
Pulmonary benign metastasizing leiomyoma: a case description and analysis of the literature
Background
Benign metastasizing leiomyoma (BML) is a rare disorder in which mitotically inactive smooth muscle tumors appear in extrauterine sites in patients who have or have had uterine leiomyomas. BML, initially presenting as fibroleiomyomatous hamartoma, was first discovered by Steiner in 1939 (1). Steiner reported a patient who died from the effects of extensive pulmonary metastases that were histologically identical to uterine leiomyomas (1). Barnaś et al. conducted a comprehensive review of 161 BML cases documented in the literature from January 1, 1965, to April 10, 2016 (2). BML mainly affects late reproductive-age women. The lung is the most frequently involved organ of metastases, with other sites including the heart, lymph nodes, skeletal muscle, and, occasionally, the pancreas (3). Depending on the location of tumors, different terminologies including pulmonary benign metastasizing leiomyoma (when found in the lung), intravenous leiomyomatosis (when found in the vessels), and disseminated peritoneal leiomyomatosis (when found in the peritoneal cavity) have been used to describe leiomyomas found outside the uterus.
Uterine leiomyomas, composed of smooth muscle tissue and excess extracellular fibrous supporting tissue, are the most common benign uterine tumor in females with or without symptoms (4). Usually, it cannot be distinguished easily from uterine sarcomas using clinical criteria or imaging techniques. The treatment for leiomyoma is often conservative or involves minimally invasive surgery, while that for uterine sarcoma often requires more radical debulking surgery (5). The misdiagnosis of uterine sarcoma for leiomyoma might result in treatment delays and increased mortality. Interestingly, it is also easy to mistake BML for metastasis from uterine sarcoma or cervical cancer (6), which might lead to unnecessary surgeries. Appropriate screening programs for early cervical cancer diagnosis might help reduce misdiagnosis (7).
Pulmonary metastases in BML are often identified incidentally during preoperative assessments, with patients typically not manifesting symptoms such as cough, chest pain, hemoptysis, or dyspnea; however, a few symptomatic cases have also been documented (8). The differential diagnosis of BML includes lymphangioleiomyomatosis (LAM) (9) and pulmonary metastases from leiomyosarcoma or other malignancies (10). The mechanism of leiomyoma dissemination remains unclear. Usually, the ability to metastasize is a feature of malignancy. In this article, the metastasis of uterine leiomyoma to the lung is paradoxically described as benign in histological terms, mirroring the biological behavior of endometriosis (11). BML is inherently characterized as a metastatic leiomyoma regardless of whether its occurrence is iatrogenic or noniatrogenic (12). Most documented cases of BML occur in women after hysterectomy or myomectomy. Only a small fraction of these pulmonary lesions co-occur with uterine leiomyoma. Barnaś et al. conducted a comprehensive systematic review of four databases (Medline/PubMed, Embase, Web of Science, and Cochrane) including articles published from January 1, 1965, to April 10, 2016. They identified a mere 10 instances of BML in women without prior surgical intervention (2). Subsequently, our team conducted a study of the literature pertaining to BML from January 2017 to October 2023 in PubMed, retrieving only 9 cases in women devoid of a history of prior surgery (6,13-20). Among these cases, six exhibited metastases to the lung, one to the heart, and one to the lymph node. Notably, one patient with BML had multiple leiomyomas affecting both lungs, the mediastinum, the pericardium, the spine, the peritoneum, and the left thigh. Hence, to the best of our knowledge, there are approximately 20 documented cases worldwide of BML in women who have not undergone any previous uterine surgeries. Cases of BML in the absence of prior uterine surgery and those following hysterectomy or myomectomy may have disparate pathogeneses and therapeutic considerations. One hypothesis posits surgery as a potential instigator for BML, postulating that tumors acquire venous access through surgical trauma (hematogenous spread) during procedures such as uterine dilatation and curettage, myomectomy, and hysterectomy (11).
