
Reversible blindness after stellate ganglion block
Introduction
The stellate ganglion is a part of cervical sympathetic nerve chain formed by the fusion of inferior cervical and first thoracic ganglia. The blockage of this ganglion, known as stellate ganglion block (SGB), has been widely used in clinic as a new minimally invasive treatment in which local anesthetic drugs are injected into the stellate ganglion and the surrounding loose connective tissue. This reversible blockage of the voltage-gated sodium channel in the preganglionic and postganglionic nerve fibers anaesthetizes the corresponding nerve supply in areas such as the head, neck, face, upper chest, and upper limbs (1). In most cases, SGB is safe, with its common complications including transient upper limb numbness caused by puncture needle injury, pneumothorax, accidental puncture of blood vessels (common carotid artery, vertebral artery, internal jugular vein, etc.), local anesthetic-related adverse reactions (dizziness, tinnitus, chills, local anesthetic poisoning, etc.), brachial plexus, high epidural and subarachnoid block caused by improper puncture position, and others, such as hoarseness, puncture site pain, foreign body sensation, infection, and arrhythmia (2-6). In this paper, we report a rare case of reversible binocular blindness after SGB. We propose different explanations for this complication through careful analysis of clinical and imaging data.
Case presentation
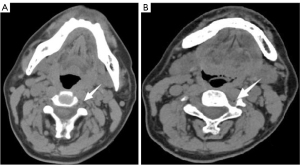
A 53-year-old male restaurant owner (height: 170 cm; weight: 65 kg) who had always been in good health began to experience left ear hearing loss 3 months prior to visiting the Ear, Nose, And Throat (ENT) department of our hospital, where he was diagnosed with sensorineural hearing. He was then transferred to the Pain Department for planetary ganglion block, where he underwent two SGBs through the left anterolateral approach performed without imaging guidance. When we completed the puncture with bare hands, we mainly touched the left sixth transverse process of the cervical spine through the second cervical crease, inserted the needle into the surface of the transverse process, and later retracted it. After it was confirmed that no blood was drawn back, a mixture of local anesthetics was injected. Both interventions went smoothly, and the patient reported that his hearing had somewhat recovered (about 20%). The left anterolateral approach was attempted for the third time, in which the patient’s neck vessel sheath was pulled and a 25-G puncture needle inserted. After negative aspiration without blood, 10 mL of 0.8% lidocaine was injected. The patient immediately experienced a splitting headache, mainly at the bilateral temporal region and forehead. After a short while, the patient’s consciousness began to deteriorate; however, he could still respond to verbal commands. The attending doctor immediately established an intravenous line, administered 500 mL of 0.9% normal saline (2.5 mL/min), initiated electrocardiographic monitoring, and observed vital signs. Upon review half an hour later, the patient’s vital signs were stable, with no obvious fluctuation. However, he still complained of severe headache. An urgent computed tomography (CT) scan of the head and neck was performed, which showed a small gas shadow in and around the left vertebral artery at the C4–6 level (Figure 1A,1B), suggesting that the puncture needle might have entered the left vertebral artery, resulting in the entry of local anesthetics and air. After completion of CT, the patient complained of fatigue and decreased visual acuity in both eyes and restlessness. He was immediately given 500 mL of 5% dextrose (6 mL/min) and intramuscular diazepam (2 mL: 10 mg). After the patient had calmed down to some extent, he underwent a cranial magnetic resonance examination. A few abnormal signals were seen in the left basal ganglia. On the diffusion-weighted imaging (DWI) sequence, the signal increased, suggesting acute cerebral infarction (Figure 2). At this time, the patient complained of headache and blindness, which had induced a state of panic. An ophthalmologist and neurologist were consulted. From the examination, it was deemed that the patient was conscious, the expression of language was clear, high-level neurological function had not declined, and binocular visual acuity included only light perception. Ocular examination yielded unremarkable findings for the conjunctiva, cornea, anterior chamber, and iris. Pupil examination showed a round, regular, and reactive pupil without relative afferent pupillary defect. Fundus examination showed no pathological changes in the optic disc, macula, and or retina. After consultation with the senior physician, the patient was immediately transferred and admitted to the Neurology Department. The blood test report indicated a glucose level of 6.34 mmol/L (3.9–6.1 mmol/L), a total glycosylated hemoglobin level of 9.77% (4.0–6.0%), a total leukocyte count of 13.25×109/L [(4–10)×109/L], a neutrophil proportion of 85.60% [(1.8–6.3)×109/L], and a lymphocyte proportion of 10.20% (20–40%), indicating that the patient was in a state of distress. At the request of the attending neurologist, CT angiography (CTA) of head and neck was again performed. The patient was extremely restless, due to which his family member had to hold his head while CTA was performed. CTA showed a developmental variation of the vertebral artery, which included an extremely thin right vertebral artery and basilar artery, with the left vertebral artery coursing in front of the transverse process of the sixth cervical vertebra. There was no definite bleeding observed in the brain parenchyma (Figure 3A-3C). Symptomatic treatment was provided and included antiplatelet drugs, statins, intravenous fluids, analgesics, and nerve nourishing medications, among other measures. In the early morning following after overnight observation, the patient’s binocular appeared to be recovered. During the subsequent 6-month follow-up, the patient exhibited no abnormalities. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.


