW, Tai HC, Cheng NC. Developing a clinical scoring system to differentiate deep-seated atypical lipomatous tumor from lipoma of soft tissue. Asian J Surg 2019;42:832-8. [Crossref] [PubMed]
- Asano Y, Miwa S, Yamamoto N, Hayashi K, Takeuchi A, Igarashi K, Yonezawa H, Araki Y, Morinaga S, Nojima T, Ikeda H, Tsuchiya H. A scoring system combining clinical, radiological, and histopathological examinations for differential diagnosis between lipoma and atypical lipomatous tumor/well-differentiated liposarcoma. Sci Rep 2022;12:237. [Crossref] [PubMed]
- Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW. Prospective change in renal volume and function in children with ADPKD. Clin J Am Soc Nephrol 2009;4:820-9. [Crossref] [PubMed]
- Dollinger M, Bäumler W, Brunner SM, Stroszczynski C, Georgieva M, Müller K, Schicho A, Müller-Wille R. Role of clinical and CT findings in the identification of adult small-bowel intussusception requiring surgical intervention. BJS Open 2021;5:zrab076. [Crossref] [PubMed]
- Lvoff N, Breiman RS, Coakley FV, Lu Y, Warren RS. Distinguishing features of self-limiting adult small-bowel intussusception identified at CT. Radiology 2003;227:68-72. [Crossref] [PubMed]
- Silva AC, Pimenta M, Guimarães LS. Small bowel obstruction: what to look for. Radiographics 2009;29:423-39. [Crossref] [PubMed]
- Khurana B, Ledbetter S, McTavish J, Wiesner W, Ros PR. Bowel obstruction revealed by multidetector CT. AJR Am J Roentgenol 2002;178:1139-44. [Crossref] [PubMed]
- Manouras A, Lagoudianakis EE, Dardamanis D, Tsekouras DK, Markogiannakis H, Genetzakis M, Pararas N, Papadima A, Triantafillou C, Katergiannakis V. Lipoma induced jejunojejunal intussusception. World J Gastroenterol 2007;13:3641-4. [Crossref] [PubMed]
- Fang SH, Dong DJ, Chen FH, Jin M, Zhong BS. Small intestinal lipomas: diagnostic value of multi-slice CT enterography. World J Gastroenterol 2010;16:2677-81. [Crossref] [PubMed]
- Taylor AJ, Stewart ET, Dodds WJ. Gastrointestinal lipomas: a radiologic and pathologic review. AJR Am J Roentgenol 1990;155:1205-10. [Crossref] [PubMed]
- Somma F, Faggian A, Serra N, Gatta G, Iacobellis F, Berritto D, Reginelli A, Di Mizio V, Cappabianca S, Di Mizio R, Grassi R. Bowel intussusceptions in adults: the role of imaging. Radiol Med 2015;120:105-17. [Crossref] [PubMed]
- Akagi I, Miyashita M, Hashimoto M, Makino H, Nomura T, Tajiri T. Adult intussusception caused by an intestinal lipoma: report of a case. J Nippon Med Sch 2008;75:166-70. [Crossref] [PubMed]
- Siu S, Oliphant R, Benstock S, Keshava A, Rickard MJFX. Colonic lipoma causing intussusception: a case for colonoscopic surveillance? ANZ J Surg 2019;89:428-30. [Crossref] [PubMed]
- Panahi SE, Mehanna D. Adult intussusception involving colonic lipoma: a case study. ANZ J Surg 2019;89:E272-3. [Crossref] [PubMed]
- Aydin HN, Bertin P, Singh K, Arregui M. Safe techniques for endoscopic resection of gastrointestinal lipomas. Surg Laparosc Endosc Percutan Tech 2011;21:218-22. [Crossref] [PubMed]
- Ako E, Morisaki T, Hasegawa T, Hirakawa T, Tachimori A, Nakazawa K, Yamagata S, Kanehara I, Nishimura S, Taenaka N. Laparoscopic resection of ileal lipoma diagnosed by multidetector-row computed tomography. Surg Laparosc Endosc Percutan Tech 2010;20:e226-9. [Crossref] [PubMed]