We encountered a patient with a highly uncommon manifestation of rare uterine leiomyoma variant. In this article, we detail a case of concurrent pulmonary BML and uterine myoma and propose a treatment regimen involving total abdominal hysterectomy and bilateral salpingo-oophorectomy, followed by three months of gonadotropin-releasing hormone agonist (GnRH) analog treatment. This approach has demonstrated efficacy in disease management, obviating the need for thoracic surgery and thereby enhancing the patient’s overall quality of life. Given the infrequency of BML and its limited documentation, there exists no consensus on the optimal treatment modalities, and this is further complicated by classifications based on prior uterine surgery history, age at disease onset, time elapsed between BML diagnosis and prior uterine surgery, and other factors. The establishment of clinical guidelines necessitates a greater volume of case reports on BML. Therefore, our case report may contribute to future systematic reviews and the development of clinical protocols.
Case presentation
A 54-year-old woman, in the perimenopausal stage, planned to undergo the removal of an intrauterine contraceptive device (IUD) at the hospital. She did not have any symptoms and had not undergone any prior uterine surgeries such as cesarean section. She had one vaginal delivery of a baby boy. Vaginal ultrasound at a local clinic revealed a uterine myoma measuring about 7 cm in the central pelvis. Given her age and the size of the uterine fibroids, the procedure changed from the original removal of the contraceptive ring to a hysterectomy. Subsequent preoperative examinations were conducted. A preoperative chest computed tomography (CT) examination revealed the presence of multiple lung nodules of various sizes, with the largest one measuring approximately 21×32 mm situated in the right middle lobe, suggestive of malignancy. She was thus referred to Shanghai General Hospital for further treatment. Remarkably, she did not experience weight loss or any respiratory symptoms, such as hemoptysis, purulent sputum, or exertional dyspnea.
She had a healthy lifestyle and a regular menstrual cycle. The levels of estradiol, progesterone, and serum lactate dehydrogenase (LDH) were 818.85 pmol/L, 0.79 ng/mL, and 134.7 U/L. Routine laboratory tests showed that the values for serum tumor markers such as alpha-fetoprotein (AFP), cancer antigen 19-9 (CA19-9), cancer antigen 125 (CA125), carcinoembryonic antigen (CEA), human epididymis protein 4 (HE4), normal squamous epithelium (NSE), and squamous cell carcinoma (SCC) were within the normal ranges. A positron emission tomography-computed tomography (PET-CT) scan demonstrated only a slight elevation in glucose metabolism within the multiple lung nodules and the anterior uterine mass. Among the lung nodules, there was a mass in the medial middle lobe of the right lung measuring about 3.2×2.0 cm in size, with shallow lobulated borders and a maximum standardize uptake value (SUV) of about 1.7. There was also a low-density mass with unclear boundaries in the anterior wall of the uterus measuring about 5.8×5.6×7.1 cm in size with an SUV of about 3.9 (Figure 1).

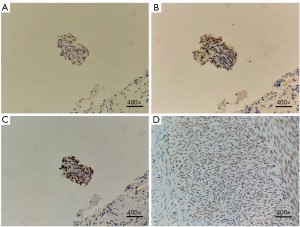
A CT-guided pulmonary biopsy was conducted, revealing no signs of malignancy. Subsequent bronchoscopy identified external pressure-induced compression of the right B5a branch in the middle lobe of the lung. Radial endobronchial ultrasound with a guide sheath (REBUS-GS) exhibited an unusual extramucosal echo, signifying an intact mucosa and suggesting the possibility of a mass situated beyond the bronchial mucosa. No phagocytic cells, fungi, bacteria, or parasites were found in the cytological examination of bronchoalveolar lavage fluid, and the special staining was negative for Cryptococcus. Transbronchial biopsy was performed in the right B5a branch of the middle lobe of the lung, and there was notable proliferation of bland spindle cells on microscopy but no evidence of necrosis, cytologic atypia, or increased mitotic activity. The immunohistochemical results included positivity for desmin, h-Caldesmon, Ki-67 (5%), estrogen receptor (ER), and progesterone receptor (PR) but not for CD10, P16, or P53 (Figure 2). The staining for human melanoma black (HMB) 45 and melan-A were also negative (Figure 3). Considering the immunohistochemical findings and the patient’s medical history, we could not rule out the potential metastasis of a uterine smooth muscle tumor.