Discussion
Many studies have shown that the sympathetic nervous system (SNS) is closely related to neuropathic pain: however, the exact role of the SNS in the development of neuropathic pain is unclear. The mechanism of sympathetic modulation is mainly vasoconstriction and pain conduction. The sympathetic nerve is involved in regulating blood vessel relaxation and contraction. When the sympathetic nerve is excited, peripheral vasoconstriction occurs, resulting in a relative decrease in blood flow and insufficient blood supply. This is in turn increases the anaerobic metabolism of muscles and produces lactic acid and other metabolites, which eventually leads to pain. When the sympathetic nerve is damaged by high temperature or mechanical or chemical effects, stimulation of the sympathetic nerve decreases, causing relative vasodilation, decreased peripheral vascular resistance, and increased collateral and peripheral blood volume, thereby increasing skin and muscle blood perfusion (7,8). The sympathetic nerve is also involved in producing and releasing pain-related mediators, such as nerve growth factor, interleukin-8, and bradykinin (9). In addition, the SNS participates in the reflex regulation of systemic inflammation (10,11). After the sympathetic nerve is blocked, it can block the transmission of pain stimuli to the central nervous system through nerve fibers, thereby inhibiting pain transmission and reducing neuropathic pain (12). Therefore, sympathetic nerve block plays an important role in the treatment of various painful and ischemic diseases.
The cervical sympathetic chain consists of the upper, middle, and lower cervical ganglia. In approximately 80% of the population, the inferior cervical ganglion fuses with the first thoracic ganglion to form the cervicothoracic ganglion, which is also known as the stellate ganglion (13). It is located in the front between the base of the transverse process of the seventh cervical vertebra and the neck of the first rib, behind the vertebral artery, inside the scalene muscle group, and below the lung apex. It is oval in shape, about 2 cm long, and 1 cm wide (8). By the inferior cervical sympathetic ganglion and the first thoracic ganglion, the stellate ganglion connects the seventh and eighth cervical nerves and the first thoracic nerve by sending out gray communicating branches. It also sends out branches to surround the subclavian artery forming the subclavian artery plexus, which reaches the first segment of the axillary artery. Some of its branches also form the vertebral artery plexus around the vertebral artery and course along the vertebral artery, while other branches enter the skull cavity, surround the vertebral artery and basilar artery, and reach the posterior cerebral artery, where they meet with the nerve plexus from the internal carotid artery. In addition, its subcardiac nerve descends behind the subclavian artery and in front of the trachea, joins the cardiac plexus. and participates in the activity of the heart. Therefore, the stellate ganglion can innervate the sweat glands and blood vessels of the face with the external carotid artery plexus; innervate the fundus, pupil, eyelid smooth muscle, and the skin near the eyebrow arch through the internal carotid artery plexus; innervate the blood vessels of the brainstem, cerebellum, temporal lobe, and the base of the occipital lobe and inner ear through the vertebral artery plexus; and regulate the activity of the heart and its blood vessels by participating in the formation of cardiac plexus. In addition, the stellate ganglion has extensive neuronal connections with the hypothalamus and amygdala, as well as the lower marginal and ventral medial temporal cortex (14). A basic mechanism of action of SGB is the blocking of the sympathetic nerves in the head, neck, and other areas, which can improve the blood supply of these parts. Its analgesic mechanism can be explained by the blocking of the neuronal connections within its innervation (15,16).