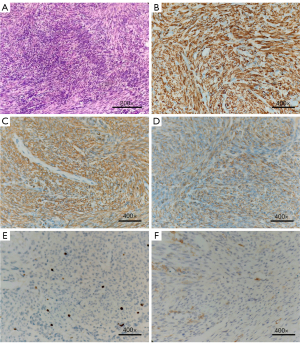
The patient then underwent laparoscopic total hysterectomy and bilateral salpingo-oophorectomy. The gross section was gray, sallow, and lobulated. On microscopy, the endometrium showed a secretory phase image, and the cervical mucosa was chronically inflamed. The immunohistochemical results included positivity for desmin, smooth muscle actin (SMA), h-Caldesmon, CD34 (vascular), and Ki-67 (5%) but not for CD10 in the uterine mass (Figure 4). The staining for high-mobility group AT-hook 2 (HMGA2) was consistent between the lung nodules and the uterine mass (Figure 3). The tumor cells found in the lung tissue were histopathologically the same as the tumor cells found in the uterine corpus, leading to the diagnosis of BML.
After the surgery, the patient was injected with GnRH analogues every four weeks, and a regular follow-up was scheduled. A chest CT was conducted three months after the operation, which showed that some nodules in both lungs had regressed slightly, with most of the lung nodules remaining unchanged. The maximum lung nodule in the right lobe was still 21×32 mm.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
BMLs are slow-growing “metastatic” tumors outside the uterus and are characterized by a benign histology with low mitotic indices, minimal atypical nuclei, the absence of coagulative necrosis, and no evidence of invasion despite multiple distant lesions. The immunohistochemical profile of BML is similar to that of primary uterine leiomyoma, with positive staining for SMA, desmin, ER, and PR (21). Generally, estrogen is known to stimulate tumor growth, while progesterone is thought to inhibit tumor growth. Consistent with this theory, BML tends to subside when patients become pregnant or postmenopausal (22).
The pathological origins of BML have been speculated to be the metastasis of existing smooth muscle cells from the uterus to distant locations, derivation from a low-grade uterine leiomyosarcoma, and derivation from multifocal but independent smooth muscle proliferation (23). Evidence supporting the theory that BML is low-grade leiomyosarcoma is weak given that there are no histologic features of malignancy such as cytological atypia, increased mitotic index, or necrosis present. The majority of theories include an origin of BML derived from the metastasis of existing benign uterine leiomyoma. Tietze et al. found an identical X-chromosome inactivation pattern in the tumors of a patient with BML involving the lungs and uterus, suggesting that the BML was monoclonal in origin and that the pulmonary lesions were metastatic deposits (12). Similarly, Patton et al. analyzed the immunohistochemical features, clonality, and telomere length of multiple lung and uterine tumors in three patients with BML, concluding that BML is clonally derived from a benign-appearing uterine leiomyoma (24). Nucci et al. reported 19q and 22q terminal deletions in all five cases of BML that they examined (25). Additionally, Wu et al. revealed loss of chromosome 3q and 11q in paired pulmonary and uterine leiomyomata from two patients with BML (26). Identical chromosomal changes between pulmonary and uterine leiomyoma further support the pathological origin of BML as being a benign uterine leiomyoma.
Metastasis is typically considered a malignant behavior, leads to the majority of cancer-related deaths, and is responsible for significant clinical complications. However, in endometriosis, a benign disease, the normal endometrium can migrate to and colonize distant organs, such as the ovaries, lungs, or pelvic floor. In this regard, there may be similarities between the possible pathogenesis of BML and endometriosis such as peritoneal seeding at the time of pelvic surgery, lymphatic or vascular spread, or metaplastic transformation of the coelomic epithelium (11). Both BML and endometriosis lesions have a positive expression of ER and PR, leading to a favorable response to endocrine therapy. Tong et al. described a case of histologically benign lymph nodal metastases from a uterine leiomyoma in a 50-year-old woman and attributed this to lymphatic or vascular spread (27). Coelomic metaplasia might be another pathogenic source of BML of the peritoneal cavity and digestive tract (28).
It is almost impossible to diagnosis BML through imaging alone because imaging findings of BLM are nonspecific and overlap with other diseases. In thoracic CT, pulmonary BML lesions often present as multiple bilateral well-defined noncalcified solid nodules of variable size with no enhancement with intravenous contrast. PET-CT with 18F-fluorodeoxyglucose (18F-FDG PET-CT) is helpful in assessing the significant metabolic activity indicative of malignant disease. Moreover, weak or no significant metabolic activity of the nodules in BML on 18F-FDG PET-CT may be helpful in differentiating BML from sarcomas (14). Recently, Wu et al. presented a case of faint 18F-FDG (a low level of glucose metabolism) but elevated gallium 68-labeled fibroblast-activation protein inhibitor (68Ga-FAPI) activity (excessive accumulation of activated fibroblasts) in a 37-year-old woman with BML to the lung and pelvis (29). PET with 68Ga-FAPI might be helpful for further differentiating BML from malignancies and avoiding risks of pneumothorax complicated by pulmonary biopsies. Simsek et al. employed 16α-[18F]-fluoro-17ß-estradiol (FES) PET-CT to evaluate the in vivo ER status of a 41-year-old woman with benign leiomyoma metastasizing to the lung. The high expression of ER receptors in BML can be decisive for the initiation of antihormonal therapies, particularly in premenopausal women (30).
Definite diagnosis still requires pulmonary histopathological examination and the comparison with the pathologic findings of leiomyoma through transbronchial or transthoracic lung biopsy. The histological diagnosis of BML involves the identification of characteristic features in tissue specimens. Key histopathological findings include a smooth muscle origin, benign-appearing cells, and a low mitotic index. The origin of the tumor cells as smooth muscle is typically established through positive immunohistochemical staining for smooth muscle markers such as SMA and desmin. The cells are typically elongated, spindle-shaped, and arranged in fascicles. Uterine leiomyomas typically express ER and PR. Positive staining for these receptors in the lung nodules can suggest a uterine origin. Ki-67 is a marker of cell proliferation and can be used to assess the growth fraction of a tumor by identifying actively dividing cells. Uterine leiomyomas generally have a low proliferation index (low percentage of Ki-67-positive cells). To prove the tumor in the uterine body and the tumor in the lung organs are the same tumor, we employed HMGA2 as a key mesenchymal tumor marker. HMGA2, known for its consistent expression in uterine leiomyomas, has been used for the immunohistochemical analysis of both uterine and lung tissues (31,32). The staining patterns of HMGA2 have been found to be remarkably consistent between the two sites, providing compelling evidence that the lung nodules are indeed metastatic lesions of the uterine leiomyoma. This integrated approach, combining histopathological and molecular analyses, serves to robustly confirm the shared origin of the tumors, contributing to a more comprehensive understanding of BML and its unique behavior.
Immunohistochemistry plays a pivotal role in distinguishing between uterine leiomyoma and uterine leiomyosarcoma, providing valuable insights into the nature of these uterine smooth muscle tumors. In uterine leiomyoma, immunohistochemistry typically reveals positive staining for desmin, SMA, and h-Caldesmon, reflecting the smooth muscle origin and differentiation. Additionally, these benign tumors often exhibit low Ki-67 proliferation indices. Conversely, uterine leiomyosarcoma exhibits a distinct immunohistochemical profile, characterized by increased cellularity, atypical mitoses, and a higher Ki-67 proliferation index. Leiomyosarcomas commonly display positive staining for desmin, SMA, and h-Caldesmon, mirroring their smooth muscle lineage, but the intensity and distribution of these markers may vary. Importantly, uterine leiomyosarcomas often present with an elevated Ki-67 index, indicative of increased cellular proliferation (33).