Traditionally, SGB is achieved by palpation of the transverse process or Chassaignac tubercle of the C6 vertebra and immediate injection of local anesthetic into the medial side without any imaging guidance. When performing SGB, we used a higher dose of 10 mL due to the location being at the C6 level. In order to reduce the toxic reactions of lidocaine, a relatively safe concentration was chosen, with a compatibility of 0.8%. The appearance of Horner syndrome was considered to be a sign of a successful block. Because the stellate ganglion is adjacent to many important structures, SGB may be related to some serious local and systemic complications. Local complications are generally related to puncture, such as pneumothorax, hematoma, brachial plexus, and injury. Systemic complications are mainly due to unexpected drug absorption leading to a systemic reaction or accidental intravascular injections when a palpation technique is used (2-6). Kimura et al. and Yokota et al. both reported the presence of severe hypertension after SGB (17,18). The researchers in both groups speculated that the increase in blood pressure was the result of vagus nerve block caused by the diffusion of local anesthetic along the carotid sheath. In addition, there are some reports of total spinal cord paralysis caused by drugs entering the subdural space and subarachnoid space (19-23), as well as of headache, seizures, dyspnea, or rare atresia syndrome caused by arterial damage and drug entering the blood (24,25).
In our case, the patient developed headache immediately after the third SGB, which was accompanied by blindness. Twenty hours following active fluid resuscitation and sedation, the patient’s binocular vision recovered. Interestingly, in 1981, Szeinfeld et al. (26) reported a similar case. A 51-year-old man was treated with SGB due to injury to his right forearm and wrist. When 1 mL of 0.25% bupivacaine was injected, the patient immediately became pale, his pupils dilated, he became blind, and he was unable to speak. After about 5 minutes, the patient’s vision recovered. We speculate that the central nervous system toxicity of local anesthetics depends on its concentration. After the local anesthetic enters the blood through the vertebral artery, the cerebral blood volume remains unchanged, while the local anesthetic concentration in the local brain tissue increases, which, in this case, led to the patient’s blindness. We reviewed the imaging data of patient. At the initial stage of symptoms (within 1 hour), we performed CT and magnetic resonance imaging (MRI) examination of the head and neck. The CT results showed a gas shadow in the left vertebral artery, which confirmed the speculation that local anesthetics had entered the vertebral artery (Figure 1A,1B). During the process of interventional therapy involving puncture, the steps of “venting” and “bloodless withdrawal” are typically crucial. However, occasionally, insufficient venting or inadequate fixation may lead to the inadvertent entry of the needle into a blood vessel. The MRI results showed that there was an acute infarct in the left thalamus, which proved that the patient had acute ischemia of the vertebrobasilar system (Figure 2). In the follow-up examination, we performed CTA imaging of the patient’s head and neck. It was found that the right vertebral artery of the patient was extremely thin, while the left vertebral artery had developmental variation, coursing in front of the anterior tubercle of the C6 transverse process (Figure 3A-3C). Therefore, we can assume that the operator accidentally penetrated the abnormally located left vertebral artery, and part of the air and local anesthetics entered the left vertebral artery. The air bubbles might have led to lumen stenosis, which along with the decreased compensatory ability of the thin right vertebral artery, ultimately reduced the blood flow of basilar artery. Moreover, after local anesthetics were injected into the blood, this could have led to small vessel spasm, which would have further decreased basilar artery blood flow. The patient was conscious and had no symptoms such as convulsion or respiratory depression, which also suggested no central neurotoxic reaction to local anesthetics had transpired. In addition, the patient’s binocular blindness occurred about 1 hour later. If it was a central neurotoxic reaction of local anesthetics, the symptoms would have appeared in the early stage.
In SGB, local anesthetic injection into the vertebral artery is a rare complication, especially the symptom of binocular blindness. In order to avoid this complication, it is suggested that SGB be performed under imaging guidance. With the application of ultrasound in pain medicine in recent years, there have been many relevant reports of success, and this is a direction that can be pursued in future research.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1421/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ikeda T, Iwase S, Sugiyama Y, Matsukawa T, Mano T, Doi M, Kikura M, Ikeda K. Stellate ganglion block is associated with increased tibial nerve muscle sympathetic activity in humans. Anesthesiology 1996;84:843-50. [Crossref] [PubMed]
- Bhatia A, Flamer D, Peng PW. Evaluation of sonoanatomy relevant to performing stellate ganglion blocks using anterior and lateral simulated approaches: an observational study. Can J Anaesth 2012;59:1040-7. [Crossref] [PubMed]
- Kapral S, Krafft P, Gosch M, Fleischmann D, Weinstabl C. Ultrasound imaging for stellate ganglion block: direct visualization of puncture site and local anesthetic spread. A pilot study. Reg Anesth 1995;20:323-8.
- Luo G, He J, Wu T, Huang Y, Miao Z, Zhao Z, Wang X, Wang Y. The Therapeutic Effect of Stellate Ganglion Block on Facial Nerve Palsy in Patients with Type 2 Diabetes Mellitus. Eur Neurol 2015;74:112-7. [Crossref] [PubMed]
- Uchida T, Nakao S, Morimoto M, Iwamoto T. Serious cervical hematoma after stellate ganglion block. J Anesth 2015;29:321. [Crossref] [PubMed]
- Goel V, Patwardhan AM, Ibrahim M, Howe CL, Schultz DM, Shankar H. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med 2019; Epub ahead of print. [Crossref]
- Ding Y, Yao P, Li H, Zhao R, Zhao G. Evaluation of combined radiofrequency and chemical blockade of multi-segmental lumbar sympathetic ganglia in painful diabetic peripheral neuropathy. J Pain Res 2018;11:1375-82. [Crossref] [PubMed]
- Gunduz OH, Kenis-Coskun O. Ganglion blocks as a treatment of pain: current perspectives. J Pain Res 2017;10:2815-26. [Crossref] [PubMed]
- Smits H, van Kleef M, Holsheimer J, Joosten EA. Experimental spinal cord stimulation and neuropathic pain: mechanism of action, technical aspects, and effectiveness. Pain Pract 2013;13:154-68. [Crossref] [PubMed]
- McAllen RM, McKinley MJ, Martelli D. Reflex regulation of systemic inflammation by the autonomic nervous system. Auton Neurosci 2022;237:102926. [Crossref] [PubMed]
- Udit S, Blake K, Chiu IM. Somatosensory and autonomic neuronal regulation of the immune response. Nat Rev Neurosci 2022;23:157-71. [Crossref] [PubMed]
- Schlereth T, Birklein F. The sympathetic nervous system and pain. Neuromolecular Med 2008;10:141-7. [Crossref] [PubMed]
- Raut MS, Maheshwari A. Stellate Ganglion Block: Important Weapon in the Anesthesiologists' Armamentarium. J Cardiothorac Vasc Anesth 2018;32:e36-7. [Crossref] [PubMed]
- Westerhaus MJ, Loewy AD. Central representation of the sympathetic nervous system in the cerebral cortex. Brain Res 2001;903:117-27. [Crossref] [PubMed]
- Kang CK, Oh ST, Chung RK, Lee H, Park CA, Kim YB, Yoo JH, Kim DY, Cho ZH. Effect of stellate ganglion block on the cerebrovascular system: magnetic resonance angiography study. Anesthesiology 2010;113:936-44. [Crossref] [PubMed]
- Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dent Anesth Pain Med 2016;16:159-63. [Crossref] [PubMed]
- Kimura T, Nishiwaki K, Yokota S, Komatsu T, Shimada Y. Severe hypertension after stellate ganglion block. Br J Anaesth 2005;94:840-2. [Crossref] [PubMed]
- Yokota S, Komatsu T, Kimura T, Shimada Y. A case of severe hypertension caused by stellate ganglion block in a patient with facial palsy. Masui 1996;45:1123-6.
- Balaban B, Baklaci K, Taskaynatan MA, Mohur H. Delayed subdural block as an unusual complication following stellate ganglion blockade. The Pain Clinic 2005;17:407-9.
- Leong MS, Mackey S. Delayed subdural block after a stellate ganglion block. Anesthesiology 2001;94:358-9. [Crossref] [PubMed]
- Bruyns T, Devulder J, Vermeulen H, De Colvenaer L, Rolly G. Possible inadvertent subdural block following attempted stellate ganglion blockade. Anaesthesia 1991;46:747-9. [Crossref] [PubMed]
- Kapral S, Krafft P, Gosch M, Fridrich P, Weinstabl C. Subdural, extra-arachnoid block as a complication of stellate ganglion block: documentation with ultrasound. Anasthesiol Intensivmed Notfallmed Schmerzther 1997;32:638-40. [Crossref] [PubMed]
- Sari S, Aydin ON. Intraspinal blockade after stellate ganglion blockade in trauma patient. Reg Anesth Pain Med 2012;37:E239-40.
- Corsaro ME, Averni F, Messina A, Messina S, Corsaro S, Averni R, Azzolina R. Clinical evidence on risk and complications of the stellate ganglion block: transient locked-in syndrome. Acta Medica Mediterranea 2009;25:7-8.
- Chaturvedi A, Dash H. Locked-in syndrome during stellate ganglion block. Indian J Anaesth 2010;54:324-6. [Crossref] [PubMed]
- Szeinfeld M, Laurencio M, Pallares VS. Total reversible blindness following attempted stellate ganglion block. Anesth Analg 1981;60:689-90.