LAM and BML are distinct entities, and while they share some similarities, they differ in terms of pathogenesis, clinical features, and behavior. LAM is a rare multisystem disease, belonging to the family of tumors with perivascular epithelioid cell (PEC) differentiation, and is characterized by smooth muscle proliferation along blood vessels. LAM primarily affects the lungs and lymphatics and is often associated with tuberous sclerosis complex (TSC) but can also occur sporadically (34). On the other hand, BML involves the metastasis of uterine leiomyomas to extragenital sites, including the lungs. The exact pathogenesis is not fully understood, but it is not related to TSC. LAM predominantly affects women of childbearing age. Symptoms may include dyspnea, pneumothorax, and chylous effusions. Pulmonary function tests often reveal obstructive patterns. BML typically presents in women with a history of uterine leiomyomas. Lung involvement may be asymptomatic or present with nonspecific respiratory symptoms. Unlike LAM, BML does not show a predilection for women of childbearing age. Radiologically, LAM is characterized by thin-walled cysts, ground-glass opacities, and pleural effusions on imaging studies such as high-resolution CT. BML can present as well-defined nodules or masses on imaging, often resembling metastatic lesions. The appearance may vary and can mimic other pulmonary conditions. Histologically, LAM is characterized by the proliferation of abnormal smooth muscle cells and cystic changes in the lung parenchyma. LAM exhibits immunohistochemical positivity for HMB45 and melan-A, indicating the presence of abnormal smooth muscle-like cells with melanocytic differentiation (35). BML is characterized by the presence of benign-appearing smooth muscle cells consistent with uterine leiomyomas at the metastatic sites. Immunohistochemical staining can help confirm the smooth muscle origin.
Due to the rarity of BML, it is almost impossible to define a standard treatment protocol through the use of clinical trials. Treatment strategies are typically individualized according to the size and location of the fibroids, the severity of the symptoms, the patient’s age, and the patient’s desire for future fertility. Smaller subcentimeter lesions without any symptoms can be followed up with surveillance CT scans. The presence of ER and PR in BML makes tumors susceptible to hormonal manipulation, including surgical sterilization and pharmacological treatment such as high-dose progestogens, GnRH analogues, selective estrogen modulators, and aromatase-P450 inhibitors. Some BML nodules may regress after hormonal treatment, without the necessity of surgical excision of the nodules (36). For young women who desire to retain fertility, hormonal treatment can be a therapeutic alternative. For women who have no reproductive intentions or have recurrent nodules, perform total abdominal hysterectomy, bilateral salpingo-oophorectomy, and debulking are recommended. Furthermore, hormonal treatment might be necessary due to continued disease progression despite oophorectomy or menopause.
Conclusions
We report a case of pulmonary BML arising concurrently with uterine myoma. Total abdominal hysterectomy and bilateral salpingo-oophorectomy were performed and were followed by treatment with GnRH analogues. After three months of combination therapy, a few nodules in both lungs had slightly regressed. Regular follow-up has been scheduled and will be strictly observed.
Acknowledgments
We would like to thank Shanghai General Hospital for providing a valuable platform for scientific and clinical research.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1863/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Steiner PE. Metastasizing fibroleiomyoma of the uterus: Report of a case and review of the literature. Am J Pathol 1939;15:89-110.7.
- Barnaś E, Książek M, Raś R, Skręt A, Skręt-Magierło J, Dmoch-Gajzlerska E. Benign metastasizing leiomyoma: A review of current literature in respect to the time and type of previous gynecological surgery. PLoS One 2017;12:e0175875. [Crossref] [PubMed]
- Sapmaz F, Ergin M, Katrancioglu O, Gonlugur T, Gonlugur U, Elagoz S. Benign metastasizing leiomyoma. Lung 2008;186:271-3. [Crossref] [PubMed]
- Machado-Lopez A, Simón C, Mas A. Molecular and Cellular Insights into the Development of Uterine Fibroids. Int J Mol Sci 2021;22:8483. [Crossref] [PubMed]
- Giannini A, Golia D'Augè T, Bogani G, Laganà AS, Chiantera V, Vizza E, Muzii L, Di Donato V. Uterine sarcomas: A critical review of the literature. Eur J Obstet Gynecol Reprod Biol 2023;287:166-70. [Crossref] [PubMed]
- Whang SG, Gholson M, Rushing RS. Benign metastasizing leiomyoma, a rare imposter of metastatic cervical cancer. Gynecol Oncol Rep 2021;38:100893. [Crossref] [PubMed]
- D’Augè TG, Giannini A, Bogani G, Di Dio C, Laganà AS, Di Donato V, Salerno MG, Caserta D, Chiantera V, Vizza E, Muzii L, D’Oria O. Prevention, Screening, Treatment and Follow-Up of Gynecological Cancers: State of Art and Future Perspectives. Clin Exp Obstet Gynecol 2023;50:160. [Crossref]
- Fan R, Feng F, Yang H, Xu K, Li S, You Y, Wan X, Zhu L. Pulmonary benign metastasizing leiomyomas: a case series of 23 patients at a single facility. BMC Pulm Med 2020;20:292. [Crossref] [PubMed]
- Pitts S, Oberstein EM, Glassberg MK. Benign metastasizing leiomyoma and lymphangioleiomyomatosis: sex-specific diseases? Clin Chest Med 2004;25:343-60. [Crossref] [PubMed]
- Makhoul K, Miller D, Ilyas U, Hosna A, Baig MA. Leiomyosarcoma: Lung Metastasis. Cureus 2023;15:e34373. [PubMed]
- Awonuga AO, Shavell VI, Imudia AN, Rotas M, Diamond MP, Puscheck EE. Pathogenesis of benign metastasizing leiomyoma: a review. Obstet Gynecol Surv 2010;65:189-95. [Crossref] [PubMed]
- Tietze L, Günther K, Hörbe A, Pawlik C, Klosterhalfen B, Handt S, Merkelbach-Bruse S. Benign metastasizing leiomyoma: a cytogenetically balanced but clonal disease. Hum Pathol 2000;31:126-8. [Crossref] [PubMed]
- Dossegger JM, Carneiro LH, Rodrigues RS, Barreto MM, Marchiori E. Pulmonary benign metastasizing leiomyoma presenting as small, diffuse nodules. J Bras Pneumol 2019;45:e20180318. [Crossref] [PubMed]
- Sawai Y, Shimizu T, Yamanaka Y, Niki M, Nomura S. Benign metastasizing leiomyoma and 18-FDG-PET/CT: A case report and literature review. Oncol Lett 2017;14:3641-6. [Crossref] [PubMed]
- Wu Y, Fan L, Niu Y, Wu Y, Gao W. Pulmonary benign metastasizing leiomyoma simultaneously diagnosed with uterine leiomyoma at first visit before hysteromyomectomy. Transl Cancer Res 2021;10:567-70. [Crossref] [PubMed]
- Wojtyś ME, Kacalska-Janssen O, Ptaszyński K, Lisowski P, Kunc M, Wójcik J, Grodzki T. Benign Metastasizing Leiomyoma of the Lung: Diagnostic Process and Treatment Based on Three Case Reports and a Review of the Literature. Biomedicines 2022;10:2465. [Crossref] [PubMed]
- Adair LB 2nd. CT findings of pathology proven benign metastasizing leiomyoma. Radiol Case Rep 2020;15:2120-4. [Crossref] [PubMed]
- Jaber M, Winner PJ, Krishnan R, Shu R, Khandelwal KM, Shah S. Benign Metastasizing Leiomyoma Causing Severe Tricuspid Regurgitation and Heart Failure. J Investig Med High Impact Case Rep 2023;11:23247096231173397. [Crossref] [PubMed]
- Glassman D, Patel JM, Hathout L, Thomas S, Girda E. Benign metastasizing leiomyoma in retroperitoneal lymph nodes with concurrent early stage cervical cancer. Gynecol Oncol Rep 2022;40:100975. [Crossref] [PubMed]
- Liu S, Zhou W, Fu W. Multiple Leiomyomas in a Patient with Benign Metastasizing Leiomyoma: A Case Report. Curr Med Imaging 2022;18:996-9. [Crossref] [PubMed]
- Jautzke G, Müller-Ruchholtz E, Thalmann U. Immunohistological detection of estrogen and progesterone receptors in multiple and well differentiated leiomyomatous lung tumors in women with uterine leiomyomas (so-called benign metastasizing leiomyomas). A report on 5 cases. Pathol Res Pract 1996;192:215-23. [Crossref] [PubMed]
- Horstmann JP, Pietra GG, Harman JA, Cole NG, Grinspan S. Spontaneous regression of pulmonary leiomyomas during pregnancy. Cancer 1977;39:314-21. [Crossref] [PubMed]
- Bakkensen JB, Samore W, Bortoletto P, Morton CC, Anchan RM. Pelvic and pulmonary benign metastasizing leiomyoma: A case report. Case Rep Womens Health 2018;18:e00061. [Crossref] [PubMed]
- Patton KT, Cheng L, Papavero V, Blum MG, Yeldandi AV, Adley BP, Luan C, Diaz LK, Hui P, Yang XJ. Benign metastasizing leiomyoma: clonality, telomere length and clinicopathologic analysis. Mod Pathol 2006;19:130-40. [Crossref] [PubMed]
- Nucci MR, Drapkin R, Dal Cin P, Fletcher CD, Fletcher JA. Distinctive cytogenetic profile in benign metastasizing leiomyoma: pathogenetic implications. Am J Surg Pathol 2007;31:737-43. [Crossref] [PubMed]
- Wu RC, Chao AS, Lee LY, Lin G, Chen SJ, Lu YJ, Huang HJ, Yen CF, Han CM, Lee YS, Wang TH, Chao A. Massively parallel sequencing and genome-wide copy number analysis revealed a clonal relationship in benign metastasizing leiomyoma. Oncotarget 2017;8:47547-54. [Crossref] [PubMed]
- Tong T, Fan Q, Wang Y, Li Y. Benign metastasizing uterine leiomyoma with lymphatic and pulmonary metastases: a case report and literature review. BMC Womens Health 2023;23:154. [Crossref] [PubMed]
- Awonuga AO, Rotas M, Imudia AN, Choi C, Khulpateea N. Recurrent benign metastasizing leiomyoma after hysterectomy and bilateral salpingo-oophorectomy. Arch Gynecol Obstet 2008;278:373-6. [Crossref] [PubMed]
- Wu S, Cai J, Chen Q, Wu J, Chen H. Increased 68 Ga-FAPI Uptake in Benign Metastasizing Leiomyoma. Clin Nucl Med 2023;48:809-11. [Crossref] [PubMed]
- Has Simsek D, Kuyumcu S, Ozkan ZG, Vuralli Bakkaloglu D, Bayram A, Topuz S, Aydıner A, Sanli Y. Demonstration of in vivo estrogen receptor status with 16α- [18F]fluoro-17ß-oestradiol (FES) PET/CT in a rare case of benign metastasizing leiomyoma. Eur J Nucl Med Mol Imaging 2021;48:4101-02. [Crossref] [PubMed]
- Hayden MA, Ordulu Z, Gallagher CS, Quade BJ, Anchan RM, Middleton NR, Srouji SS, Stewart EA, Morton CC. Clinical, pathologic, cytogenetic, and molecular profiling in self-identified black women with uterine leiomyomata. Cancer Genet 2018;222-223:1-8. [Crossref] [PubMed]
- Hodge JC, Morton CC. Genetic heterogeneity among uterine leiomyomata: insights into malignant progression. Hum Mol Genet 2007;16:R7-13. [Crossref] [PubMed]
- Sparić R, Andjić M, Babović I, Nejković L, Mitrović M, Štulić J, Pupovac M, Tinelli A. Molecular Insights in Uterine Leiomyosarcoma: A Systematic Review. Int J Mol Sci 2022;23:9728. [Crossref] [PubMed]
- McCarthy C, Gupta N, Johnson SR, Yu JJ, McCormack FX. Lymphangioleiomyomatosis: pathogenesis, clinical features, diagnosis, and management. Lancet Respir Med 2021;9:1313-27. [Crossref] [PubMed]
- Ataya A, Brantly M, Riley L. Lymphangioleiomyomatosis (LAM). Am J Respir Crit Care Med 2018;198:7-8. [Crossref] [PubMed]
- Mizuno M, Nawa A, Nakanishi T, Yatabe Y. Clinical benefit of endocrine therapy for benign metastasizing leiomyoma. Int J Clin Oncol 2011;16:587-91. [Crossref] [PubMed]


